Methane Emissions Regulated by Microbial Community Response to the Addition of Monensin and Fumarate in Different Substrates
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. In Vitro Rumen Incubation
2.3. Chemical Analyses
2.4. Microbial Analyses
2.4.1. 16S rRNA Sequencing
2.4.2. Real-Time PCR Analysis
2.5. Data Analysis
3. Results
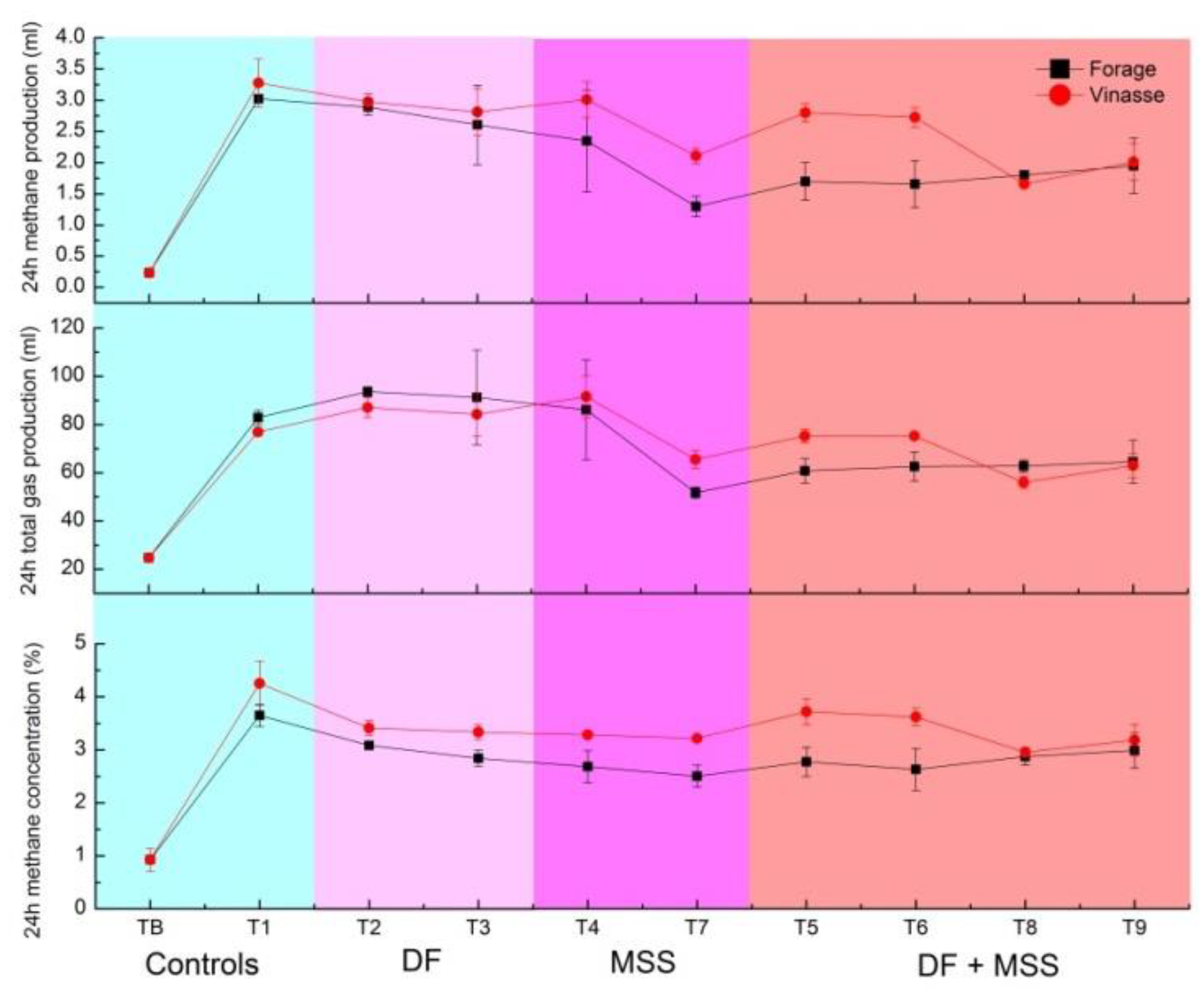
3.1. Effect of MSS and DF on In Vitro Rumen Fermentation and Methanogenesis
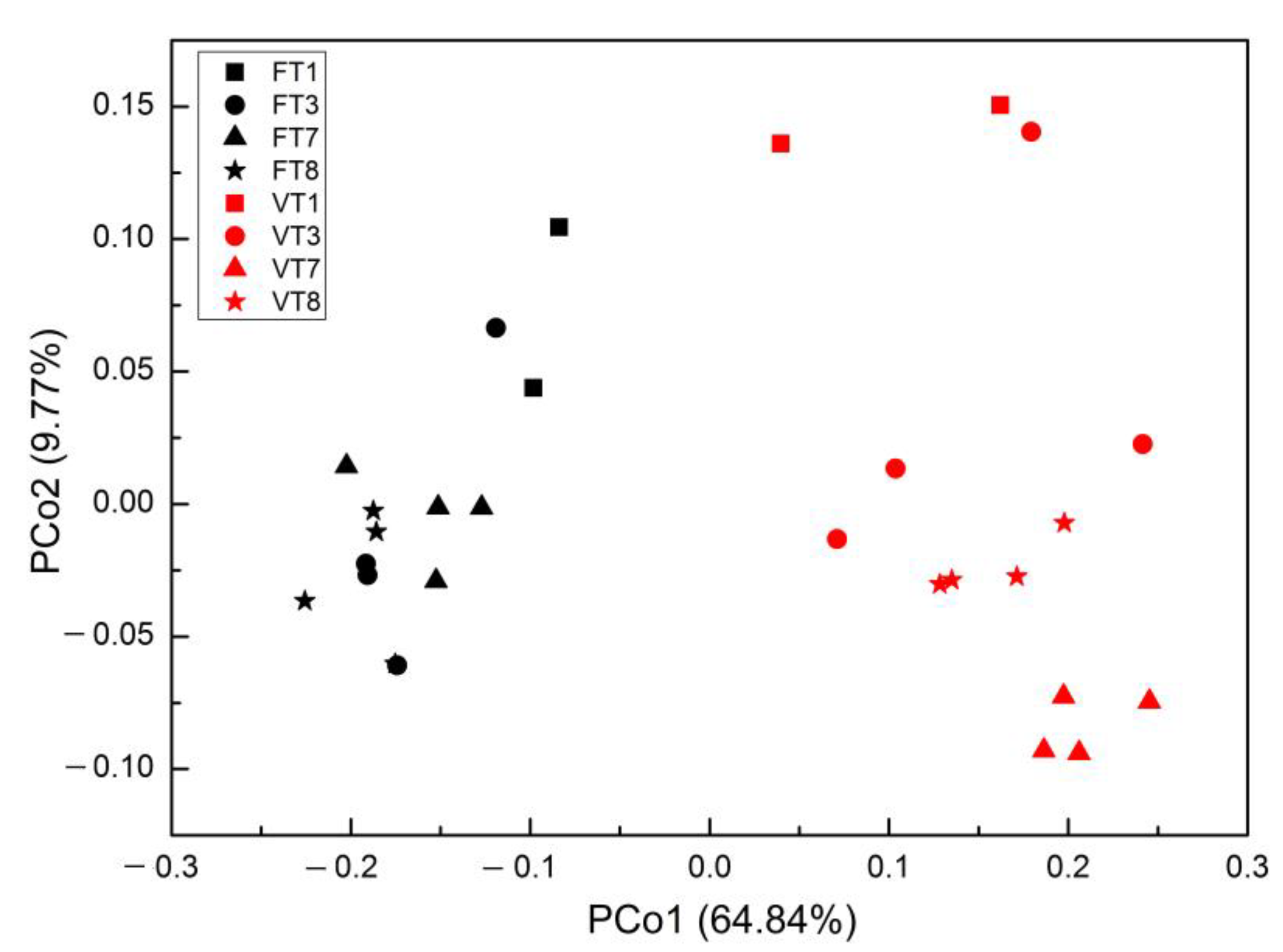
3.2. Effect of MSS and DF Supplementation on Prokaryotic Community Diversity and Structure
3.3. Effect of MSS and DF Supplementation on the Composition of Rumen Prokaryotic Community
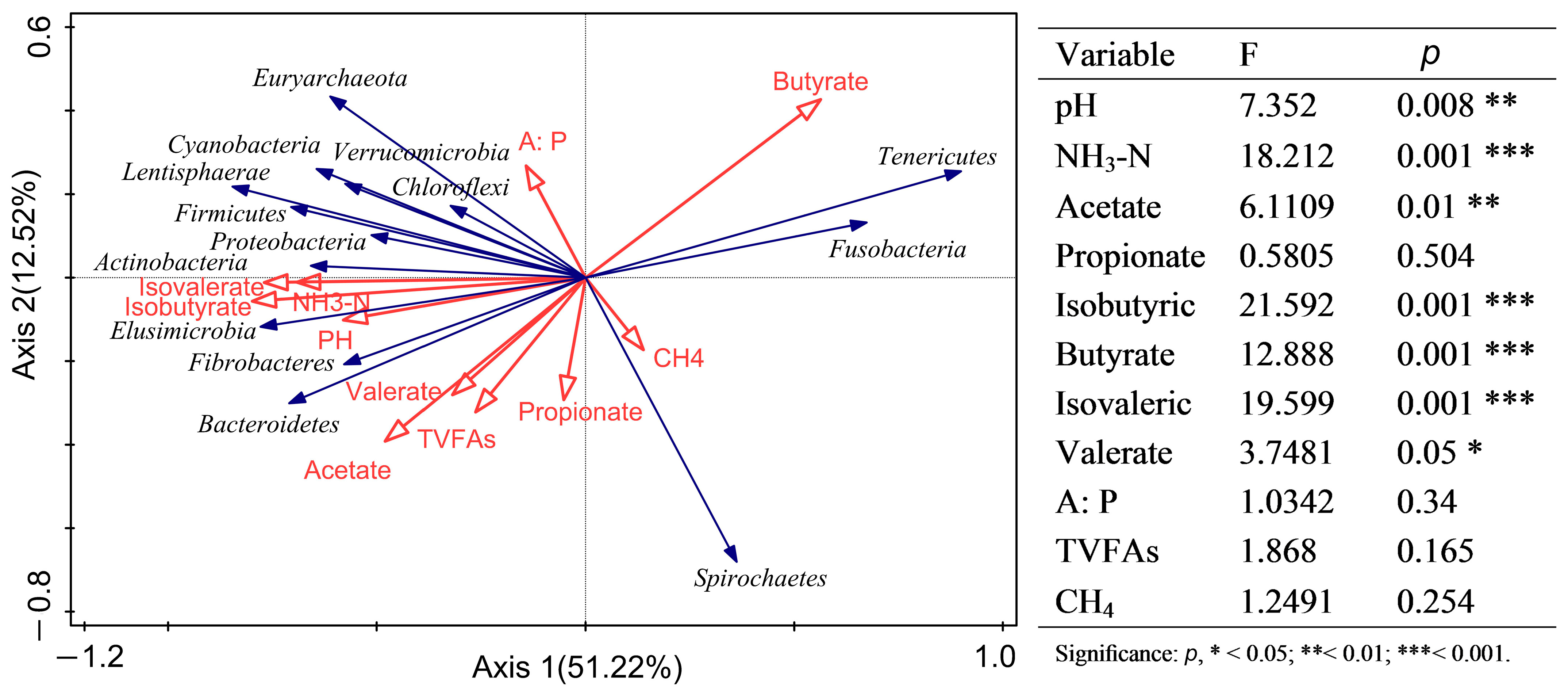
3.4. Relationships between Rumen Fermentation Parameters and Prokaryotic Communities
3.5. Effect of MSS and DF Supplementation on the Abundance of Selected Microbes
4. Discussion
4.1. Methane Emissions Regulated by Microbial Community under Different Substrates
4.2. Effect of Methane Mitigation Additives on In Vitro Methane Production, Rumen Fermentation, and Microbes
4.3. Interactions between Substrates and Methane Mitigation Additives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lassey, K.R. Livestock methane emission: From the individual grazing animal through national inventories to the global methane cycle. Agric. For. Meteorol. 2007, 142, 120–132. [Google Scholar] [CrossRef]
- Chang, J.; Peng, S.; Ciais, P.; Saunois, M.; Dangal, S.R.S.; Herrero, M.; Havlík, P.; Tian, H.; Bousquet, P. Revisiting enteric methane emissions from domestic ruminants and their δ13CCH4 source signature. Nat. Commun. 2019, 10, 3420. [Google Scholar] [CrossRef] [PubMed]
- Denman, K.L.; Brasseur, G.; Chidthaisong, A.; Ciais, P.; Cox, P.M.; Dickinson, R.E.; Hauglustaine, D.; Heinze, C.; Holland, E.; Jacob, D.; et al. Couplings Between Changes in the Climate System and Biogeochemistry. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Mayer, M.; Hyman, R.; Harnisch, J.; Reilly, J.M.; No, T.N. Emissions Inventories and Time Trends for Greenhouse Gases and Other Pollutants; MIT Joint Program on the Science and Policy of Global Change: Cambridge, MA, USA, 2000; p. 49. [Google Scholar]
