Co-Production of Hydrogen and Methanol Using Fuel Mix Systems: Technical and Economic Assessment
Abstract
:1. Introduction
2. Development of Simulation Model
2.1. Case 1: Coal and Biomass-Based Model for the Simultaneous Methanol and Hydrogen Production (CBMH Process)
2.2. Case 2: Co-Production of Methanol and Hydrogen from Gasification and Reformation Models Using Three Fuels (Coal, Biomass and Natural Gas)—CBNMH Process
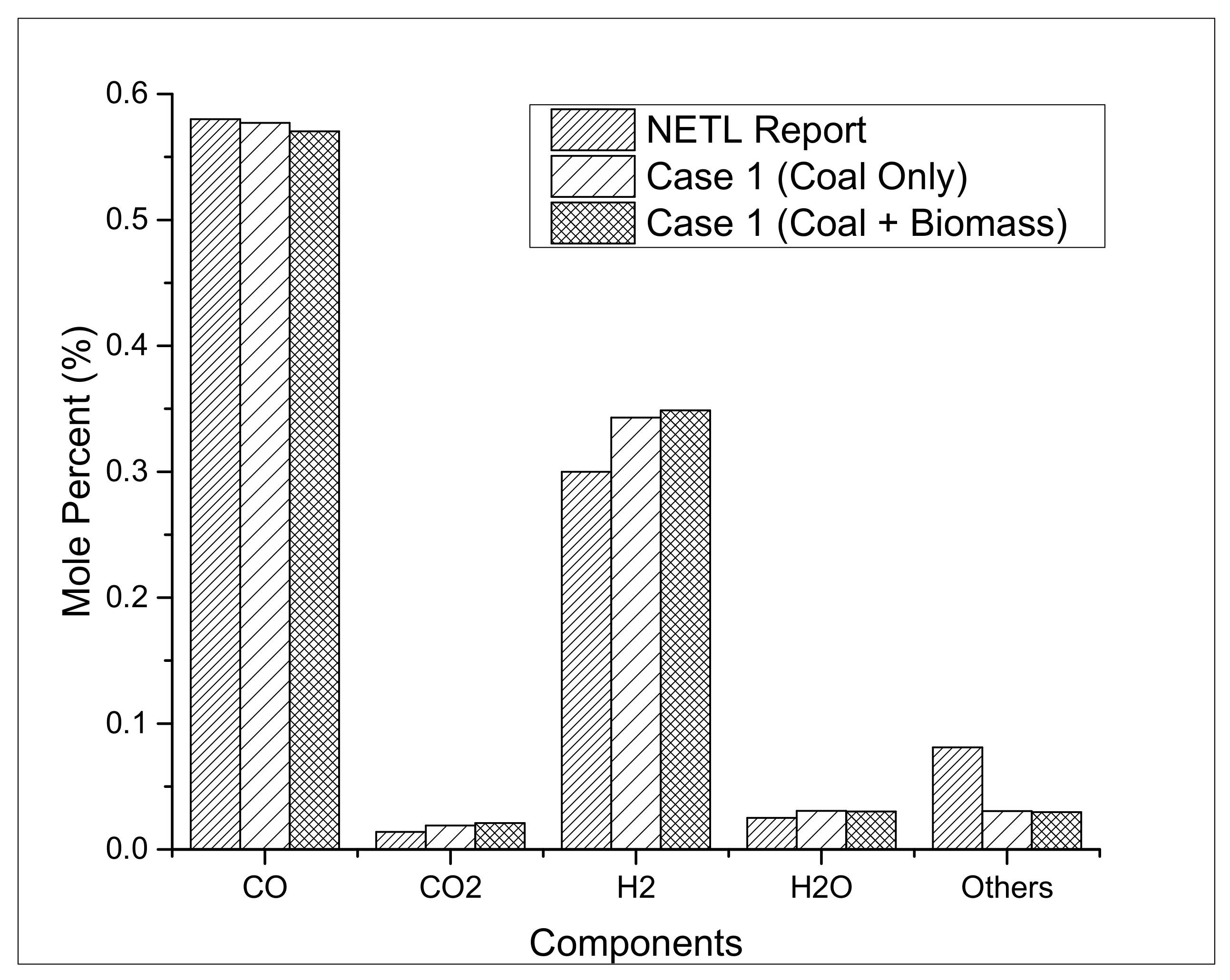
3. Results and Discussion
3.1. Equations Used for Comparative Analysis
3.1.1. Process Efficiency
3.1.2. CO2-Specific Emission
