High Dimension Granite Pavement Bio-Desalination Practical Implementation
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Pseudomonas Stutzeri Optimal Denitrification Assessment
2.3. Biomass Production
2.4. High Scale Application Protocol Implementation
- Warm 2% agar is prepared by mixing 2% agar powder in deionized water and then boiling it. Then, it must be tempered (between 40–45 °C) to become a semisolid, which is a good consistency for the application.
- Ground 2% agar is previously prepared in the lab following the warm agar protocol but leaving to become completely cold and, therefore, to jellify on a crystal recipient. Then it is ground and used or stored in a fridge for a few hours.
- Nevek® does not need any preparation because it is directly applied from the storage package.
- Infrared heat lamps are installed onto a tripod and oriented to face the treatment surface. They must be switched on for 14 h before treatment in order to reach the desired treatment temperature. The surface temperature is manually measured with an infrared thermometer (Parkside PTIA1, OWIM GmbH & Co. KG, Neckarsulm, Germany).
- The air heater must be inserted into a previously installed hothouse on the treated area in order to reduce the volume of air to be warmed. The air heater must be activated 24 h before treatment in order to obtain the adequate treatment temperature. The surface temperature is manually measured with an infrared thermometer.
- A heating electric mat (10 m long and 50 cm wide, Warmup, UK) is applied directly to the delivery system and, on top of the mat, a thermally insulating material (such as wood panels) must be added in order to avoid temperature loss to the ambient air. The heating electric mat must be activated 24 h before treatment in order to obtain the desired temperature. It has a temperature control thermostat.
2.5. High Scale Optimized Protocol
2.6. Bio-Desalination Monitoring
2.7. Digital Analysis Cleaning Study
3. Results
3.1. Selection of Best Bacterial Denitrification Conditions
3.2. Optimal High Scale Application Protocol Determination
3.3. Efficiency of High Dimension Bio-Desalination
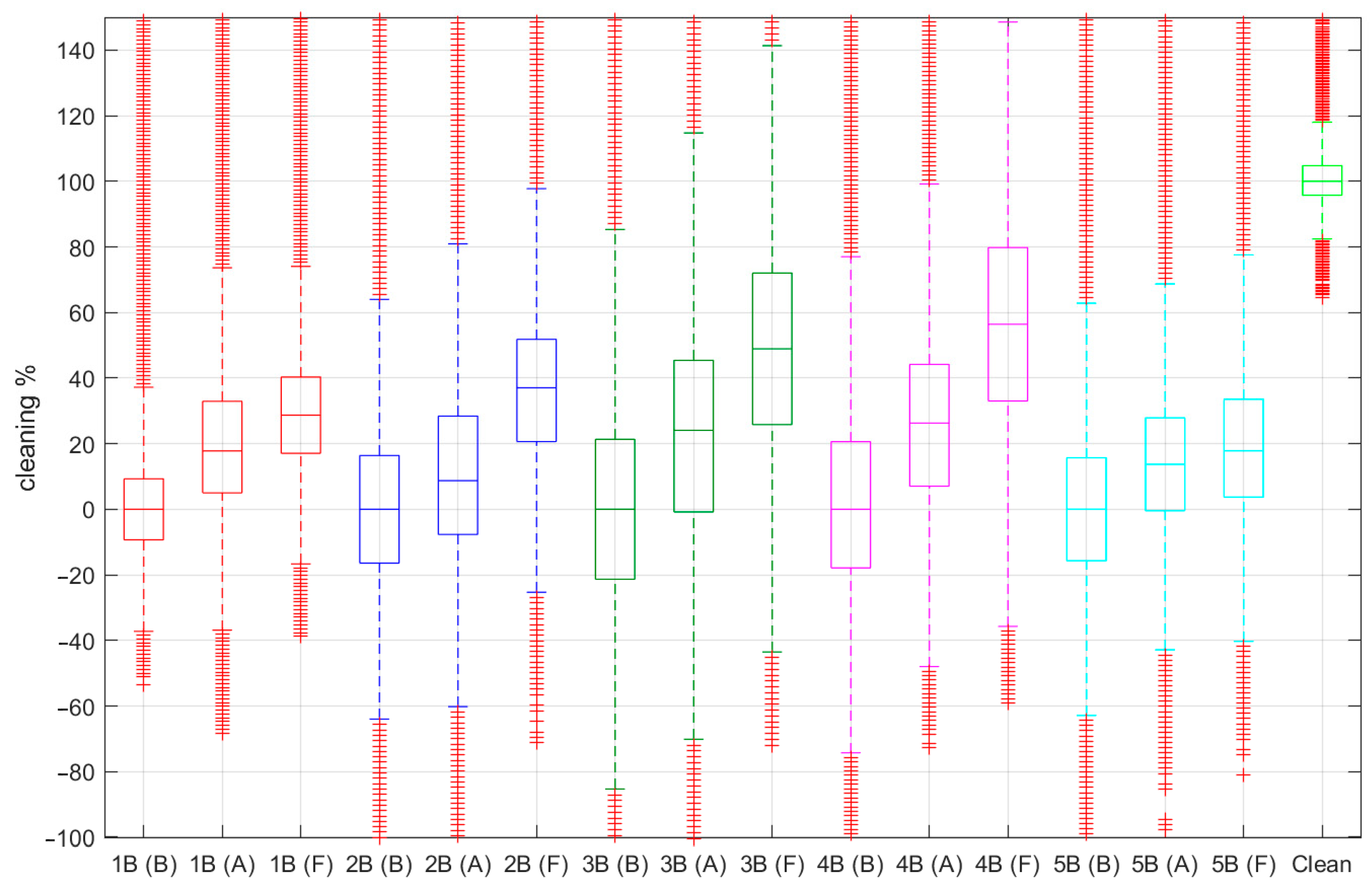
3.4. High Dimension Bio-Desalination Visual Cleaning and Digital Imaging Analysis
4. Discussion
- Reduce in situ material preparation. Any needed preparations must first be completed in the lab to reduce in situ preparation time and equipment.
- Use versatile materials, allowing them to be adapted to the building architectural needs.
- Select materials and protocols that allow a high treatment area per day and self-handling in order to reduce economic costs.
- Use methods that modify the interior environmental conditions of the monument as little as possible.
- Use electricity generation methods that are compatible with the low electrical power generally supported by the monuments and avoid impact on the surrounding environment (noise, gases, etc.).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bosch-Roig, P.; Ranalli, G. Biocleaning of Cultural Heritage treasures. In Biodeterioration and Preservation of Cultural Heritage Treasures; Mitchel, R., Ed.; Archetype Publications: London, UK, 2018; pp. 169–183. [Google Scholar]
