Abstract
We aimed to determine the association of cervical range of motion (ROM) with the clinical features of headache and neck pain and psychosocial factors in patients with migraine. Seventy women diagnosed with migraine were questioned regarding migraine onset and frequency, and the presence, frequency, and intensity of self-reported neck pain. These individuals also completed the following questionnaires: Neck Disability Index, Migraine Disability Assessment, Patient Health Questionnaire (PHQ-9), and Tampa Scale for Kinesiophobia. Active cervical ROM was assessed in the sagittal, frontal, and transverse planes using the Multi-Cervical Unit Rehabilitation® equipment. Potential associations were calculated using Pearson’s correlation test or Spearman’s correlation (p < 0.05). A weak negative correlation was observed between the PHQ-9 scores and sagittal (ρ = −0.30, p = 0.010), frontal (ρ = −0.34, p = 0.004), and transverse (ρ = −0.31, p = 0.009) cervical ROM. No correlation was found between cervical ROM and kinesiophobia, migraine-related disability, neck pain disability, or clinical features of neck pain and migraine (p > 0.05). Our findings indicated that cervical mobility was associated with the severity of depressive symptoms, but not with the clinical variables of migraine and neck pain, kinesiophobia levels, neck pain disability, and migraine-related disability in women with migraine.
1. Introduction
Migraine is a primary headache disorder reported to be the most disabling neurological disease and the sixth most prevalent condition in the world [1,2]. Recently, migraine has been revealed as a complex clinical condition owning to its variety of symptoms and comorbidities, with the headache attack being just one phase of a cascade of events [1,3,4]. The comorbid conditions increase the use of health resources and general costs. In addition, musculoskeletal signs and symptoms are often observed in patients with migraine, and these patients are referred to physical therapy as part of the multidisciplinary management of this headache [5,6].
According to the International Classification of Functioning, Disability, and Health, impairments are problems in body function or structure such as a significant deviation or loss. Impairments may also be related to a disease, disorder, or physiological state [7]. Musculoskeletal impairments in migraine are mainly related to the cervical spine. Patients with migraine often have a greater forward head posture, greater number of trigger points in the cervical region, and reduced cervical range of motion (ROM) compared to healthy individuals [8,9,10]. Despite that, no difference in morphometry of neck muscles has been observed between migraineurs and controls [11]. Neck pain is highly prevalent in migraineurs, affecting approximately 70% of patients [12,13,14]. Neck pain is associated with the worse clinical presentations of migraine, such as severe cutaneous allodynia and pain-related disability [15].
Despite that, some cervical impairments are observed in migraineurs regardless of neck pain, such as in the performance of deep cervical flexor muscles and in upper cervical mobility [15]. Therefore, in cases of migraine, it is possible that some cervical impairments may not be strictly associated with the presence of neck pain but rather with other factors, such as migraine features or its comorbidities. This indicates the relevance of understanding which musculoskeletal impairments are related to migraine itself, and which are related to a cervical musculoskeletal disorder.
In this context, cervical ROM is a functional measure used in clinical practice and is often used as an outcome in individuals with neck pain [16]. The reduction of cervical ROM is observed in patients with migraine [9,17]. According to the linear correlation data, in migraineurs, a lower cervical ROM as a function of an increased neck-pain-related disability is expected [18]. However, the cervical ROM does not seem to be linearly correlated with migraine frequency [9]. The correlation between cervical ROM and other clinical parameters of migraine and neck pain remains unclear.
Psychological aspects such as anxiety and depression are strongly associated with migraine [4], with a potential to influence disease prevalence, prognosis, treatment, and clinical outcomes [19]. The increased burden of migraine has been observed in patients who also present with both depression and anxiety comorbidities previously associated with medication overuse and fibromyalgia [20,21]. Such psychological conditions are also considered risk factors for the progression from episodic to chronic migraine [22]. Patients with migraine and concomitant depression present with increased muscle tension and tenderness [23,24] and exhibit painful physical symptoms that usually result in functional impairment, affecting their quality of life and the treatment response [25]. These physical and painful symptoms are associated with kinesiophobia in patients with neck pain and chronic musculoskeletal pain [26,27], since fear of movement, a strategy used to avoid pain, also leads to physical and functional consequences [28].
To date, no information is available regarding the relationship between cervical ROM and the clinical presentations of migraine, including neck pain, and psychosocial factors, such as fear of movement and depression. If we are to confirm the association between these factors, it needs to be considered during migraine treatment and rehabilitation. Therefore, the objective of this study was to determine the association of cervical ROM with clinical features of headache and neck pain and psychosocial factors in individuals with migraine. We hypothesized that migraineurs with worse clinical features of both headache and neck pain would present with lower cervical ROM and higher levels of fear of movement and depressive symptoms.
