Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production
Abstract
:1. Introduction
2. Methods
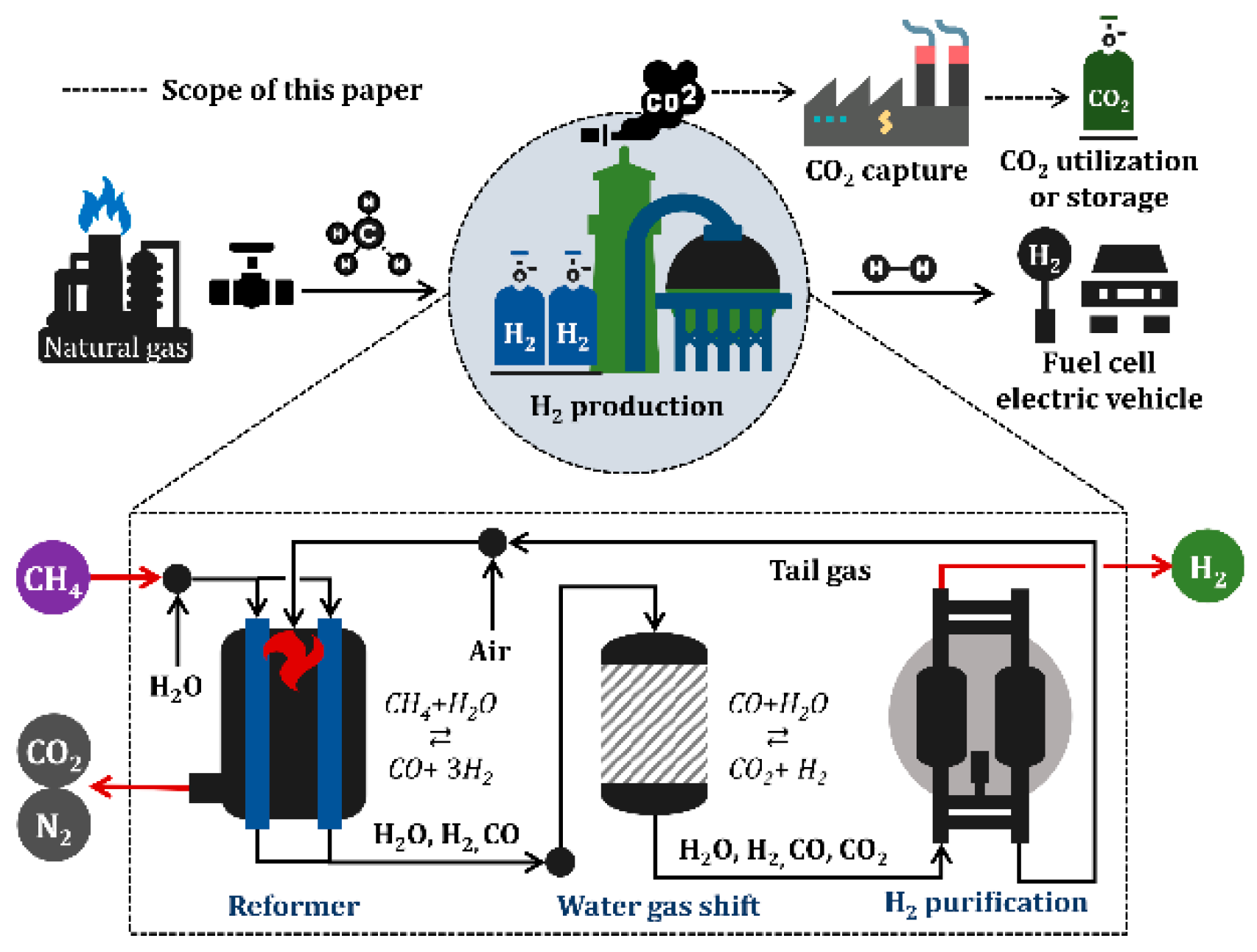
2.1. Process Description
2.2. Scenario Development
2.3. Economic Evaluation
2.4. Sensitivity and Uncertainty Analysis
3. Results and Discussion
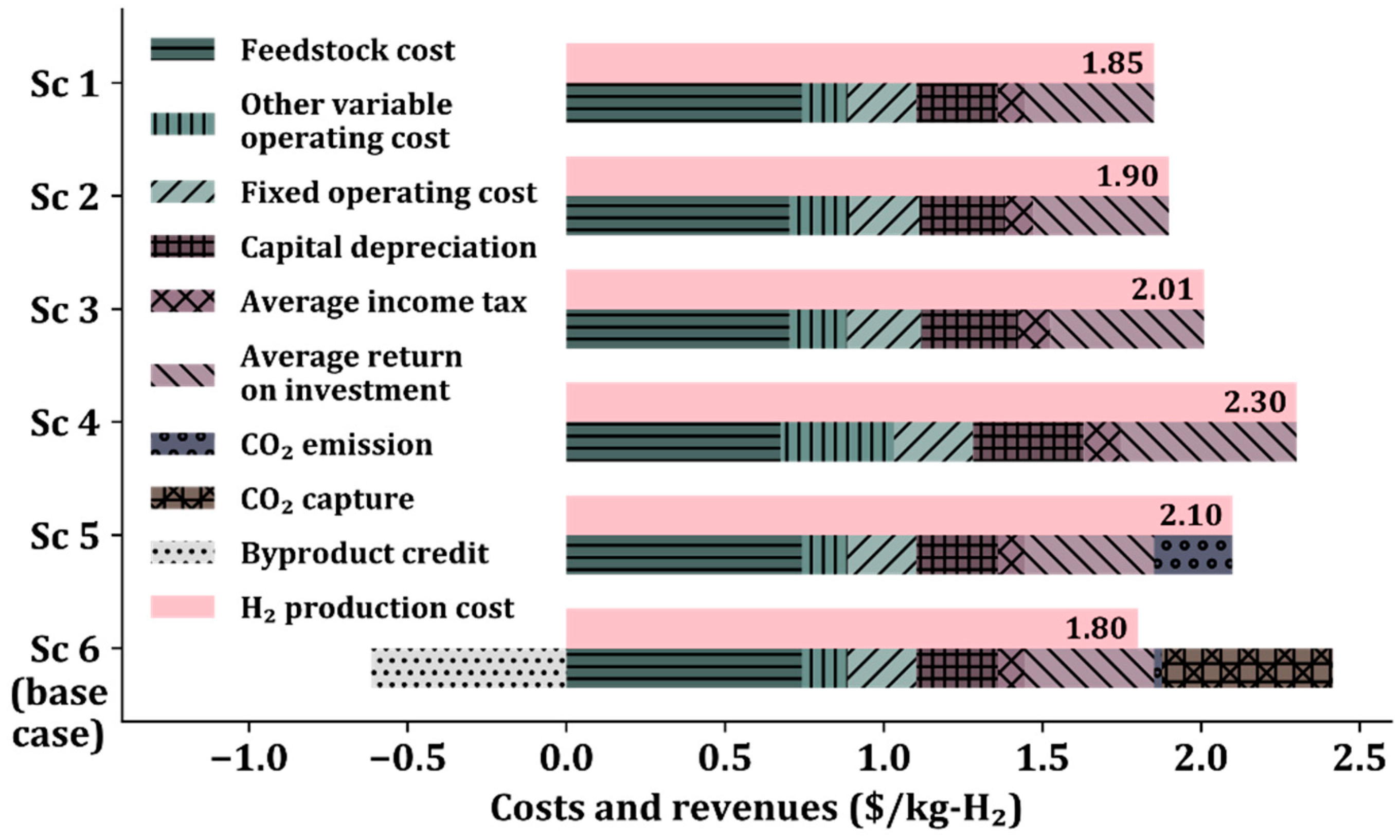
3.1. Capital and Operating Costs
3.2. Hydrogen Production Cost
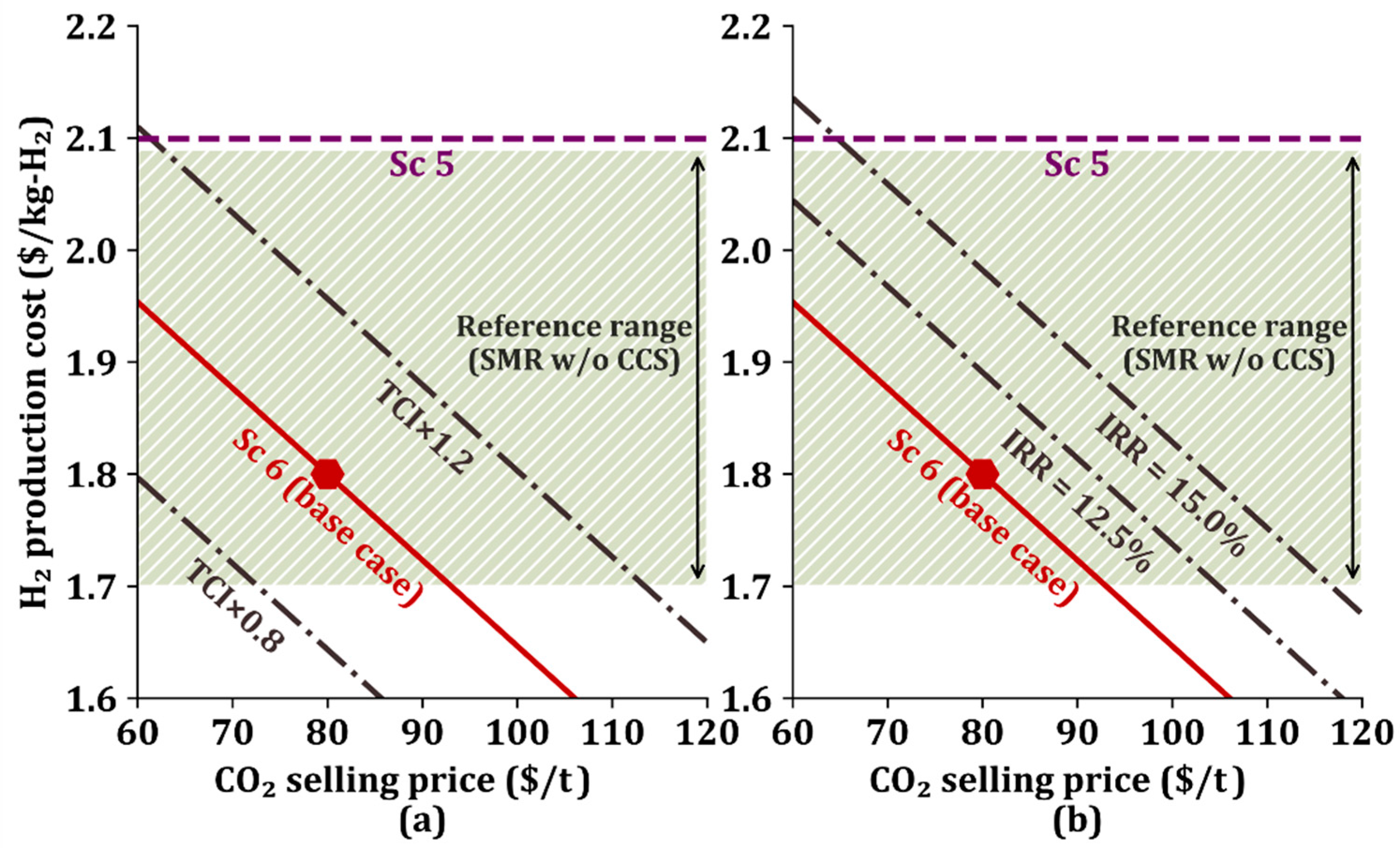
3.3. Sensitivity Analysis
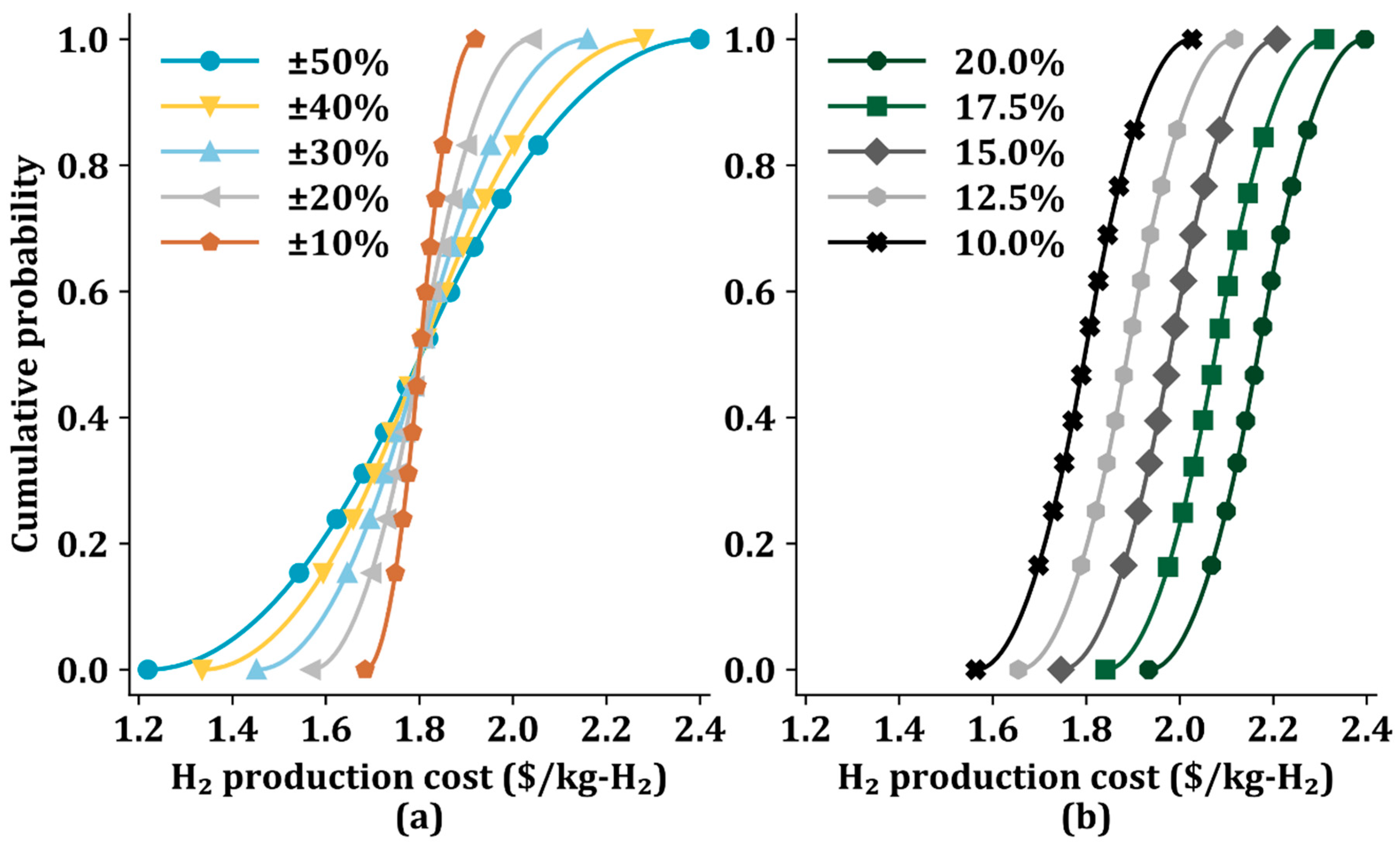
3.4. Uncertainty Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Won, W.; Motagamwala, A.H.; Dumesic, J.A.; Maravelias, C.T. A co-solvent hydrolysis strategy for the production of biofuels: Process synthesis and technoeconomic analysis. React. Chem. Eng. 2017, 2, 397–405. [Google Scholar] [CrossRef]
- Kim, M.; Won, W.; Kim, J. Integration of carbon capture and sequestration and renewable resource technologies for sustainable energy supply in the transportation sector. Energy Convers. Manag. 2017, 143, 227–240. [Google Scholar] [CrossRef]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.M.; et al. Net-zero emissions energy systems. Science 2018, 360, 6396. [Google Scholar] [CrossRef] [Green Version]