- Ding, X.Z.; Long, R.J.; Kreuzer, M.; Mi, J.D.; Yang, B. Methane emissions from yak (Bos grunniens) steers grazing or kept indoors and fed diets with varying forage:concentrate ratio during the cold season on the Qinghai-Tibetan Plateau. Anim. Feed Sci. Technol. 2010, 162, 91–98. [Google Scholar] [CrossRef]
- Joblin, K.N. Ruminal acetogens and their potential to lower ruminant methane emissions. Crop Pasture Sci. 1999, 50, 1307–1314. [Google Scholar] [CrossRef]
- Kahraman, O.; Ozbılgin, A.; Alatas, M.S.; Citil, O.B. Strategies to reduce methane production in ruminants. Sci. Pap. 2015, 58, 144–148. [Google Scholar]
- Boadi, D.; Benchaar, C.; Chiquette, J.; Massé, D. Mitigation strategies to reduce enteric methane emissions from dairy cows: Update review. Can J. Anim. Sci. 2004, 84, 319–335. [Google Scholar] [CrossRef]
- Hook, S.E.; Northwood, K.S.; Wright, A.D.; Mcbride, B.W. Long-term monensin supplementation does not significantly affect the quantity or diversity of methanogens in the rumen of the lactating dairy cow. Appl. Environ. Microb. 2009, 75, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Abrar, A.; Tsukahara, T.; Kondo, M.; Ban-Tokuda, T.; Chao, W.; Matsui, H. Effect of monensin withdrawal on rumen fermentation, methanogenesis and microbial populations in cattle. Anim. Sci. J. 2015, 86, 849–854. [Google Scholar] [CrossRef]
- Appuhamy, J.; Strathe, A.B.; Jayasundara, S.; Wagner-Riddle, C.; Dijkstra, J.; France, J.; Kebreab, E. Anti-methanogenic effects of monensin in dairy and beef cattle: A meta-analysis. J. Dairy Sci. 2013, 96, 5161–5173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odongo, N.E.; Bagg, R.; Vessie, G.; Dick, P.; Or-Rashid, M.M.; Hook, S.E.; Gray, J.T.; Kebreab, E.; France, J.; McBride, B.W. Long-term effects of feeding monensin on methane production in lactating dairy cows. J. Dairy Sci. 2007, 90, 1781–1788. [Google Scholar] [CrossRef]
- McAllister, T.A.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Abrar, A.; Kondo, M.; Kitamura, T.; Ban-Tokuda, T.; Matsui, H. Effect of supplementation of rice bran and fumarate alone or in combination on in vitro rumen fermentation, methanogenesis and methanogens. Anim. Sci. J. 2016, 87, 398–404. [Google Scholar] [CrossRef]