2. Materials and Methods
2.1. Participants
This cross-sectional study was performed between January 2018 and August 2019. Volunteers were screened from an outpatient headache clinic and the local community, and the diagnosis was confirmed by neurologists specialized in headache in accordance with the third version of the International Headache Classification [29]. Women between 18 and 55 years of age with at least 3 days of migraine headache per month in the last 3 months were included in this study. The exclusion criteria were as follows: the presence of other concomitant headaches, a history of face or neck injury, fibromyalgia, a history of a herniated disk or cervical vertebral arthrosis as per the medical records, anesthetic block in the previous 3 months of the study, and pregnancy.
2.2. Experimental Procedure
Participants who met the inclusion criteria attended a physical examination in a laboratory setting. All participants were under prescribed pharmacologic migraine prophylaxis. They were instructed to avoid any analgesic drugs 24 h before the appointment, and in the case of a migraine attack, to reschedule the appointment. All participants were evaluated on a headache-free day.
On the day of assessment, the examiner applied a structured screening to collect demographic and clinical data, including migraine frequency (days per month), migraine onset (years), and presence of self-reported neck pain. Participants who reported neck pain answered questions about neck pain frequency (days per month) and intensity (numerical pain rating scale). Subsequently, the following cross-culturally adopted questionnaires were used: Neck Disability Index (NDI), Migraine Disability Assessment (MIDAS), Patient Health Questionnaire (PHQ-9), and Tampa Scale for Kinesiophobia (TSK), to determine the migraine-related disability, depressive symptoms, and fear of movement, respectively.
The NDI is a questionnaire designed to assess how the neck pain affects the patient’s ability to perform daily activities [30,31]. The NDI includes 10 items, and each item is scored from 0 to 5. According to the total score, the patient was classified as follows: no disability (0–4), mild disability (5–14), moderate disability (15–24), severe disability (25–34), and complete disability (35 or more) [30,31]. The NDI is a valid and reliable tool to measure neck-pain-related disability and is used as a guide for clinical decision-making [31,32].
The MIDAS evaluates migraine-related disability with five questions related to the number of days in the past 3 months during which the patients had partial or complete difficulty in managing everyday life owning to migraine. The patient was classified as follows based on the final score: no disability (0–5), mild disability (6–10), moderate disability (11–20), and severe disability (21 or higher) [33]. The MIDAS has a good reliability [34] and high correlation with physicians’ judgments regarding patients’ pain, migraine-related disability, and need for medical care [35].
Depressive symptoms were measured using the PHQ-9, a tool containing nine questions to assess the occurrence of depressive symptoms in the past two weeks [36]. Each question is scored on a scale from 0 to 3, with a maximum of 27 points. The severity of depression is classified as follows: minimal (0–4), mild (5–9), moderate (10–14), moderately severe (15–20), and severe (21–27) [34]. The PHQ-9 presents excellent test–retest reliability, and a strong association between its score and functional status, disability days, and symptom-related difficulty [37].
The TSK is a 17-item tool used to assess fear of movement, with items 4, 8, 12, and 16 being reversely scored. Scoring options range from strongly disagree (1) to strongly agree (4), with a total score between 17 and 68 [38]. The questionnaire has a good reliability and correlation with depressive and catastrophic symptoms [39].
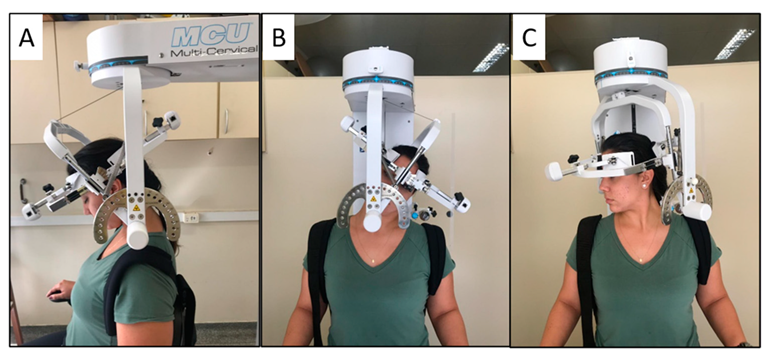
After completing the questionnaires, active cervical ROM was assessed by a second examiner, blinded to the participants’ clinical condition, using the Multi-Cervical Rehabilitation Unit® (BTE Technologies, Inc.™, Hanover, NH, USA). The equipment has excellent reliability for measuring ROM in flexion, extension, lateral flexion, and rotation [40]. The examiner positioned the participant in a seated position, with the knee and hip at 90° flexion and with the trunk restrained by bands to avoid movement compensation during the cervical movements, and the equipment elm was positioned on the head. The participants were instructed to perform the movements of flexion, extension, lateral flexion, and rotation to the maximum possible range (Figure 1) three times in each direction. The presence of self-reported neck pain during movements was also recorded. All movements were performed in approximately 4 s, guided by the examiner’s voice command, and the order was randomized. We considered the total ROM on each plane of movement for the analysis; that is, sagittal plane ROM was obtained by the sum of the flexion and extension, the frontal plane was the sum of lateral flexion on both sides, and the transverse plane was the sum of rotation on both sides.