- Thonemann, N.; Pizzol, M. Consequential life cycle assessment of carbon capture and utilization technologies within the chemical industry. Energy Environ. Sci. 2019, 12, 2253–2263. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Won, W.; Barnett, K.J.; Brentzel, Z.J.; Alonso, D.M.; Huber, G.W.; Dumesic, J.A.; Maravelias, C.T. Improving economics of lignocellulosic biofuels: An integrated strategy for coproducing 1,5-pentanediol and ethanol. Appl. Energy 2018, 213, 585–594. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Huang, K.; Maravelias, C.T.; Dumesic, J.A. Solvent system for effective near-term production of hydroxymethylfurfural (HMF) with potential for long-term process improvement. Energy Environ. Sci. 2019, 12, 2212–2222. [Google Scholar] [CrossRef]
- Urban, C.; Xu, J.; Sträuber, H.; dos Santos Dantas, T.R.; Mühlenberg, J.; Härtig, C.; Angenent, L.T.; Harnisch, F. Production of drop-in fuels from biomass at high selectivity by combined microbial and electrochemical conversion. Energy Environ. Sci. 2017, 10, 2231–2244. [Google Scholar] [CrossRef]
- Han, S.; Won, W.; Kim, J. Scenario-based approach for design and comparatively analysis of conventional and renewable energy systems. Energy 2017, 129, 86–100. [Google Scholar] [CrossRef]
- Heede, R.; Oreskes, N. Potential emissions of CO2 and methane from proved reserves of fossil fuels: An alternative analysis. Glob. Environ. Chang. 2016, 36, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Won, W.; Kwon, H.; Han, J.-H.; Kim, J. Design and operation of renewable energy sources based hydrogen supply system: Technology integration and optimization. Renew. Energy 2017, 103, 226–238. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.; Lee, H.; Byun, M.; Yoon, C.W.; Lim, H. Assessment of the economic potential: COx-free hydrogen production from renewables via ammonia decomposition for small-sized H2 refueling stations. Renew. Sustain. Energy Rev. 2019, 113, 109262. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen fuel cell vehicles; current status and future prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Won, W.; Maravelias, C.T. Thermal fractionation and catalytic upgrading of lignocellulosic biomass to biofuels: Process synthesis and analysis. Renew. Energy 2017, 114, 357–366. [Google Scholar] [CrossRef]