- Newbold, C.J.; Ouda, J.O.; Lopez, S.; Nelson, N.; Omed, H.; Wallace, R.J.; Moss, A.R. Propionate precursors as possible alternative electron acceptors to methane in ruminal fermentation. In Greenhouse Gases and Animal Agriculture, Proceedings of the 1st International Conference on Greenhouse Gases and Animal Agriculture, Obihiro, Japan, 7–11 November 2001; Elsevier Science BV: Amsterdam, The Netherlands, 2002; pp. 151–154. [Google Scholar]
- Shen, J.; Liu, Z.; Yu, Z.; Zhu, W. Monensin and Nisin Affect Rumen Fermentation and Microbiota Differently in vitro. Front. Microbiol. 2017, 8, 1111. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.J.; Mao, S.Y.; Long, L.M.; Zhu, W.Y. Effect of disodium fumarate on microbial abundance, ruminal fermentation and methane emission in goats under different forage: Concentrate ratios. Animal 2012, 6, 1788–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Wittenberg, K.M.; Ominski, K.H.; Krause, D.O. Efficacy of ionophores in cattle diets for mitigation of enteric methane. J. Anim. Sci. 2006, 84, 1896–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adesogan, A.T.; Krueger, N.K.; Kim, S.C. A novel, wireless, automated system for measuring fermentation gas production kinetics of feeds and its application to feed characterization. Anim. Feed Sci. Technol. 2005, 123, 211–223. [Google Scholar] [CrossRef]
- Martínez-Sibaja, A.; Alvarado-Lassman, A.; Astorga-Zaragoza, C.M.; Adam-Medina, M.; Posada-Gómez, R.; Rodríguez-Jarquin, J.P. Volumetric gas meter for laboratory-scale anaerobic bioreactors. Measurement 2011, 44, 1801–1805. [Google Scholar] [CrossRef]
- Ishii, S.; Kosaka, T.; Hori, K.; Hotta, Y.; Watanabe, K. Coaggregation facilitates interspecies hydrogen transfer between Pelotomaculum thermopropionicum and Methanothermobacter thermautotrophicus. Appl. Environ. Microbl. 2005, 71, 7838–7845. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Li, P.H.; Hao, S.S.; Zhang, Y.Q.; Kim, S.W.; Li, H.Z.; Ma, X.; Gao, S.; He, L.C.; Wu, W.J.; et al. Dynamic Distribution of the Gut Microbiota and the Relationship with Apparent Crude Fiber Digestibility and Growth Stages in Pigs. Sci. Rep. 2015, 5, 9938. [Google Scholar] [CrossRef] [Green Version]
- Xue, D.; Chen, H.; Chen, F.; He, Y.; Zhao, C.; Zhu, D.; Zeng, L.; Li, W. Analysis of the rumen bacteria and methanogenic archaea of yak (Bos grunniens) steers grazing on the Qinghai-Tibetan Plateau. Livest. Sci. 2016, 188, 61–71. [Google Scholar]