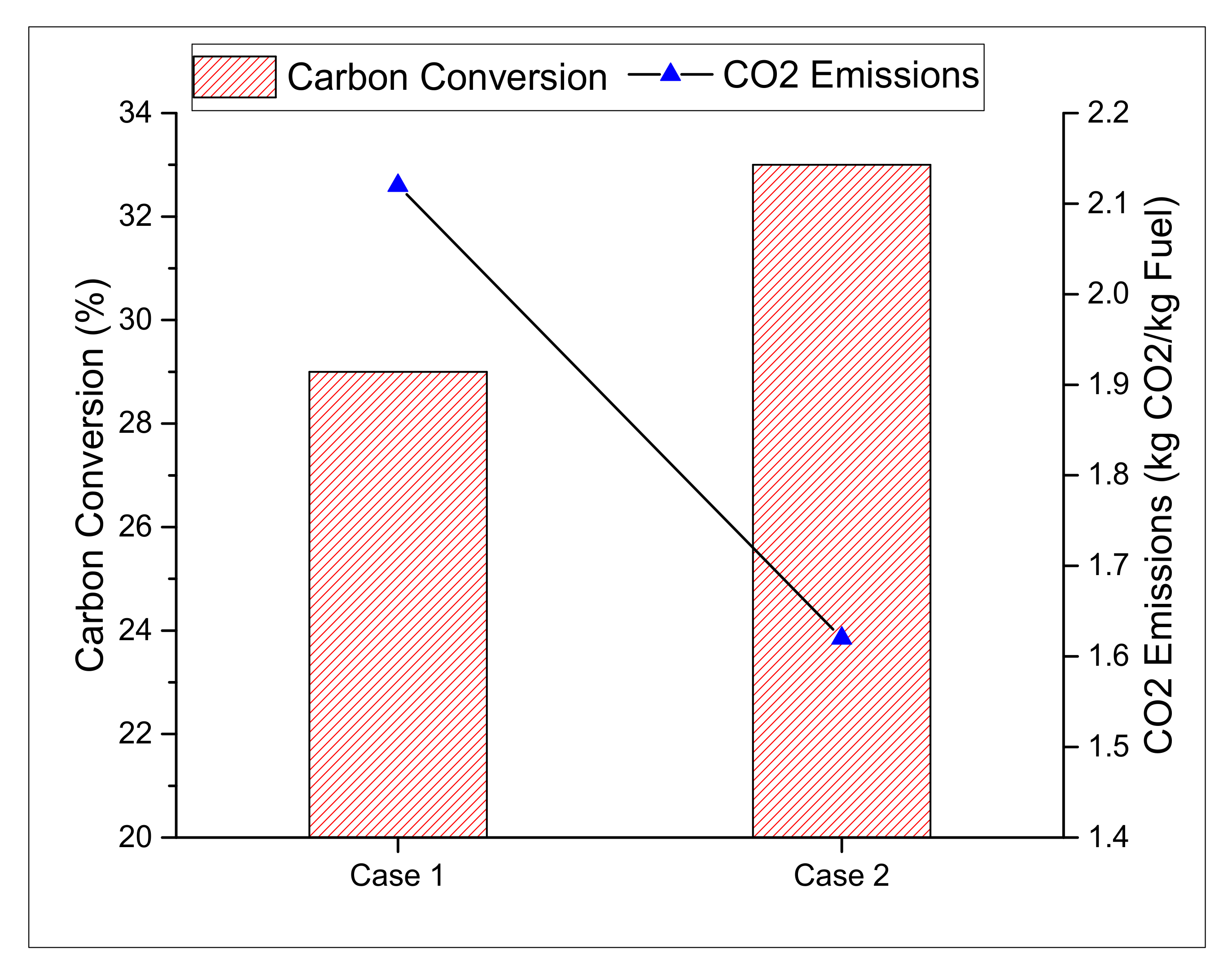
3.1.3. Carbon Conversion Efficiency
3.2. Process Performance Analysis
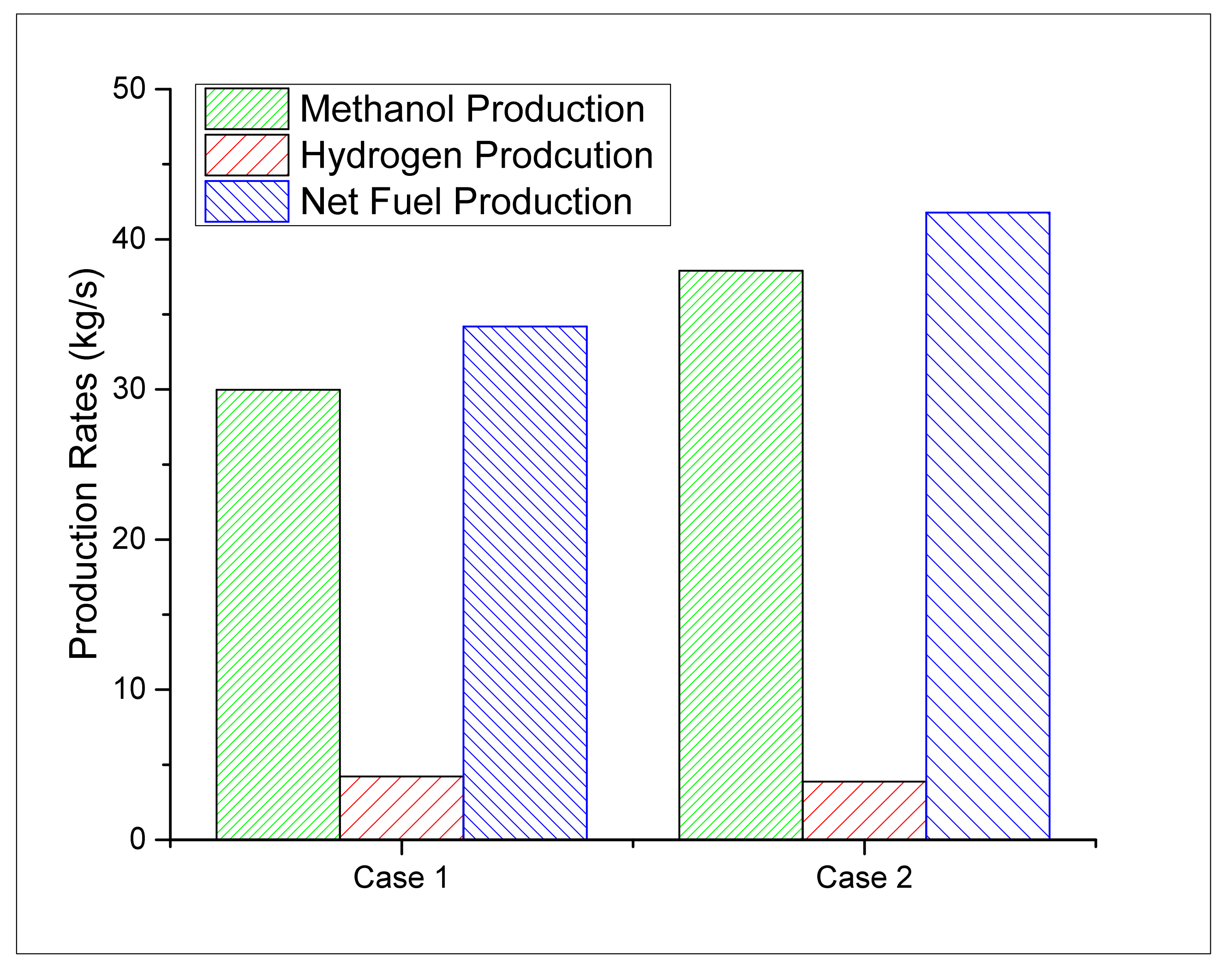
3.2.1. Methanol and Hydrogen Production Rates
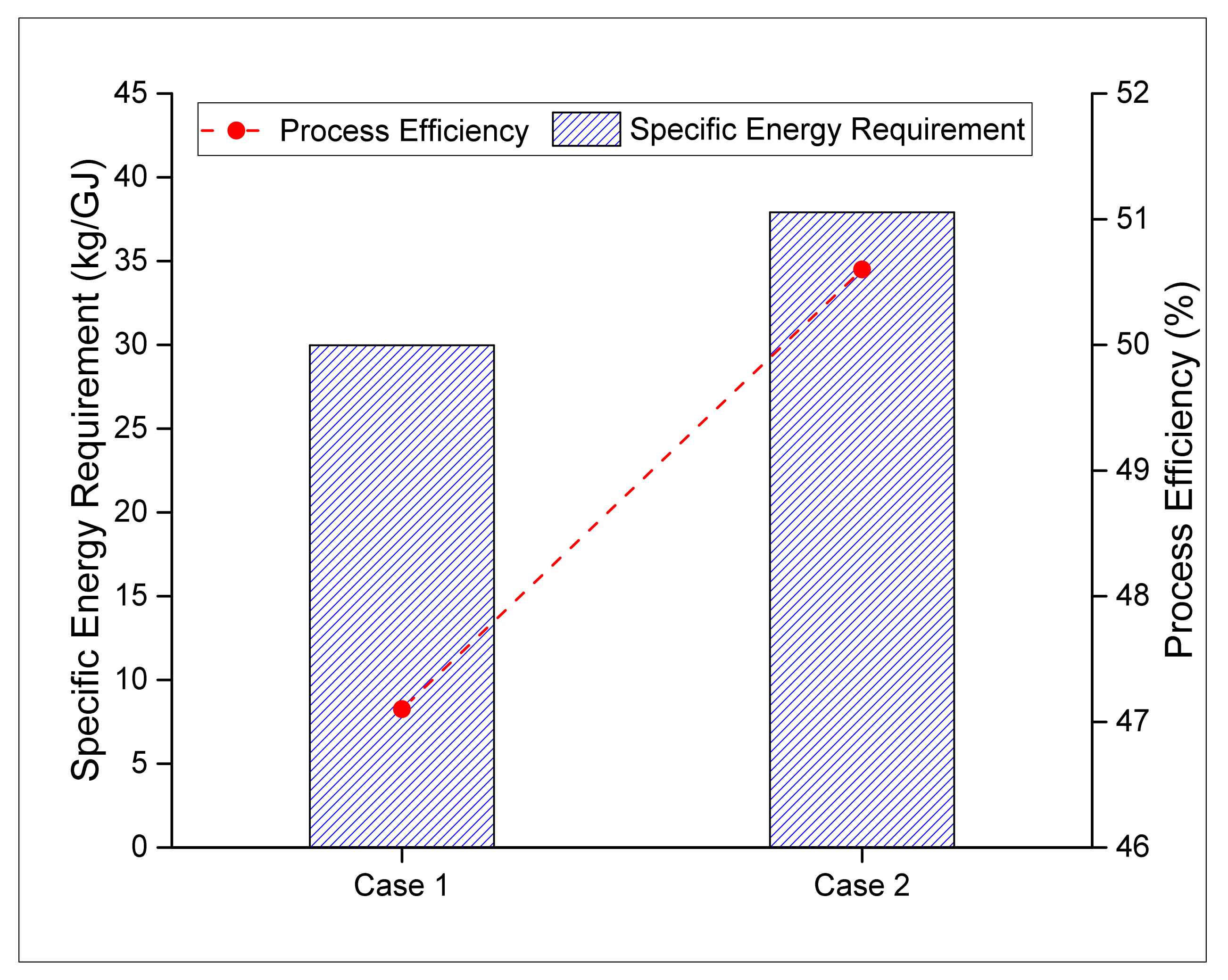
3.2.2. Specific Energy Consumption and Process Efficiency
3.2.3. Carbon Conversion and Emissions
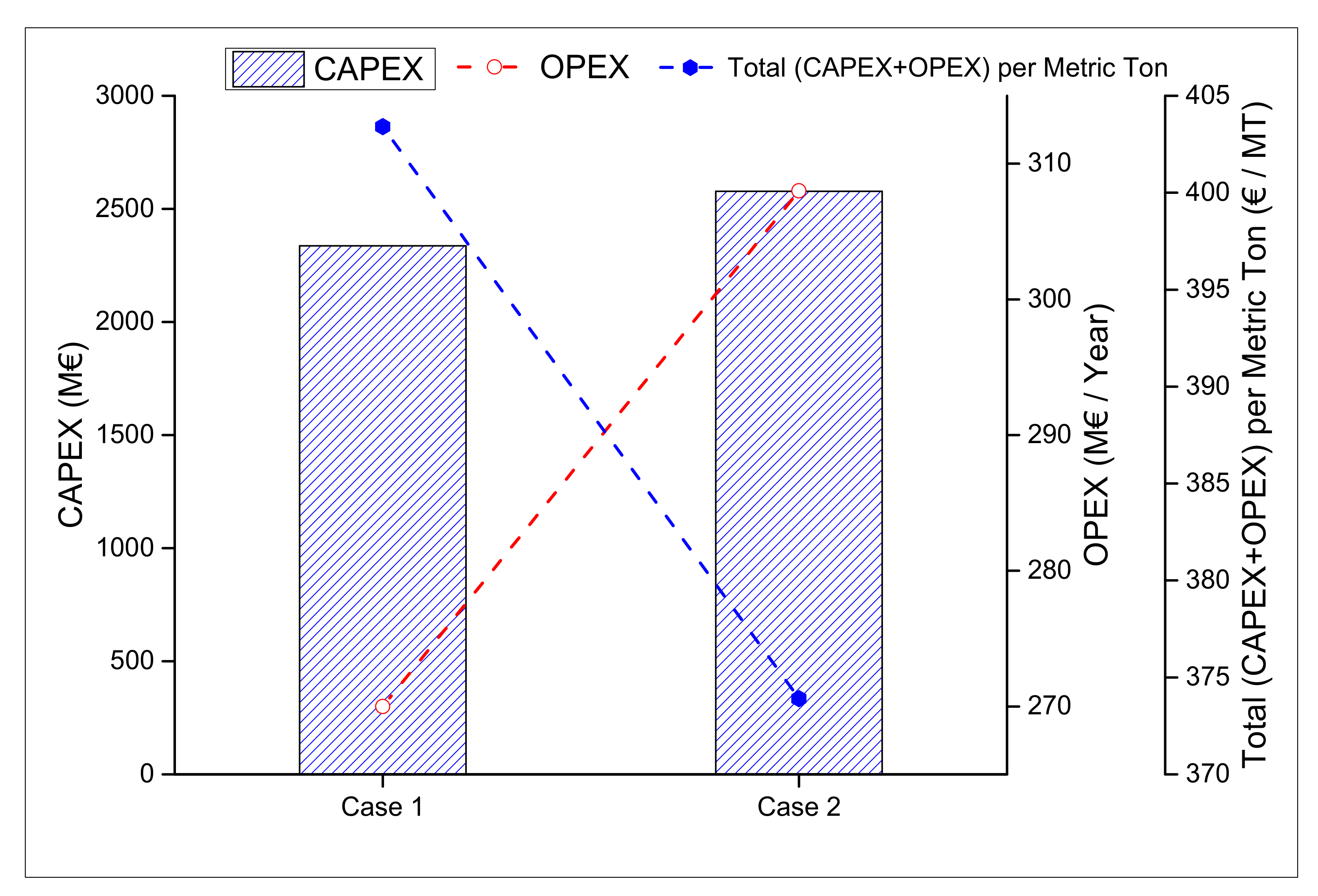
3.3. Economic Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AGR | Acid Gas Removal |
| CAPEX | Capital Expenditure |
| CBMH | Coal and Biomass to Methanol and Hydrogen |
| CBNMH | Coal Biomass and Natural Gas to Methanol and Hydrogen |