- Ayodele, T.R.; Munda, J.L. Potential and economic viability of green hydrogen production by water electrolysis using wind energy resources in South Africa. Int. J. Hydrogen Energy 2019, 44, 17669–17687. [Google Scholar] [CrossRef]
- Nguyen, T.; Abdin, Z.; Holm, T.; Mérida, W. Grid-connected hydrogen production via large-scale water electrolysis. Energy Convers. Manag. 2019, 200, 112108. [Google Scholar] [CrossRef]
- Chen, J.; Han, J.; Xu, D. Efficient operation of autothermal microchannel reactors for the production of hydrogen by steam methane reforming. Int. J. Hydrogen Energy 2019, 44, 11546–11563. [Google Scholar] [CrossRef]
- Boyano, A.; Blanco-Marigorta, A.M.; Morosuk, T.; Tsatsaronis, G. Exergoenvironmental analysis of a steam methane reforming process for hydrogen production. Energy 2011, 36, 2202–2214. [Google Scholar] [CrossRef]
- Saeidi, S.; Fazlollahi, F.; Najari, S.; Iranshahi, D.; Klemeš, J.J.; Baxter, L.L. Hydrogen production: Perspectives, separation with special emphasis on kinetics of WGS reaction: A state-of-the-art review. J. Ind. Eng. Chem. 2017, 49, 1–25. [Google Scholar] [CrossRef]