- Warton, D.I.; Wright, S.T.; Wang, Y. Distance-based multivariate analyses confound location and dispersion effects. Methods Ecol. Evol. 2012, 3, 89–101. [Google Scholar] [CrossRef]
- Eun, J.S.; Fellner, V.; Gumpertz, M.L. Methane production by mixed ruminal cultures incubated in dual-flow fermentors. J. Dairy Sci. 2004, 87, 112–121. [Google Scholar] [CrossRef]
- Giraldo, L.A.; Tejido, M.L.; Ranilla, M.J.; Carro, M.D. Effects of exogenous fibrolytic enzymes on in vitro ruminal fermentation of substrates with different forage: Concentrate ratios. Anim. Feed Sci. Technol. 2008, 141, 306–325. [Google Scholar] [CrossRef]
- Serment, A.; Giger-Reverdin, S.; Schmidely, P.; Dhumez, O.; Broudiscou, L.P.; Sauvant, D. In vitro fermentation of total mixed diets differing in concentrate proportion: Relative effects of inocula and substrates. J. Sci. Food Agric. 2016, 96, 160–168. [Google Scholar] [CrossRef]
- Martinez, M.E.; Ranilla, M.J.; Tejido, M.L.; Saro, C.; Carro, M.D. The effect of the diet fed to donor sheep on in vitro methane production and ruminal fermentation of diets of variable composition. Anim. Feed Sci. Technol. 2010, 158, 126–135. [Google Scholar] [CrossRef]
- Børsting, C.F.; Brask, M.; Hellwing, A.L.F.; Weisbjerg, M.R.; Lund, P. Enteric methane emission and digestion in dairy cows fed wheat or molasses. J. Dairy Sci. 2020, 103, 1448–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hook, S.E.; Steele, M.A.; Northwood, K.S.; Wright, A.D.; Mcbride, B.W. Impact of high-concentrate feeding and low ruminal pH on methanogens and protozoa in the rumen of dairy cows. Microb. Ecol. 2011, 62, 94. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, R.; Schnürer, A.; Arthurson, V.; Bertilsson, J. Methanogenic population and CH4 production in swedish dairy cows fed different levels of forage. Appl. Environ. Microbl. 2012, 78, 6172. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Hernandezsanabria, E.; Guan, L.L. Characterization of variation in rumen methanogenic communities under different dietary and host feed efficiency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Appl. Environ. Microbl. 2010, 76, 3776–3786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, A.K.; Yu, Z. Effects of garlic oil, nitrate, saponin and their combinations supplemented to different substrates on in vitro fermentation, ruminal methanogenesis, and abundance and diversity of microbial populations. J. Appl. Microbiol. 2015, 119, 127–138. [Google Scholar] [CrossRef]