| CTM | Coal to Methanol |
| GHG | Global Greenhouse Gas |
| HCR | Hydrogen-to-Carbon Ratio |
| IPCC | Intergovernmental Panel on Climate Change |
| NGTM | Natural Gas to Methanol |
| OPEX | Operational Expenditure |
| SMR | Steam Methane Reforming |
| WGS | Water–Gas Shift |
References
- Jameel, A.G.A.; van Oudenhoven, V.; Emwas, A.-H.; Sarathy, S.M. Predicting Octane Number Using Nuclear Magnetic Resonance Spectroscopy and Artificial Neural Networks. Energy Fuels 2018, 32, 6309–6329. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, U. Techno-economic analysis of dual methanol and hydrogen production using energy mix systems with CO2 capture. Energy Convers. Manag. 2021, 228, 113663. [Google Scholar] [CrossRef]
- Ahmed, U. Techno-economic feasibility of methanol synthesis using dual fuel system in a parallel process design configuration with control on green house gas emissions. Int. J. Hydrogen Energy 2020, 45, 6278–6290. [Google Scholar] [CrossRef]
- Ahmed, U.; Zahid, U.; Lee, Y. Process simulation and integration of IGCC systems for H2/syngas/electricity generation with control on CO2 emissions. Int. J. Hydrogen Energy 2019, 44, 7137–7148. [Google Scholar] [CrossRef]
- AlNouss, A.; McKay, G.; Al-Ansari, T. A techno-economic-environmental study evaluating the potential of oxygen-steam biomass gasification for the generation of value-added products. Energy Convers. Manag. 2019, 196, 664–676. [Google Scholar] [CrossRef]
- Bazzanella, A.M.; Ausfelder, F. Low Carbon Energy and Feedstock for the European Chemicalindustry (Technical Study). In Dechema, Ges. für Chem. Tech. Biotechnol. eV; Frankfurt am Main, Germany, 2017; Available online: https://dechema.de/en/Low_carbon_chemical_industry-path-123212,124930.html (accessed on 29 June 2021).