- Lai, G.H.; Lak, J.H.; Tsai, D.H. Hydrogen production via low-temperature steam–methane reforming using Ni–CeO2–Al2O3 hybrid nanoparticle clusters as catalysts. ACS Appl. Energy Mater. 2019, 2, 7963–7971. [Google Scholar] [CrossRef]
- Jung, W.; Park, J.; Won, W.; Lee, K.S. Simulated moving bed adsorption process based on a polyethylenimine-silica sorbent for CO2 capture with sensible heat recovery. Energy 2018, 150, 950–964. [Google Scholar] [CrossRef]
- Son, Y.; Won, W.; Lee, T.; Lee, K.S. An intermittently moving bed adsorption process for carbon dioxide capture. Int. J. Greenh. Gas Control 2016, 49, 34–46. [Google Scholar] [CrossRef]
- Park, J.; Won, W.; Jung, W.; Lee, K.S. One-dimensional modeling of a turbulent fluidized bed for a sorbent-based CO2 capture process with solid-solid sensible heat exchange. Energy 2019, 168, 1168–1180. [Google Scholar] [CrossRef]
- Nexant Inc. Equipment Design and Cost Estimation for Small Modular Biomass Systems, Synthesis Gas Cleanup, and Oxygen Separation Equipment; National Renewable Energy Laboratory: Golden, CO, USA, 2006.