- Popova, M.; Morgavi, D.P.; Doreau, M.; Martin, C. Methane production and ruminal microbial interactions. Prod. Anim. 2011, 24, 447–460. [Google Scholar]
- Manatbay, B.; Cheng, Y.F.; Mao, S.Y.; Zhu, W.Y. Effect of Gynosaponin on Rumen In vitro Methanogenesis under Different Forage-Concentrate Ratios. Asian Australas. J. Anim. 2014, 27, 1088–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asanuma, N.; Iwamoto, M.; Hino, T. Effect of the Addition of Fumarate on Methane Production by Ruminal Microorganisms In Vitro. J. Dairy Sci. 1999, 82, 780–787. [Google Scholar] [CrossRef]
- Mamuad, L.; Kim, S.H.; Jeong, C.D.; Choi, Y.J.; Jeon, C.O.; Lee, S.S. Effect of Fumarate Reducing Bacteria on In Vitro Rumen Fermentation, Methane Mitigation and Microbial Diversity. J. Microbiol. 2014, 52, 120–128. [Google Scholar] [CrossRef]
- Tejido, M.L.; Ranilla, M.J.; Giraldo, L.A.; Carro, M.D. Influence of disodium fumarate on methane production and microbial activity in Rusitec fermenters fed a mixed diet. J. Anim. Feed Sci. 2007, 16, 609–614. [Google Scholar] [CrossRef]
- Lopez, S.; Valdes, C.; Newbold, C.J.; Wallace, R.J. Influence of sodium fumarate addition on rumen fermentation in vitro. Br. J. Nutr. 1999, 81, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Russell, J.B.; Houlihan, A.J. Ionophore resistance of ruminal bacteria and its potential impact on human health1. FEMS Microbiol. Rev. 2003, 27, 65–74. [Google Scholar] [CrossRef]
- Wolin, M.J. A Theoretical Rumen Fermentation Balance. J. Dairy Sci. 1960, 43, 1452–1459. [Google Scholar] [CrossRef]
- Zhou, Y.W.; McSweeney, C.S.; Wang, J.K.; Liu, J.X. Effects of disodium fumarate on ruminal fermentation and microbial communities in sheep fed on high-forage diets. Animal 2012, 6, 815–823. [Google Scholar] [CrossRef]
- Janssen, P.H.; Kirs, M. Structure of the archaeal community of the rumen. Appl. Environ. Microbl. 2008, 74, 3619–3625. [Google Scholar] [CrossRef] [Green Version]