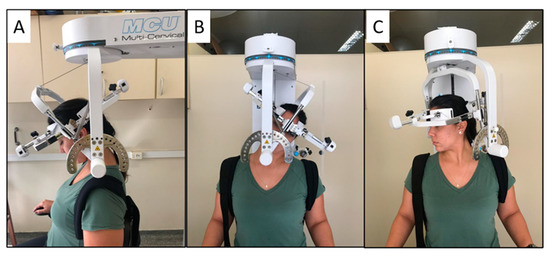
Figure 1.
Illustration of the subject’s position in the Multi Cervical Rehabilitation Unit® performing the cervical range of motion assessment on the sagittal plane (A), frontal plane (B), and transverse plane (C).
2.3. Statistical Analysis
The sample size calculation was performed using G*Power 3.1.9.7. A minimum of 64 participants were required in order to detect significant moderate associations (ρ = 0.3) with an α of 0.05 and a desired power of 0.80. Descriptive data analyses are reported as mean, median, standard deviation, and interquartile range. After verifying the data distribution using the Shapiro–Wilk test, the Pearson’s correlation test was used to verify the association of cervical ROM with TSK scores, and the Spearman’s correlation was used to evaluate the association of cervical ROM with migraine onset, migraine frequency, neck pain frequency, neck pain intensity, and the MIDAS, NDI, and PHQ-9 scores. The association between the cervical ROM and both the self-report of neck pain and neck pain during the movements assessed was performed using a point–biserial correlation. Correlation values of <0.40 indicated a weak correlation; 0.40 to 0.69, moderate correlation; and >0.70, strong correlation [41]. The statistical analysis was performed using the Statistical Package for the Social Sciences software, version 20.0 (IBM, New York, NY, USA), and the significance level was set at 0.05.
3. Results
Of the 81 eligible participants who agreed to participate, 70 were included in the study. The reasons for exclusions were concomitant headache (n = 7), receipt of an anesthetic block in the previous 3 months (n = 2), and a history of previous neck trauma (n = 2).
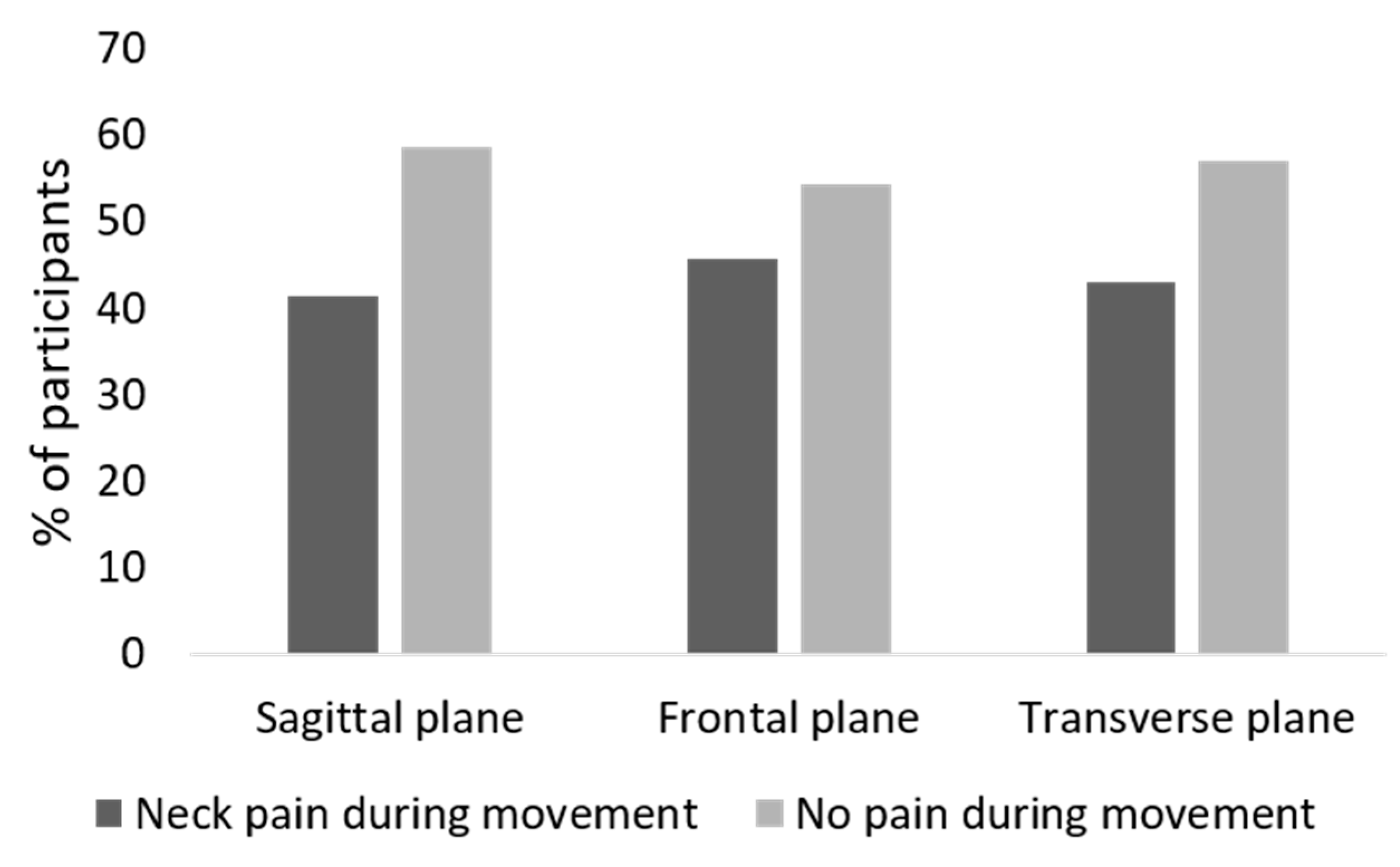
Table 1 summarizes the demographic and clinical features of the sample population. A total of 75.7% of participants reported neck pain, 78% of patients presented severe migraine-related disability according to the MIDAS score, and approximately 67% of the sample reported depressive symptoms. During the cervical ROM assessment, the proportion of participants that reported neck pain during movement was 41.4% for the sagittal plane, 45.7% for the frontal plane, and 42.9% for the transverse plane (Figure 2).