- Blumberg, T.; Tsatsaronis, G.; Morosuk, T. On the economics of methanol production from natural gas. Fuel 2019, 256. [Google Scholar] [CrossRef]
- Castellani, B.; Gambelli, A.M.; Morini, E.; Nastasi, B.; Presciutti, A.; Filipponi, M.; Nicolini, A.; Rossi, F. Experimental Investigation on CO2 Methanation Process for Solar Energy Storage Compared to CO2-Based Methanol Synthesis. Energies 2017, 10, 855. [Google Scholar] [CrossRef]
- Castellani, B.; Rinaldi, S.; Morini, E.; Nastasi, B.; Rossi, F. Flue gas treatment by power-to-gas integration for methane and ammonia synthesis—Energy and environmental analysis. Energy Convers. Manag. 2018, 171, 626–634. [Google Scholar] [CrossRef]
- Edenhofer, O. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: New York, NY, USA, 2015; Volume 3. [Google Scholar]
- Zoelle, A.; Keairns, D.; Turner, M.J.; Woods, M.; Kuehn, N.; Shah, V.; Chou, V.; Pinkerton, L.L.; Fout, T. Cost and Performance Baseline for Fossil Energy Plants Volume 1b: Bituminous Coal (IGCC) to Electricity Revision 2b—Year Dollar Update; United States Departmeant of Energy: Washington, DC, USA, 2015.
- Hamid, U.; Rauf, A.; Ahmed, U.; Shah, S.A.S.; Ahmad, N. Techno-economic assessment of process integration models for boosting hydrogen production potential from coal and natural gas feedstocks. Fuel 2020, 266, 117111. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Cui, P. Design concept for coal-based polygeneration processes of chemicals and power with the lowest energy consumption for CO2 capture. Energy Convers. Manag. 2018, 157, 186–194. [Google Scholar] [CrossRef]
- Jameel, A.; Gani, A. Predicting Sooting Propensity of Oxygenated Fuels Using Artificial Neural Networks. Processes 2021, 9, 1070. [Google Scholar] [CrossRef]
- Jana, K.; Ray, A.; Majoumerd, M.M.; Assadi, M.; De, S. Polygeneration as a future sustainable energy solution—A comprehensive review. Appl. Energy 2017, 202, 88–111. [Google Scholar] [CrossRef]
- Khalafalla, S.S.; Zahid, U.; Jameel, A.G.A.; Ahmed, U.; Alenazey, F.S.; Lee, C.-J. Conceptual Design Development of Coal-To-Methanol Process with Carbon Capture and Utilization. Energies 2020, 13, 6421. [Google Scholar] [CrossRef]