- Forsberg, C.W.; Cheng, K.J.; White, B.A. Polysaccharide Degradation in the Rumen and Large Intestine. In Gastrointestinal Microbiology; Springer: Boston, MA, USA, 1997. [Google Scholar]
- Giraldo, L.A.; Ranilla, M.J.; Tejido, M.L.; Carro, M.D. Influence of exogenous fibrolytic enzymes and fumarate on methane production, microbial growth and fermentation in Rusitec fermenters. Br. J. Nutr. 2007, 98, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castromontoya, J.; De, C.S.; Van, R.G.; Fievez, V. Interactions between methane mitigation additives and basal substrates on in vitro methane and VFA production. Anim. Feed Sci. Technol. 2012, 176, 47–60. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhao, G.Y.; Zheng, W.S.; Niu, W.J.; Wei, C.; Lin, S.X. Effects of rare earth element lanthanum on rumen methane and volatile fatty acid production and microbial flora in vitro. J. Anim. Physiol. Anim. Nutr. 2015, 99, 442–448. [Google Scholar] [CrossRef] [PubMed]

| Monensin Sodium Salt (mg/kg) | Disodium Fumarate (mmol/L) | ||
|---|---|---|---|
| 0 | 7 | 14 | |
| 0 | T1 | T2 | T3 |
| 40 | T4 | T5 | T6 |
| 80 | T7 | T8 | T9 |
| Parameters | Substrates | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 |
|---|---|---|---|---|---|---|---|---|---|---|
| pH value | Forage | 7.35 ± 0.04 ab | 7.28 ± 0.08 ab | 7.4 ± 0.06 a | 7.22 ± 0.24 ab | 7.41 ± 0.04 a | 7.39 ± 0.08 ab | 7.33 ± 0.12 ab | 7.25 ± 0.09 b | 7.35 ± 0.05 ab |
| Vinasse | 7.21 ± 0.00 bc | 7.32 ± 0.01 a | 7.27 ± 0.04 ab | 7.17 ± 0.08 c | 7.24 ± 0.04 bc | 7.22 ± 0.06 bc | 7.16 ± 0.03 d | 7.25 ± 0.03 bc | 7.23 ± 0.1 bc | |
| NH3-N (mg/dL) | Forage | 31.95 ± 3.07 a | 28.93 ± 2.31 a | 29.31 ± 1.99 a | 34.83 ± 6.78 a | 29 ± 0.63 a | 28.74 ± 0.96 a | 26.29 ± 3.54 a | 36.07 ± 3.18 a | 31.16 ± 3.56 a |
| Vinasse | 22.61 ± 0.06 a | 17.95 ± 0.86 c | 20.49 ± 0.38 b | 23.05 ± 1.13 ab | 21.72 ± 1.2 ab | 23.77 ± 3.02 ab | 24.93 ± 1.00 a | 23.41 ± 0.45 a | 23.31 ± 1.32 ab | |
| Acetate (mmol/L) | Forage | 36.75 ± 0.2 a | 38.61 ± 4.67 a | 41.22 ± 5.1 a | 44.31 ± 10.94 a | 34.54 ± 1.2 a | 36.23 ± 3.14 a | 30.37 ± 1.6 a | 37.74 ± 4.63 a | 38.55 ± 1.21 a |
| Vinasse | 33.27 ± 0.83 abc | 34.67 ± 2.54 ab | 36.11 ± 0.85 a | 33.66 ± 2.27 abc | 37.03 ± 2.07 a | 32.42 ± 3.51 abc | 31.35 ± 2.54 bc | 26.66 ± 2.47 D | 30.91 ± 2.39 c | |