Table 1.
Sample clinical and demographic characteristics.
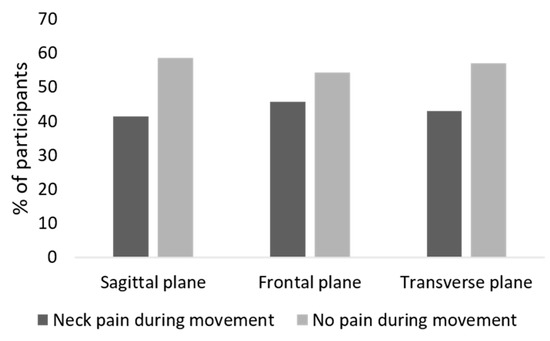
Figure 2.
Self-reported neck pain during cervical motion in the three planes of motion.
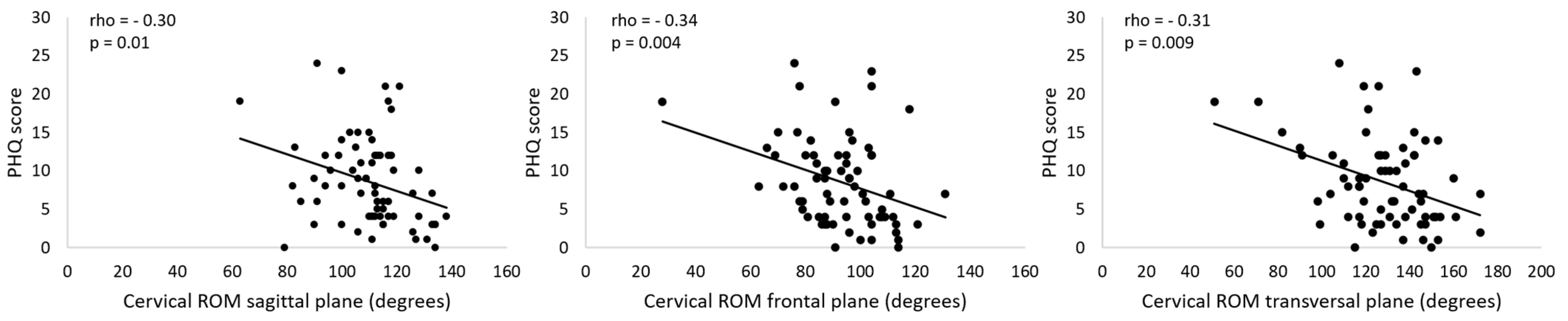
The correlation analysis revealed weak negative associations between PHQ-9 score and cervical ROM in all planes (Table 2, Figure 3); the higher the depressive symptoms, the lower was the cervical ROM. No significant associations between cervical ROM and migraine pain features, self-report of neck pain, neck pain during cervical movement, neck pain features, kinesiophobia, neck pain disability, and migraine-related disability were observed (Table 2). The correlation analysis performed considering only the individuals who reported neck pain also revealed no significant associations between cervical ROM and neck pain disability, neck pain frequency, and neck pain intensity (Table 3).

Table 2.
Correlation (95% CI) between cervical range of motion and migraine clinical presentation, depressive symptoms, and kinesiophobia levels in patients with migraine (n = 70).
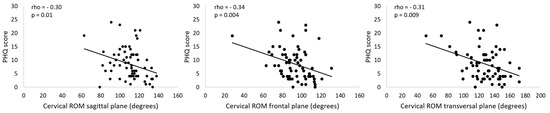
Figure 3.
Association between PHQ scores and cervical range of motion (ROM). PHQ: Patient Health Questionnaire.

Table 3.
Correlation (95%CI) between cervical range of motion and neck pain features in patients with migraine who report neck pain (n = 53).