- Bosch-Roig, P.; Ranalli, G. The safety of biocleaning technologies for Cultural Heritage. Front. Microbiol. 2014, 5, 155. [Google Scholar] [CrossRef] [Green Version]
- Ranalli, G.; Zanardini, E. Biocleaning on Cultural Heritage: New frontiers of microbial biotechnologies. J. Appl. Microbiol. 2021, 1–21. [Google Scholar] [CrossRef]
- Lombardi, E.; Balloi, A.; Troiano, F.; Gulotta, D.; Polo, A.; Gioventù, E.; Sorlini, C.; Cappitelli, F.; Daffonchio, D. Strategies for increasing the scale of biocleaning treatment through sulfate crust bioremoval. In Proceedings of the Built Heritage 2013 Monitoring Conservation Management, Milano, Italy, 18–20 November 2013; Politecnico di Milano: Milano, Italy, 2013; pp. 1424–1430. [Google Scholar]
- Romano, I.; Abbate, M.; Poli, A.; D’Orazio, L. Bio-cleaning of nitrate salt efflorescence on stone samples using extremophilic bacteria. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Soffritti, I.; D’Accolti, M.; Lanzoni, L.; Volta, A.; Bisi, M.; Mazzacane, S.; Caselli, E. The Potential Use of Microorganisms as Restorative Agents: An Update. Sustainability 2019, 11, 3853. [Google Scholar] [CrossRef] [Green Version]
- Gioventù, E.; Lorenzi, P.F.; Villa, F.; Sorlini, C.; Rizzi, M.; Cagnini, A.; Griffo, A.; Cappitelli, F. Comparing the bioremoval of black crusts on colored artistic lithotypes of the Cathedral of Florence with chemical and laser treatment. Int. Biodeterior. Biodegrad. 2011, 65, 832–839. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Allegue, H.; Bosch, I. Granite Pavement Nitrate Desalination: Traditional Methods vs. Biocleaning Methods. Sustainability 2019, 11, 4227. [Google Scholar] [CrossRef] [Green Version]
- Cappitelli, F.; Zanardini, E.; Toniolo, L.; Abbruscato, P.; Ranalli, G.; Sorlini, C. Bioconservation of the marble base of the Pietà Rondanini by Michelangelo Buonarroti. Geophys. Res. Abstr. 2005, 7, 06675. [Google Scholar]
- Polo, A.; Cappitelli, F.; Brusetti, L.; Principi, P.; Villa, F.; Giacomucci, L.; Ranalli, G.; Sorlini, C. Feasibility of Removing Surface Deposits on Stone Using Biological and Chemical Remediation Methods. Microb. Ecol. 2010, 60, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Troiano, F.; Gulotta, D.; Balloi, A.; Polo, A.; Toniolo, L.; Lombardi, E.; Daffonchio, D.; Sorlini, C.; Cappitelli, F. Successful combination of chemical and biological treatments for the cleaning of stone artworks. Int. Biodeterior. Biodegrad. 2013, 85, 294–304. [Google Scholar] [CrossRef]
- Martino, M.; Schiavone, S.; Palla, F.; Pellegrino, L.; De Castro, E.; Balloi, A. Bioremoval of sulphate layer from a 15th century polycrome marble artifact. Conserv. Sci. Cult. Herit. 2015, 15, 235–243. [Google Scholar]
- Bosch-Roig, P.; Regidor-Ros, J.L.; Soriano-Sancho, P.; Doménec-Carbó, M.T.; Montes-Estelle s, R.M. Ensayos de biolimpieza con bacterias en pinturas murales. Arché 2010, 4–5, 115–122. [Google Scholar]