| Propionate (mmol/L) | Forage | 8.32 ± 0.33 b | 15.37 ± 0.89 a | 17.91 ± 2.43 a | 12.11 ± 3.71 ab | 13.76 ± 1.33 ab | 17.91 ± 0.77 a | 8.45 ± 0.49 b | 14.87 ± 2.25 ab | 19.82 ± 2.22 a |
| Vinasse | 6.32 ± 0.03 b | 15.76 ± 2.86 ab | 14.91 ± 6.18 ab | 9.58 ± 1.3 b | 15.69 ± 0.27 a | 16.7 ± 2.65 ab | 10.67 ± 0.86 b | 13.11 ± 2.42 ab | 18.03 ± 0.98 a | |
| Isobutyrate (mmol/L) | Forage | 0.77 ± 0.04 a | 0.71 ± 0.16 a | 0.75 ± 0.09 a | 0.99 ± 0.28 a | 0.7 ± 0.02 a | 0.71 ± 0.09 a | 0.73 ± 0.05 a | 0.87 ± 0.11 a | 0.82 ± 0.06 a |
| Vinasse | 0.64 ± 0.02 ab | 0.60 ± 0.03 ab | 0.63 ± 0.05 a | 0.65 ± 0.02 a | 0.64 ± 0.02 a | 0.56 ± 0.06 b | 0.62 ± 0.05 ab | 0.56 ± 0.03 c | 0.59 ± 0.03 ab | |
| Butyrate (mmol/L) | Forage | 6.73 ± 0.28 ab | 5.65 ± 1.46 c | 5.97 ± 0.83 c | 9.79 ± 3.34 a | 6.25 ± 0.21 b | 6.16 ± 0.92 b | 7.27 ± 0.56 ab | 8.34 ± 1.24 a | 7.53 ± 0.61 a |
| Vinasse | 11.23 ± 0.08 a | 8.11 ± 0.06 d | 9.39 ± 1.24 bc | 10.31 ± 0.29 ab | 9.58 ± 0.52 bc | 7.93 ± 0.65 d | 9.38 ± 0.67 bc | 8.03 ± 0.8 d | 8.8 ± 0.91 cd | |
| Isovalerate (mmol/L) | Forage | 1.42 ± 0.06 c | 1.54 ± 0.17 b | 1.61 ± 0.14 b | 2.05 ± 0.75 ab | 1.79 ± 0.24 ab | 1.7 ± 0.21 ab | 1.63 ± 0.13 ab | 2.31 ± 0.24 a | 2.23 ± 0.39 ab |
| Vinasse | 1.14 ± 0.06 c | 1.38 ± 0.12 ab | 1.42 ± 0.09 a | 1.15 ± 0.06 c | 1.25 ± 0.11 bc | 1.11 ± 0.17 c | 1.1 ± 0.09 c | 1.05 ± 0.06 d | 1.2 ± 0.12 c | |
| Valerate (mmol/L) | Forage | 1.25 ± 0.03 ab | 1.13 ± 0.27 b | 1.19 ± 0.15 ab | 1.65 ± 0.52 a | 1.14 ± 0.05 ab | 1.13 ± 0.16 b | 1.06 ± 0.11 c | 1.28 ± 0.16 ab | 1.24 ± 0.1 ab |
| Vinasse | 1.08 ± 0.03 ab | 1.04 ± 0.08 b | 1.05 ± 0.02 b | 1.24 ± 0.13 a | 1.27 ± 0.07 a | 1.01 ± 0.13 b | 1.21 ± 0.09 a | 0.99 ± 0.09 b | 1.14 ± 0.18 ab | |
| Acetate:propionate | Forage | 4.42 ± 0.2 a | 2.52 ± 0.32 ab | 2.31 ± 0.03 ab | 3.69 ± 0.23 a | 2.52 ± 0.18 ab | 2.02 ± 0.09 ab | 3.59 ± 0.1 a | 2.55 ± 0.23 ab | 1.97 ± 0.27 b |
| Vinasse | 5.27 ± 0.11 a | 2.23 ± 0.25 ab | 3.01 ± 1.93 ab | 3.53 ± 0.23 ab | 2.36 ± 0.12 ab | 1.95 ± 0.14 b | 2.94 ± 0.04 ab | 2.06 ± 0.17 b | 1.72 ± 0.06 b |
| Target Species | Forage | Vinasse | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T3 | T7 | T8 | T1 | T3 | T7 | T8 | |
| Total bacteria (×107 copies/mL) | 3.51 ± 1.07 abc | 2.54 ± 1.23 c | 2.83 ± 1.42 c | 3.34 ± 2.12 bc | 9.53 ± 3.22 ab | 10.34 ± 7.47 a | 9.97 ± 3.01 a | 5.43 ± 1.93 abc |