4. Discussion
The hypothesis tested in this study was that migraineurs with worse clinical presentations of headache and higher levels of fear of movement and depression would demonstrate less cervical ROM. Our results did not confirm this hypothesis. Among all the correlations analyzed, only depressive symptoms presented as significant; however, weak correlations with the cervical ROM were noted in our sample of migraineurs—the higher the severity of depressive symptoms, the lower the cervical ROM. No other significant correlation was observed between cervical ROM and the remaining clinical variables.
The lack of significant correlation between cervical ROM and frequency of migraine have already been reported previously [9,18]. It contrasts with the lower cervical ROM observed in patients with chronic migraine or transformed migraine (15 or more headache-days per month) compared to controls [9,42]. Therefore, we suggest that the relationship between cervical ROM and more frequent migraine attacks is not linear. Furthermore, it seems that there is no linear correlation between cervical ROM and the migraine characteristics such as years with migraine and migraine-related disability, or neck pain features, such as pain frequency, intensity, and related disability.
The relationship between musculoskeletal disorders and depression has already been demonstrated [43]. They are not only comorbid conditions, but also mutually exacerbating and predictors of negative prognosis [26,27,44,45,46]. Depression is also a well-known comorbidity of migraine with negative repercussions on the patient’s life and prognosis [22,47]. Our results indicated a correlation between depressive symptoms, and cervical ROM, as a higher severity of depressive symptoms in female patients with migraine correlated with lower cervical ROM. This correlation may be owing to the greater muscle tension and tenderness that was already demonstrated in the patients with migraine and comorbid depression [23,24]. In addition, motivational factors during the assessment could be involved.
The results of this study should be interpreted with caution, as the correlations between cervical ROM and depressive levels were weak. Moreover, the wide confidence intervals ranging from −0.54 to −0.05, meant that the correlations could be considered between moderate and weak; however, they even presented a correlation close to 0, which indicates the absence of any correlation between these variables. Further, other associated factors observed in patients with both migraine and depression, such as fibromyalgia [21] and medication overuse [20], were excluded and could increment the correlation results. To the best of our knowledge, there is no published report regarding a significant correlation between cervical ROM and depression in patients with headache or neck pain. However, cervical ROM appears to be weakly to moderately correlated with patient-reported outcome measures, such as pain and neck-related disability [18,48,49,50]. Based on these findings, we assumed that the clinical implication of the results might be limited since the values of the association coefficients were low.
In addition, cervical range of motion may be influenced by other factors that we did not control in this study, such as head and neck posture and type of work occupation, for example. The absence of significant correlations between cervical ROM and pain and disability-related measures suggests that the cervical ROM may be just an impairment not immediately related to the clinical presentation of migraine or neck pain. It means that a unique impairment does not necessarily characterize a cervical musculoskeletal disorder. Although the pain cannot be a determinant factor to characterize a musculoskeletal disorder overall, it is suggested that cervical disorders would be better defined by a combination of neck pain and a cluster of symptoms and impairments of body function [51].
Another aspect that might contribute to the absence of significant correlation between cervical ROM and pain- and disability-related variables is the range of cervical ROM observed in our cohort. We did not have a control group without pain to assume any restriction of cervical ROM. However, using distinct methods for measuring the ROM reference data from controls, as reported by Liang et al. (2019) [17] in their metanalysis, could be of benefit to our understanding. The median and interquartile ranges of our cohort’s ROM at the sagittal and transverse planes are predominantly lower than their respective means in the referred metanalysis. This suggests that the majority of our cohort would be classified as having a limited ROM at the sagittal and transverse planes. A cohort with a narrow range composed mostly of those with restricted ROM would not properly reflect a linear correlation.
The fear-avoidance model of pain suggests that when pain is perceived as harmful, this may lead to catastrophic thoughts, which generate fear of movement-related pain that in turn leads to avoidance of activities, resulting in disability [38]. Previous reports in patients with neck pain support this model and have demonstrated a significant correlation between TSK and cervical ROM in individuals with neck pain [47,48]. However, in our sample, the active cervical ROM did not present a significant linear correlation with the fear of movement-related pain measured by TSK, even when more than 40% of the sample reported neck pain during active cervical movement. Behavioral and physical factors involved in these assessments may play a complex role in patients with migraine.
This study assessed the patients in the interictal phase, i.e., out of a migraine headache attack, and our results indicate that in migraine patients, fear of movement-related pain may not be associated with a constant neck and head movement avoidance that could lead to lower cervical ROM. These characteristics may be restricted to ictal phases (i.e., during the attacks), since aggravation of pain by cervical movement and avoidance of head movement during a headache attack have been indicated as sensitive and specific manifestations of migraine [52]. This may also occur because fear related to movement in patients with migraine is not only restricted to head and neck motion [53]. Therefore, the lack of correlation between TSK and cervical ROM in our sample of female patients with migraine could be a consequence of the content of TSK items, which do not particularly consider head and neck movement. Furthermore, correlation analysis is better characterized when it includes a great variability of observations. As the interquartile range of TSK indicates a narrow amplitude of this variable, it may not have been enough to demonstrate a linear correlation. Although the TSK was not developed or validated in a sample of migraineurs, it is the only scale that measures fear of movement, and since kinesiophobia is a behavior caused by a negative belief, it is not conditioned to the individual’s clinical condition. Nevertheless, we consider the use of TSK as one of the limitations of this study. The development of a migraine-specific scale should be considered in future studies.