- Lustrato, G.; Alfano, G.; Andreotti, A.; Colombini, M.P.; Ranalli, G. Fast biocleaning of mediaeval frescoes using viable bacterial cells. Int. Biodeterior. Biodegrad. 2012, 69, 51–61. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Regidor-Ros, J.L.; Soriano-Sancho, P.; Montes-Estellés, R.M. Biocleaning of animal glue on wall paintings by Pseudomonas stutzeri. Chem. Today 2013, 31, 50–53. [Google Scholar]
- Roig, P.B.; Ros, J.L.R.; Montes-Estelles, R.M. Biocleaning of nitrate alterations on wall paintings by Pseudomonas stutzeri. Int. Biodeterior. Biodegrad. 2013, 84, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Metaldi, S. Applicazione sperimentale di batteri solfato riduttori sul pigmento azzurrite. In Proceedings of the I batteri nel restauro, Thiene, Italy, 28 September 2013. [Google Scholar]
- Mazzoni, M.; Alisi, C.; Tasso, F.; Cecchini, A.; Marconi, P.; Sprocati, A.R. Laponite micro-packs for the selective cleaning of multiple coherent deposits on wall paintings: The case study of Casina Farnese on the Palatine Hill (Rome-Italy). Int. Biodeterior. Biodegrad. 2014, 94, 1–11. [Google Scholar] [CrossRef]
- Balloi, A.; Lombardi, E.; Troiano, F.; Polo, A.; Cappitelli, F.; Gulotta, D.; Toniolo, L.; Lucchini, A. Sulfate reducing bacteria as bio-cleaning agents: Development of new methodologies and study cases. Conserv. Sci. Cult. Herit. 2015, 15, 109–119. [Google Scholar]
- Panella, E.; Giovannone, C.; Bartolini, M.; Fondi, V. Biotechnology in the conservation field: Removal of sulphates using bacteria and bioconsolidation of paintings and stuccos. Conserv. Sci. Cult. Herit. 2019, 19, 157–175. [Google Scholar]
- Ranalli, G.; Zanardini, E.; Rampazzi, L.; Corti, C.; Andreotti, A.; Colombini, M.P.; Bosch-Roig, P.; Lustrato, G.; Giantomassi, C.; Zari, D.; et al. Onsite advanced biocleaning system for historical wall paintings using new agar-gauze bacteria gel. J. Appl. Microbiol. 2019, 126, 1785–1796. [Google Scholar] [CrossRef]
- Alfano, G.; Lustrato, G.; Belli, C.; Zanardini, E.; Cappitelli, F.; Mello, E.; Sorlini, C.; Ranalli, G. The bioremoval of nitrate and sulfate alterations on artistic stonework: The case-study of Matera Cathedral after six years from the treatment. Int. Biodeterior. Biodegrad. 2011, 65, 1004–1011. [Google Scholar] [CrossRef]
- Rampazzi, L.; Andreotti, A.; Bressan, M.; Colombini, M.P.; Corti, C.; Cuzman, O.; D’Alessandro, N.; Liberatore, L.; Palombi, L.; Raimondi, V.; et al. An interdisciplinary approach to a knowledge-based restoration: The dark alteration on Matera Cathedral (Italy). Appl. Surf. Sci. 2018, 458, 529–539. [Google Scholar] [CrossRef]
- Ranalli, G.; Alfano, G.; Belli, C.; Lustrato, G.; Colombini, M.P.; Bonaduce, I.; Zanardini, E.; Abbruscato, P.; Cappitelli, F.; Sorlini, C. Biotechnology applied to cultural heritage: Borestoration of frescoes using viable bacterial cells and enzymes. J. Appl. Microbiol. 2005, 98, 73–83. [Google Scholar] [CrossRef] [PubMed]
- García Morales, S.; Otero Ortiz de Cosca, R.; Allegue Castelos, H. Investigación Sobre El Oscurecimiento Húmedo Que Afecta Al Enlosado De La Capilla Del Cristo De Santa María De Conxo; Cuadernos técnicos; Consorcio de Santiago Oficina Técnica: Santiago de Compostela, Spain, 2016; Available online: https://issuu.com/consorciodesantiago/docs/conxo_oscurecimiento_humedo (accessed on 16 April 2021).