- Liu, K.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Sharma, I.; Friedrich, D.; Golden, T.; Brandani, S. Exploring the opportunities for carbon capture in modular, small-scale steam methane reforming: An energetic perspective. Int. J. Hydrogen Energy 2019, 44, 14732–14743. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Wagner, J.L. Selective Adsorption Process. U.S. Patent 3430418, 4 March 1969. [Google Scholar]
- Fuderer, A.; Rudelstorfer, E. Selective Adsorption Process. U.S. Patent 3986849, 19 October 1976. [Google Scholar]
- Kim, H.; Lee, S.; Lee, J.; Won, W. Simultaneous production of 1,6-hexanediol, furfural, and high-purity lignin from white birch: Process integration and techno-economic evaluation. Bioresour. Technol. 2021, 331, 125009. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; You, H.; Choi, K.S.; Han, S. A Study on Interconnecting to the power grid of new energy using the natural gas pressure. J. Electr. Eng. Technol. 2020, 15, 307–314. [Google Scholar] [CrossRef]
- Park, H.G.; Han, S.Y.; Jun, K.W.; Woo, Y.; Park, M.J.; Kim, S.K. Bench-scale steam reforming of methane for hydrogen production. Catalysts 2019, 9, 615. [Google Scholar] [CrossRef] [Green Version]
- Haryanto, A.; Fernando, S.D.; Filip To, S.D.; Steele, P.H.; Pordesimo, L.; Adhikari, S. High temperature water gas shift reaction over nickel catalysts for hydrogen production: Effect of supports, GHSV, metal loading, and dopant materials. J. Thermodyn. Catal. 2011, 2, 645–2153. [Google Scholar] [CrossRef] [Green Version]
- Jeong, D.W.; Potdar, H.S.; Roh, H.S. Comparative study on nano-sized 1 wt% Pt/Ce0.8Zr0.2O2 and 1 wt% Pt/Ce0.2Zr0.8O2 catalysts for a single stage water gas shift reaction. Catal. Lett. 2012, 142, 439–444. [Google Scholar] [CrossRef]
- Carbon Dioxide (CO2) Market Share, Size, Trends, & Industry Analysis Report, By Sources (Hydrogen, Ethyl Alcohol, Ethylene Oxide, Substitute Natural Gas), By Application (Food & Beverages, Oil & Gas, Medical, Rubber, Fire Fighting), By Regions: Segment Forecast, 2018–2026. Available online: https://www.polarismarketresearch.com/industry-analysis/carbon-dioxide-market (accessed on 8 June 2021).
- Zhang, Z.; Pan, S.Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Liang, F.; Lindberg, P.; Lindblad, P. Engineering photoautotrophic carbon fixation for enhanced growth and productivity. Sustain. Energy Fuels 2018, 2, 2583–2600. [Google Scholar] [CrossRef] [Green Version]
- The Chemical Engineering Plant Cost Index. Available online: https://www.chemengonline.com/pci-home (accessed on 3 May 2021).
- Steward, D.; Ramsden, T.; Zuboy, J. H2A Central Hydrogen Production Model, Version 3 User Guide (DRAFT); National Renewable Energy Laboratory: Golden, CO, USA, 2012.