Another limitation is the study design, as a cross-sectional study, no causality between variables can be assumed. Finally, considering the sample, the results reported here are sex-specific, so they may not be the same when assessing male patients with migraine. However, this study was the first to assess the correlation between cervical ROM, psychosocial factors, and clinical presentations of migraine. The results confirm that cervical ROM is not linearly related to the clinical aspects of migraine or the presence of neck pain, or to fear of movement-related pain as measured by the TSK; however, it seems to be weakly related to the depressive symptoms. Based on these findings, we suggest that physiotherapists investigate the presence and severity of depressive symptoms since it may be associated with reduced mobility and consequently impair rehabilitation progress. Further, depressive symptoms suggest the necessity of psychological approaches. Futures research can contribute to a better understanding of other associations between psychosocial conditions and musculoskeletal impairments (i.e., muscle function) in patients with migraine.
5. Conclusions
In female patients with migraine, active cervical mobility was weakly associated with the severity of depressive symptoms; however, it was not correlated with the clinical variables of migraine, neck pain features, and fear of movement.
Author Contributions
Conceptualization, C.F.P., A.S.O., L.L.F., F.D., C.F.-d.-l.-P. and D.B.-G.; methodology, C.F.P., J.C.S.M. and L.L.F.; formal analysis, C.F.P., J.C.S.M., A.S.O. and L.L.F.; data curation, C.F.P. and L.L.F.; writing—original draft preparation, C.F.P. and J.C.S.M.; writing—review and editing, C.F.P., A.S.O., F.D., C.F.-d.-l.-P. and D.B.-G.; supervision, A.S.O. and D.B.-G.; project administration, D.B.-G.; funding acquisition, C.F.P. and D.B.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation, grant numbers 2018/23832-5 and 2015/18031-5, and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Ribeirão Preto Medical School of University of São Paulo (protocol code 12145/2016, approved 23-November-2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to containing information that could compromise the privacy of research participants.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine—A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef] [PubMed]
- GBD. 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef]
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, burden, and comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Luedtke, K.; Starke, W.; May, A. Musculoskeletal dysfunction in migraine patients. Cephalalgia 2018, 38, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.F.; Schwarz, A.; Szikszay, T.M.; Adamczyk, W.M.; Bevilaqua-Grossi, D.; Luedtke, K. Physical therapy and migraine: Musculoskeletal and balance dysfunctions and their relevance for clinical practice. Braz. J. Phys. Ther. 2020, 24, 306–317. [Google Scholar] [CrossRef]
- ICF. International Classification of Functioning, Disability, and Health; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Fernández-de-las-Peñas, C.; Cuadrado, M.L.; Pareja, J.A. Myofascial trigger points, neck mobility and forward head posture in unilateral migraine. Cephalalgia 2006, 26, 1061–1070. [Google Scholar] [CrossRef]
- Bevilaqua-Grossi, D.; Pegoretti, K.S.; Goncalves, M.C.; Speciali, J.G.; Bordini, C.A.; Bigal, M.E. Cervical mobility in women with migraine. Headache 2009, 49, 726–731. [Google Scholar] [CrossRef]
- Ferracini, G.N.; Florencio, L.L.; Dach, F.; Chaves, T.C.; Palacios-Ceña, M.; Fernández-de-las-Peñas, C.; Bevilaqua-Grossi, D.; Speciali, J.G. Myofascial trigger points and migraine-related disability in women with episodic and chronic migraine. Clin. J. Pain 2017, 33, 109–115. [Google Scholar] [CrossRef]
- Hvedstrup, J.; Amin, F.M.; Hougaard, A.; Ashina, H.; Christensen, C.E.; Larsson, H.B.W.; Ashina, M.; Schytz, H.W. Volume of the rectus capitis posterior minor muscle in migraine patients: A cross-sectional structural MRI study. J. Headache Pain 2020, 21, 57. [Google Scholar] [CrossRef]
- Calhoun, A.H.; Ford, S.; Millen, C.; Finkel, A.G.; Truong, Y.; Nie, Y. The prevalence of neck pain in migraine. Headache 2010, 50, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Ashina, S.; Bendtsen, L.; Lyngberg, A.C.; Lipton, R.B.; Hajiyeva, N.; Jensen, R. Prevalence of neck pain in migraine and tension-type headache: A population study. Cephalalgia 2015, 35, 211–219. [Google Scholar] [CrossRef]