- Ranalli, G.; Chiavarini, M.; Guidetti, V.; Marsala, F.; Matteini, M.; Zanardini, E.; Sorlini, C. The use of microorganisms for the removal of nitrates and organic substances on artistic stoneworks. In Proceedings of the 8th International Congress on Deterioration and Conservation of Stone, Berlin, Germany, 30 September–4 October 1996. [Google Scholar]
- May, E.; Webster, A.M.; Inkpen, R.; Zamarreno, D.; Kuever, J.; Rudolph, C.; Warcheid, T.; Sorlini, C.; Cappitelli, F.; Zanardini, E.; et al. The BIOBRUSH Project for bioremediation of heritage stone. In Heritage Microbiology and Science. Microbes, Monuments and Maritime Materials; May, E., Jones, M., Mitchell, J., Eds.; RCS Publishing: Cambridge, UK, 2008; pp. 76–93. [Google Scholar]
- Vidakovic, A.; Sovljanski, O.; Vucurovic, D.; Racic, G.; Djilas, M.; Curcic, N.; Markov, S. Novel denitrifying bacteria Pseudomonas stutzeri strain D1—From isolation to the biomass production. Chem. Ind. Chem. Eng. Q. 2019, 25, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Noble, R.C.; Overman, S.B. Pseudomonas stutzeri infection a review of hospital isolates and a review of the literature. Diagn. Microbiol. Infect. Dis. 1994, 19, 51–56. [Google Scholar] [CrossRef]
- Atlas, R.M.; Chowdhury, A.N.; Gauri, K.L. Microbial calcification of gypsum-rock and sulfated marble. J. Stud. Conserv. 1988, 33, 149–153. [Google Scholar]
- Bosch-Roig, P.; Lustrato, G.; Zanardini, E.; Ranalli, G. Biocleaning of Cultural Heritage stone surfaces and frescoes: Which delivery system can be the most appropriate? Ann. Microbiol. 2014, 65, 1227–1241. [Google Scholar] [CrossRef]
- Ranalli, G.; Bosch-Roig, P.; Crudele, S.; Rampazzi, L.; Corti, C.; Zanardini, E. Dry biocleaning of artwork: An innovative methodology for Cultural Heritage recovery? Microb. Cell 2021, 8, 91–105. [Google Scholar] [CrossRef]
- Yang, J.; Feng, L.; Pi, S.; Cui, D.; Ma, F.; Zhao, H.-P.; Li, A. A critical review of aerobic denitrification: Insights into the intracellular electron transfer. Sci. Total. Environ. 2020, 731, 139080. [Google Scholar] [CrossRef] [PubMed]
- Franco, B.; Gisbert, J.; Mateos, I.; Navarro, P. Deterioro de los materiales pétreos por sales: Cinética del proceso, cartografía y métodos de extracción. In Proceedings of the I Congreso GEIIC, Valencia, Spain, 25–27 November 2002; pp. 287–294. Available online: https://www.ge-iic.com/wp-content/uploads/2006/06/Franco_Belen.pdf (accessed on 20 May 2021).