- Davis, R.E.; Grundl, N.J.; Tao, L.; Biddy, M.J.; Tan, E.C.D.; Beckham, G.T.; Humbird, D.; Thompson, D.N.; Roni, M.S. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels and Coproducts: 2018 Biochemical Design Case Update; National Renewable Energy Laboratory: Golden, CO, USA, 2018.
- Davis, R.; Tao, L.; Scarlata, C.; Tan, E.C.D.; Ross, J.; Lukas, J.; Sexton, D. Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute-Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons; National Renewable Energy Laboratory: Golden, CO, USA, 2015.
- Turton, R.; Shaeiwitz, J.A.; Bhattacharyya, D.; Whiting, W.B. Analysis, Synthesis and Design of Chemical Processes, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2018. [Google Scholar]
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Farris, P.W.; Bendle, N.; Pfeifer, P.E.; Reibstein, D. Marketing Metrics: The Definitive Guide to Measuring Marketing Performance, 2nd ed.; Pearson Education: London, UK, 2010. [Google Scholar]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Choe, B.; Lee, S.; Won, W. Process integration and optimization for economical production of commodity chemicals from lignocellulosic biomass. Renew. Energy 2020, 162, 242–248. [Google Scholar] [CrossRef]
- Choe, B.; Lee, S.; Lee, H.; Lee, J.; Lim, H.; Won, W. Integrated strategy for coproducing bioethanol and adipic acid from lignocellulosic biomass. J. Clean. Prod. 2021, 311, 127849. [Google Scholar] [CrossRef]



| Parameters | Values | References |
|---|---|---|
| Area 100: Hydrogen production | ||
| Natural gas pressure (kPa) | 800 | [31] |
| Steam reformer temperature (°C) | 800 | [25] |
| GHSV of steam reformer (m3/h⋅kg-cat.) | 2.0 | [32] |
| GHSV of high temperature of WGS reactor (m3/h⋅kg-cat.) | 1.0 | [33] |
| GHSV of low temperature of WGS reactor (m3/h⋅m3-cat.) | 6000 | [34] |
| Area 200: Steam production | ||
| Steam to CH4 molar ratio | 3.0 | [25] |
| Area 300: Hydrogen purification | ||
| H2 recovery | 75% | [24] |
| H2 purification | 99.99% | [24] |
| Operating pressure range (kPa) | 2–800 | Assumed |
| Area 400: Feed intake | ||
| Temperature of exhaust gas (°C) | 900 | Assumed |
| Steam Reformer Pressure (kPa) | Number of WGS Reactors | WGS Reactor Temperature (°C) | CO2 Emission Allowances | CO2 Capture and Sales | |
|---|---|---|---|---|---|
| Sc 1 | 800 | 1 | 450 | N | N |
| Sc 2 | 800 | 1 | 250 | N | N |
| Sc 3 | 800 | 2 | 450, 250 | N | N |
| Sc 4 | 100 | 1 | 450 | N | N |
| Sc 5 | 800 | 1 | 450 | Y | N |
| Sc 6 | 800 | 1 | 450 | Y | Y |
| Parameters | Values | References |
|---|---|---|
| Natural gas price ($/t) | 239.0 | [41] |
| Ru–Ni/Al2O3 catalyst (steam reformer) ($/kg) * | 25.0 | [24] |
| Cu/Fe3O4–Cr2O3 catalyst (WGS reactor) ($/kg) * | 25.0 | [24] |
| Adsorbent ($/kg) * | 2.0 | [24] |
| Deionized water ($/t) | 14.5 | [42] |
| CO2 emission allowances ($/t) | 29.0 | [43] |
| CO2 capture cost ($/t) | 70.0 | [43] |
| CO2 selling price ($/t) | 80.0 | [43] |
| Plant financing by equity (%) | 40.0 | [39] |
| Plant life (y) | 20 | [39] |
| Income tax rate (%) | 35.0 | [39] |
| Internal rate of return (%) | 10.0 | [39] |
| Interest rate for debt financing (%) | 8.0 | [39] |