- Lamp, C.; Rudolph, M.; Deligianni, C.I.; Mitsikostas, D.D. Neck pain in episodic migraine: Premonitory symptom or part of the attack? J. Headache Pain 2015, 16, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bragatto, M.M.; Bevilaqua-Grossi, D.; Benatto, M.T.; Lodovichi, S.S.; Pinheiro, C.F.; Carvalho, G.F.; Dach, F.; Fernández-de-las-Peñas, C.; Florencio, L.L. Is the presence of neck pain associated with more severe clinical presentation in patients with migraine? A cross-sectional study. Cephalalgia 2019, 39, 1500–1508. [Google Scholar] [CrossRef]
- Howell, E.R. The association between neck pain, the Neck Disability Index and cervical ranges of motion: A narrative review. J. Can. Chiropr. Assoc. 2011, 55, 211–221. [Google Scholar]
- Liang, Z.; Galea, O.; Thomas, L.; Jull, G.; Treleaven, J. Cervical musculoskeletal impairments in migraine and tension type headache: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2019, 42, 67–83. [Google Scholar] [CrossRef]
- Carvalho, G.F.; Chaves, T.C.; Gonçalves, M.C.; Florencio, L.L.; Braz, C.A.; Dach, F.; Fernández-de-las-Peñas, C.; Bevilaqua-Grossi, D. Comparison between neck pain disability and cervical range of motion in patients with episodic and chronic migraine: A cross-sectional study. J. Manip. Physiol. Ther. 2014, 37, 641–646. [Google Scholar] [CrossRef]
- Louter, M.A.; Pijpers, J.A.; Wardenaar, K.J.; Van Zwet, E.W.; Hemert, A.M.; Zitman, F.G.; Ferrari, M.D.; Penninx, B.W.; Terwindt, G.M. Symptom dimensions of affective disorders in migraine patients. J. Psychosom. Res. 2015, 79, 458–463. [Google Scholar] [CrossRef]
- Martínez, L.M.; Román, P.J.; Hernández, J.M.D.; Unturbe, C.M.; Ramírez-Castillejo, M.C. Chronic Migraine with medication overuse: Clinical pattern and evolution from a retrospective cohort in Seville, Spain. SN Compr. Clin. Med. 2020, 2, 1514–1525. [Google Scholar] [CrossRef]
- Patel, U.K.; Malik, P.; Sheth, R.; Malhi, P.; Kapoor, A.; Rasul, B.M.; Saiyed, S.; Kavi5, T.; Kapoor, A. Fibromyalgia and myositis linked to higher burden and disability in patients with migraine. SN Compr. Clin. Med. 2019, 1, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Kong, F.; Buse, D.C. Predictors of episodic migraine transformation to chronic migraine: A systematic review and meta-analysis of observational cohort studies. Cephalalgia 2020, 40, 503–516. [Google Scholar] [CrossRef]
- Hung, C.-I.; Liu, C.-Y.; Chen, J.-J.; Wang, S.-J. Migraine predicts self-reported muscle tension in patients with major depressive disorder. Psychosomatics 2008, 49, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Mongini, F.; Ciccone, G.; Deregibus, A.; Ferrero, L.; Mongini, T. Muscle tenderness in different headache types and its relation to anxiety and depression. Pain 2004, 112, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-I.; Liu, C.-Y.; Yang, C.-H.; Wang, S.-J. Migraine with active headache was associated with other painful physical symptoms at two-year follow-up among patients with major depressive disorder. PLoS ONE 2019, 14, e0216108. [Google Scholar] [CrossRef] [PubMed]
- Luque-Suarez, A.; Martinez-Calderon, J.; Falla, D. Role of kinesiophobia on pain, disability and quality of life in people suffering from chronic musculoskeletal pain: A systematic review. Br. J. Sports Med. 2019, 53, 554–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, L.J.; Holm, L.W.; Hogg-Johnson, S.; Côté, P.; Cassidy, J.D.; Haldeman, S.; Nordin, M.; Hurwitz, E.L.; Carragee, E.J.; Van der Velde, G.; et al. Course and Prognostic Factors for Neck Pain in Whiplash-Associated Disorders (WAD). Spine 2008, 33 (Suppl. S4), S83–S92. [Google Scholar] [CrossRef]
- Hudes, K. The Tampa Scale of Kinesiophobia and neck pain, disability and range of motion: A narrative review of the literature. J. Can. Chiropr. Assoc. 2011, 55, 222–232. [Google Scholar] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders. Cephalalgia 2018, 38, 1–211. [CrossRef]
- Cook, C.; Richardson, J.K.; Braga, L.; Menezes, A.; Soler, X.; Kume, P.; Zaninelli, M.; Socolows, F.; Pietrobon, R. Cross-cultural adaptation and validation of the Brazilian Portuguese version of the neck disability index and neck pain and disability scale. Spine 2006, 31, 1621–1627. [Google Scholar] [CrossRef]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1992, 14, 409–415. [Google Scholar]
- McCarthy, M.J.H.; Grevitt, M.P.; Silcocks, P.; Hobbs, G. The reliability of the Vernon and Mior neck disability index, and its validity compared with the short form-36 health survey questionnaire. Eur. Spine J. 2007, 16, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragoso, Y. MIDAS (Migraine Disability Assessment): A valuable tool for work-site identification of migraine in workers in Brazil. Sao Paulo Med. J. 2002, 120, 118–121. [Google Scholar] [CrossRef] [Green Version]