- Randazzo, L.; Montana, G.; Castiglia, A.; La Russa, M.F. Salt extraction from lime-based mortars: An experimental study using different poultice formulations. Constr. Build. Mater. 2020, 255, 119391. [Google Scholar] [CrossRef]
- Doehne, E. Salt weathering: A selective review. Geol. Soc. Lond. Spéc. Publ. 2002, 205, 51–64. [Google Scholar] [CrossRef]
- Bromblet, P.; Verges-Belmin, V. The removal of sulfates from calcareous stone outdoor statuary: A questionable practice. In Proceedings of the Le dessalement des matériaux Poreux. 7es journées d’études de la SFIIC, Poitiers, France, 9–10 May 1996; pp. 55–63. [Google Scholar]
- Charola, A.E. Salts in the deterioration of porous materials: An overview. J. Am. Inst. Conserv. 2000, 39, 327–343. [Google Scholar] [CrossRef]
- Siedel, H. Experiences from desalting of tuffstone and sandstone monuments by compresses. In Proceedings of the Le dessalement des matériaux poreux. 7es journées d’études de la SFIIC, Poitiers, France, 9–10 May 1996; pp. 191–198. [Google Scholar]
- Zornoza-Indart, A. Técnicas de desalación. In La Conservación de los Geomateriales utilizados en el Patrimonio; Programa Geomateriales: Madrid, Spain, 2012; pp. 143–154. [Google Scholar]
- Wust, R.; McLane, J. Rock deterioration in the Royal Tomb of Seti I, Valley of the Kings, Luxor, Egypt. Eng. Geol. 2000, 58, 163–190. [Google Scholar] [CrossRef]
- Wüst, R.A.; Schlüchter, C. The Origin of Soluble Salts in Rocks of the Thebes Mountains, Egypt: The Damage Potential to Ancient Egyptian Wall Art. J. Archaeolog. Sci. 2000, 27, 1161–1172. [Google Scholar] [CrossRef]
- Arnold, A.; Zehnder, K. Decay of stony materials by salts in humid atmosphere. In Proceedings of the 6th International Congress on Deterioration and Conservation of Stone, Torun, Poland, 12–14 September 1988; Ciabach, J., Ed.; Copernicus University Press: Torun, Poland, 1988; pp. 138–148. [Google Scholar]
- Cantón, Y.; Solé-Benet, A.; Queralt, I.; Pini, R. Weathering of a gypsum-calcareous mudstone under semi-arid environment at Tabernas, SE Spain: Laboratory and field-based experimental approaches. Catena 2001, 44, 111–132. [Google Scholar] [CrossRef]
- Williams, R.B.G.; Robinson, D.A. Experimental frost weathering of sandstone by various combinations of salts. Earth Surf. Process. Landf. 2001, 26, 811–818. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Doehne, E.; Sebastian, E. Influencing Crystallization Damage in Porous Materials through the Use of Surfactants: Experimental Results Using Sodium Dodecyl Sulfate and Cetyldimethylbenzylammonium Chloride. Langmuir 2000, 16, 947–954. [Google Scholar] [CrossRef]
- Mukherjee, A.K.; Das, K. Microbial Surfactants and Their Potential Applications: An Overview. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; Volume 672. [Google Scholar]
- Gazzano, C.; Favero-longo, S.E.; Matteucci, E.; Piervittori, R. Image analysis for measuring lichen colonization and within stonework. Lichenologist 2009, 41, 299–313. [Google Scholar] [CrossRef]
- Miller, A.Z.; Rogerio-Candelera, M.A.; Dionísio, A.; Macedo, M.F.; Saiz-Jimenez, C. Microalgae, as biodeteriogens of stone cultural heritage: Qualitative and quantitative research by non-contact techniques. In Microalgae: Biotechnology, Microbiology and Energy; Johanssen, M.N., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 345–358. [Google Scholar]