| Methanogenic archaea (×105 copies/mL) | 2.2 ± 0.16 bCd | 0.93 ± 0.42 e | 1.28 ± 0.63 d | 1.76 ± 0.99 Cd | 4.34 ± 0.44 a | 2.95 ± 1.74 abc | 3.75 ± 0.63 ab | 2.63 ± 0.65 bCd |
| Ciliate protozoa (×102 copies/mL) | 6.46 ± 0.22 ab | 2.62 ± 1.73 b | 6.36 ± 4.3 ab | 10.24 ± 6.21 a | 7.04 ± 0.71 ab | 9.06 ± 6.45 ab | 9.9 ± 2.97 a | 11.25 ± 5.79 a |
| Anaerobic fungi (copies/mL) | 7.8 ± 0.48 a | 7.24 ± 0.91 a | 12.23 ± 5.47 a | 8.05 ± 1.42 a | 10.68 ± 1.47 a | 6.65 ± 2.99 a | 6.47 ± 2.08 a | 11.91 ± 6.39 a |
| Fibrobacter succinogenes (×104 copies/mL) | 13.89 ± 6.19 ab | 16.08 ± 6.27 a | 17.76 ± 8.31 a | 33.65 ± 21.54 a | 0.37 ± 0.09 D | 1.9 ± 1.48 c | 2.91 ± 1.03 bc | 1.21 ± 0.54 c |
| Selenomonas ruminantium (×105 copies/mL) | 2.15 ± 0.05 C | 2.43 ± 0.25 C | 2.75 ± 0.73 d | 3.55 ± 1.8 C | 7.28 ± 1.73 BC | 15 ± 8.83 B | 98.94 ± 44.96 a | 47.74 ± 11 aB |
| Ruminococcus flavefaciens (×102 copies/mL) | 5.74 ± 0.14 a | 2.08 ± 0.47 b | 2.34 ± 1.43 b | 1.85 ± 0.85 b | 1.82 ± 0.11 b | 2.84 ± 1.19 ab | 2.87 ± 0.93 ab | 2.09 ± 0.47 b |
| Ruminococcus albus (×102 copies/mL) | 4.63 ± 0.56 a | 2.42 ± 0.24 b | 1.46 ± 0.28 C | 1.52 ± 0.26 C | 0.76 ± 0.02 d | 1.46 ± 0.08 C | 0.34 ± 0.16 e | 0.50 ± 0.20 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, D.; Chen, H.; Luo, X. Methane Emissions Regulated by Microbial Community Response to the Addition of Monensin and Fumarate in Different Substrates. Appl. Sci. 2021, 11, 6282. https://doi.org/10.3390/app11146282
Xue D, Chen H, Luo X. Methane Emissions Regulated by Microbial Community Response to the Addition of Monensin and Fumarate in Different Substrates. Applied Sciences. 2021; 11(14):6282. https://doi.org/10.3390/app11146282
Chicago/Turabian StyleXue, Dan, Huai Chen, and Xiaolin Luo. 2021. "Methane Emissions Regulated by Microbial Community Response to the Addition of Monensin and Fumarate in Different Substrates" Applied Sciences 11, no. 14: 6282. https://doi.org/10.3390/app11146282
APA StyleXue, D., Chen, H., & Luo, X. (2021). Methane Emissions Regulated by Microbial Community Response to the Addition of Monensin and Fumarate in Different Substrates. Applied Sciences, 11(14), 6282. https://doi.org/10.3390/app11146282