| Term for debt financing (y) | 10.0 | [39] |
| Plant depreciation (y) | 7.0 | [39] |
| Average labor rate ($/h) | 20.0 | [39] |
| Land (% of fixed capital investment) | 2.0 | [39] |
| Working capital (% of fixed capital investment) | 5.0 | [39] |
| On-stream percentage after startup (%) | 90.0 | [39] |
| Startup time (y) | 0.5 | [39] |
| Revenue and costs during startup | ||
| Revenue (% of normal) | 50.0 | [39] |
| Variable costs (% of normal) | 50.0 | [39] |
| Fixed costs (% of normal) | 75.0 | [39] |
| Construction period (y) | 1.0 | [39] |
| Capital Costs ($) | ||||||
|---|---|---|---|---|---|---|
| Sc 1 | Sc 2 | Sc 3 | Sc 4 | Sc 5 | Sc 6 | |
| A100: Hydrogen production | 307,888 | 326,817 | 393,578 | 289,849 | 307,888 | 307,888 |
| A200: Steam production | 23,608 | 25,331 | 26,493 | 10,759 | 23,608 | 23,608 |
| A300: Hydrogen purification | 102,747 | 104,032 | 104,032 | 295,521 | 102,747 | 102,747 |
| A400: Feed intake | 12,347 | 11,842 | 12,210 | 11,827 | 12,347 | 12,347 |
| Total installed equipment costs | 446,590 | 468,021 | 536,312 | 607,956 | 446,590 | 446,590 |
| Total direct costs | 526,976 | 552,265 | 627,231 | 717,388 | 526,976 | 526,976 |
| Total indirect costs | 316,186 | 331,359 | 376,338 | 430,433 | 316,186 | 316,186 |
| Fixed capital investment | 843,162 | 883,624 | 1,003,569 | 1,147,821 | 843,162 | 843,162 |
| Total capital investment | 902,183 | 945,478 | 1,073,819 | 1,228,168 | 902,183 | 902,183 |
| Operating Costs ($/y) | ||||||
| Sc 1 | Sc 2 | Sc 3 | Sc 4 | Sc 5 | Sc 6 | |
| Feedstock | 121,977 | 121,860 | 120,588 | 119,368 | 121,977 | 121,977 |
| Ru–Ni/Al2O3 catalyst | 462 | 438 | 438 | 420 | 462 | 462 |
| Cu/Fe3O4–Cr2O3 catalyst | 513 | 665 | 879 | 478 | 513 | 513 |
| Adsorbent | 1776 | 1813 | 1813 | 1940 | 1776 | 1776 |
| Electricity | 6215 | 6391 | 6391 | 30,434 | 6215 | 6215 |
| Cooling water | 834 | 783 | 696 | 834 | 834 | 834 |
| Deionized water | 13,652 | 14,434 | 14,434 | 16,056 | 13,652 | 13,652 |
| CO2 emission allowances | - | - | - | - | 40,632 | 40,632 |
| CO2 capture | - | - | - | - | - | 88,270 |
| Fixed operating costs | 35,634 | 36,346 | 38,456 | 40,994 | 35,634 | 35,634 |
| Total operating costs | 181,063 | 182,730 | 183,695 | 210,524 | 221,696 | 273,397 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kim, H.S.; Park, J.; Kang, B.M.; Cho, C.-H.; Lim, H.; Won, W. Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production. Appl. Sci. 2021, 11, 6021. https://doi.org/10.3390/app11136021
Lee S, Kim HS, Park J, Kang BM, Cho C-H, Lim H, Won W. Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production. Applied Sciences. 2021; 11(13):6021. https://doi.org/10.3390/app11136021
Chicago/Turabian StyleLee, Shinje, Hyun Seung Kim, Junhyung Park, Boo Min Kang, Churl-Hee Cho, Hankwon Lim, and Wangyun Won. 2021. "Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production" Applied Sciences 11, no. 13: 6021. https://doi.org/10.3390/app11136021
APA StyleLee, S., Kim, H. S., Park, J., Kang, B. M., Cho, C.-H., Lim, H., & Won, W. (2021). Scenario-Based Techno-Economic Analysis of Steam Methane Reforming Process for Hydrogen Production. Applied Sciences, 11(13), 6021. https://doi.org/10.3390/app11136021