- Coutinho, M.L.D.; Miller, A.Z.; Gutierrez-Patricio, S.; Hernandez-Marine, M.; Gómez-Bolea, A.; Rogerio-Candelera, M.A.; Philips, A.; Jurado, V.; Saiz-Jimenez, C.; Macedo, M.F. Microbial communities on deteriorated artistic tiles from Pena National Palace (Sintra, Portugal). Int. Biodeterior. Biodegrad. 2013, 84, 322–332. [Google Scholar] [CrossRef]




| Time (h) | Temperature (°C) | Turbidity (+/−) | CFU mL−1 | Nitrates (mg L−1) | Nitrites (mg L−1) | Gas (+/−) |
|---|---|---|---|---|---|---|
| 24 | 4 | − | 2.0 × 101 | 500 | 23 | − |
| 20 | − | 2.0 × 102 | 500 | 18 | − | |
| 26 | + | 8.0 × 103 | 0 | 77 | + | |
| 48 | 4 | − | 1.0 × 102 | 500 | 24 | − |
| 20 | + | 3.0 × 106 | 0 | 63 | + | |
| 26 | + | 1.0 × 105 | 0 | 64 | + | |
| 72 | 4 | − | 1.9 × 102 | 500 | 26 | − |
| 20 | + | 3.0 × 106 | 0 | 66 | + | |
| 26 | + | 3.0 × 106 | 0 | 67 | + |
| In Situ Performance Difficulties | Economic Evaluation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Preparation Difficulties | Supporting Equipment | Time-Consuming (1) | Efficiency (2) | Complexity (3) | Treatment Area/Day | Costs €/m2 (4) | |||
| Material (5) | Personnel (6) | ||||||||
| Delivery system | Warm 2% agar | very high | very high | very high | very high | medium | 15 m2 | EUR 11.91 | EUR 104.98 |
| Ground 2% agar | low | low | medium | very high | low | 30 m2 | EUR 7.42 | EUR 18.37 | |
| Nevek | low | low | low | medium | low | 30 m2 | EUR 70.66 | EUR 14.00 | |
| Thermic systems | Infrared heat lamps | high | high | medium | very low | high | 15 m2 | EUR 3.54 | EUR 2.53 |
| Air heater | high | high | high | low | high | 15 m2 | EUR 7.40 | EUR 1.56 | |
| Heating electric mat | medium | medium | medium | very high | medium | 25 m2 | EUR 13.49 | EUR 0.98 | |
| Treated Slab | Ion Conductivity (μS/cm) | ||
|---|---|---|---|
| Before | After 1 | Follow-Up 2 | |
| 33 | 659 | 387 | 350 |
| 40 | 413 | 208 | 303 |
| 41 | 423 | 300 | 390 |
| 45 | 287 | 252 | 200 |
| 46 | 282 | 216 | 350 |
| Mean values | 412.8 | 272.6 | 318.6 |
| Standard deviation | 153.0 | 73.6 | 73.1 |
| Treated Slab | Nitrate–Nitrite Reduction (mg/L) | |||||
|---|---|---|---|---|---|---|
| Before | After 1 | Follow-Up 2 | ||||
| Nitrates | Nitrites | Nitrates | Nitrites | Nitrates | Nitrites | |
| 33 | 500 | 80 | 250 | 20 | 250 | 0 |
| 40 | 500 | 80 | 500 | 40 | 250 | 0 |
| 41 | 500 | 80 | 250 | 40 | 250 | 0 |
| 45 | 500 | 80 | 250 | 20 | 50 | 0 |
| 46 | 500 | 80 | 500 | 40 | 250 | 0 |
| Mean values | 500 | 80 | 350 | 32 | 210 | 0 |
| Standard deviation | 0.0 | 0.0 | 122.3 | 9.8 | 80.0 | 0 |
| Median ± std (%) | 1B | 2B | 3B | 4B | 5B | Media |
|---|---|---|---|---|---|---|
| After | 17.80 ± 21 | 8.73 ± 28 | 24.08 ± 34 | 26.30 ± 28 | 13.72 ± 22 | 18.12 ± 27 |
| Follow-up | 28.70 ± 18 | 37.05 ± 25 | 48.92 ± 32 | 56.41 ± 33 | 17.85 ± 22 | 37.78 ± 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch-Roig, P.; Pérez-Castro, L.; Fernández-Santiago, Á.; Bosch, I. High Dimension Granite Pavement Bio-Desalination Practical Implementation. Appl. Sci. 2021, 11, 6458. https://doi.org/10.3390/app11146458
Bosch-Roig P, Pérez-Castro L, Fernández-Santiago Á, Bosch I. High Dimension Granite Pavement Bio-Desalination Practical Implementation. Applied Sciences. 2021; 11(14):6458. https://doi.org/10.3390/app11146458
Chicago/Turabian StyleBosch-Roig, Pilar, Lourdes Pérez-Castro, Ángeles Fernández-Santiago, and Ignacio Bosch. 2021. "High Dimension Granite Pavement Bio-Desalination Practical Implementation" Applied Sciences 11, no. 14: 6458. https://doi.org/10.3390/app11146458