- Stewart, W.F.; Lipton, R.B.; Dowson, A.J.; Sawyer, J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001, 56 (Suppl. S1), S20–S28. [Google Scholar] [CrossRef]
- Lipton, R.B.; Stewart, W.F.; Sawyer, J.; Edmeads, J.G. Clinical utility of an instrument assessing migraine disability: The migraine disability assessment (MIDAS) questionnaire. Headache 2001, 41, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Osório, F.L.; Mendes, A.V.; Crippa, J.A.; Loureiro, S.R. Study of the discriminative validity of the phq-9 and phq-2 in a sample of brazilian women in the context of primary health care. Perspect. Psychiatr. Care 2009, 45, 216–227. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Souza, F.S.; Marinho, C.S.; Siqueira, F.B.; Maher, C.G.; Costa, L.O. Psychometric testing confirms that the Brazilian-Portuguese adaptations, the original versions of the Fear-Avoidance Beliefs Questionnaire, and the Tampa Scale of Kinesiophobia have similar measurement properties. Spine 2008, 33, 1028–1033. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Kole-Snijders, A.M.J.; Rotteveel, A.M.; Ruesink, R.; Heuts, P.H.T.G. The role of fear of movement/(re)injury in pain disability. J. Occup. Rehabil. 1995, 5, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.T.W.; Sing, K.L. Evaluation of cervical range of motion and isometric neck muscle strength: Reliability and validity. Clin. Rehabil. 2002, 16, 851–858. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Calculating correlation coefficients with repeated observations: Part 2—Correlation between subjects. BMJ 1995, 310, 633. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Souza, A.I.S.; Florencio, L.L.; Carvalho, G.F.; Fernández-de-las-Peñas, C.; Dach, F.; Bevilaqua-Grossi, D. Reduced flexion rotation test in women with chronic and episodic migraine. Braz. J. Phys. Ther. 2019, 23, 387–394. [Google Scholar] [CrossRef]
- Du, L.; Luo, S.; Liu, G.; Wang, H.; Zheng, L.; Zhang, Y. The 100 top-cited studies about pain and depression. Front. Psychol. 2020, 10, 3072. [Google Scholar] [CrossRef]
- Viana, M.C.; Lim, C.C.W.; Pereira, F.G.; Aguilar-Gaxiola, S.; Alonso, J.; Bruffaerts, R.; Jonge, P.; Caldas-de-Almeida, J.M.; O’Neill, S.; Stein, D.J.; et al. Previous mental disorders and subsequent onset of chronic back or neck pain: Findings from 19 countries. J. Pain 2018, 19, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bair, M.J.; Wu, J.; Damush, T.M.; Sutherland, J.M.; Kroenke, K. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom. Med. 2008, 70, 890–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, C.; Kadriu, B.; Lanzenberger, R.; Zarate, C.A.; Kasper, S. Prognosis and improved outcomes in major depression: A review. Transl. Psychiatry 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alwhaibi, M.; Alhawassi, T.M. Humanistic and economic burden of depression and anxiety among adults with migraine: A systematic review. Depress. Anxiety 2020, 37, 1146–1159. [Google Scholar] [CrossRef]
- Sarig Bahat, H.; Weiss, P.L.; Sprecher, E.; Krasovsky, A.; Laufer, Y. Do neck kinematics correlate with pain intensity, neck disability or with fear of motion? Man. Ther. 2014, 19, 252–258. [Google Scholar] [CrossRef]
- Treleaven, J.; Chen, X.; Sarig Bahat, H. Factors associated with cervical kinematic impairments in patients with neck pain. Man. Ther. 2016, 22, 109–115. [Google Scholar] [CrossRef]
- Saavedra-Hernández, M.; Castro-Sánchez, A.M.; Cuesta-Vargas, A.I.; Cleland, J.A.; Fernández-de-las-Peñas, C.; Arroyo-Morales, M. The contribution of previous episodes of pain, pain intensity, physical impairment, and pain-related fear to disability in patients with chronic mechanical neck pain. Am. J. Phys. Med. Rehabil. 2012, 91, 1070–1076. [Google Scholar] [CrossRef]
- Childs, J.D.; Cleland, J.A.; Elliott, J.M.; Teyhen, D.S.; Wainner, R.S.; Whitman, J.M.; Sopky, B.J.; Godges, J.J.; Flynn, T.W. American Physical Therapy Association. Neck pain: Clinical practice guidelines linked to the international classification of functioning, disability, and health from the orthopedic section of the american physical therapy association. J. Orthop. Sports Phys. Ther. 2008, 38, A1–A34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, I.P.; Gouveia, R.G.; Parreira, E. Kinesiophobia in migraine. J. Pain 2006, 7, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Benatto, M.T.; Bevilaqua-Grossi, D.; Carvalho, G.F.; Bragatto, M.M.; Pinheiro, C.F.; Lodovichi, S.S.; Dach, F.; Fernández-de-las-Peñas, C.; Florencio, L.L. Kinesiophobia is associated with migraine. Pain Med. 2019, 20, 846–851. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).