- Moavenzadeh, J.; Torres-Montoya, M.; Gange, T. Repowering Transport: Project White Paper; World Economic Forum: Geneva, Switzerland, 2011. [Google Scholar]
- Ng, K.S.; Zhang, N.; Sadhukhan, J. Techno-economic analysis of polygeneration systems with carbon capture and storage and CO2 reuse. Chem. Eng. J. 2013, 219, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Pachauri, R.K.; Meyer, L. Climate Change 2014 Synthesis Report-Summary for Policymakers; Intergovernmetnal Panel on Climate Change (IPCC): Geneva, Switzerland, 2014. [Google Scholar]
- Rehfeldt, M.; Worrell, E.; Eichhammer, W.; Fleiter, T. A review of the emission reduction potential of fuel switch towards biomass and electricity in European basic materials industry until 2030. Renew. Sustain. Energy Rev. 2020, 120, 109672. [Google Scholar] [CrossRef]
- Wang, X.; Demirel, Y. Feasibility of power and methanol production by an entrained-flow coal gasification system. Energy Fuels 2018, 32, 7595–7610. [Google Scholar] [CrossRef]
- Yao, C.; Pan, W.; Yao, A. Methanol fumigation in compression-ignition engines: A critical review of recent academic and technological developments. Fuel 2017, 209, 713–732. [Google Scholar] [CrossRef]

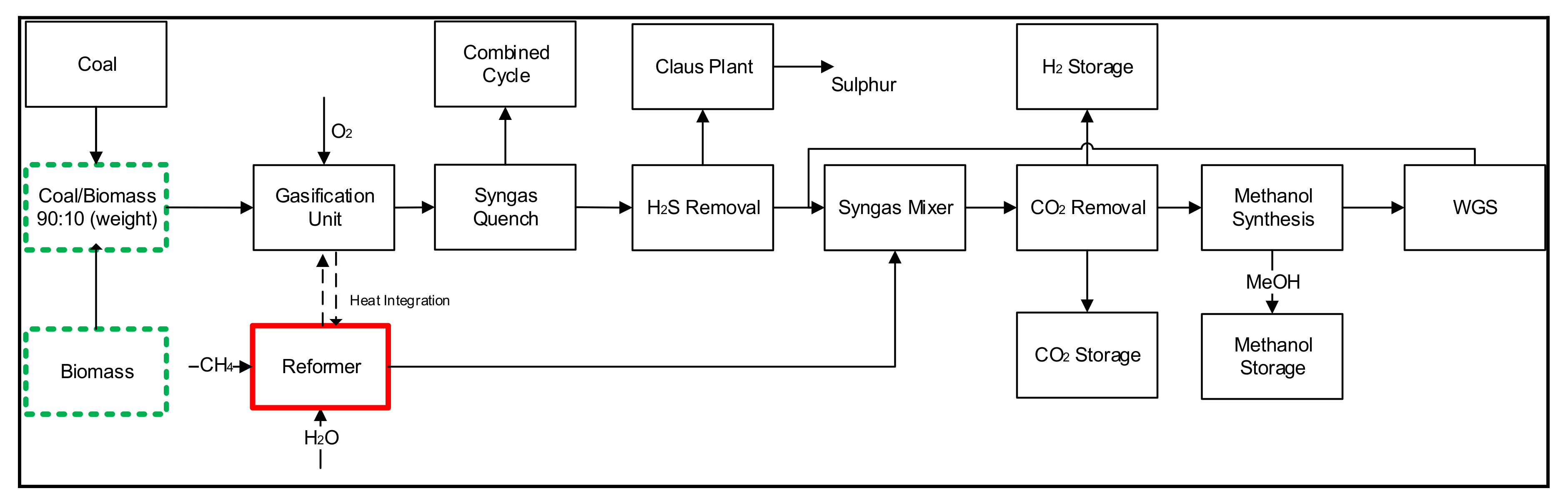
| Process Section | Simulation Unit/Conditions |
|---|---|
| Gasification | Model: RGIBBS Temperature: 1350–1370 °C Pressure: 56 bar Feed flow rate = 62.01 kg/s (coal) + 6.2 kg/s (biomass) |
| Reformer | Model: RGIBBS Temperature: 900 °C Pressure: 32 bar Feed flow rate: 5.5 kg/s (natural gas) H2O:CH4 = 3:1 Ni-based catalyst |
| Shift Conversion (WGS) | Model: REquil Reactor 2 Adiabatic reactors Co-Mo based catalyst for sour shift Steam/CO: ~2.2 CO conversion ~99% |
| Air Separation Unit (ASU) | Model: HeatX, Compr O2 purity: 95% (vol) Energy consumption: 0.25 kWh/kg |
| AGR Unit | Model: Flash, RadFrac H2S Removal = 100 ppbv CO2 Removal = 90% Rectisol Process) Temp/Pressure = −33 °C/5.5 MPa |
| Methanol Reactor | RGibbs (Reactor) Cu/ZnO/Al2O3 based catalyst Temp/Pressure = 200 °C/5.5 MPa |
| Heat Exchangers | Heater, HeatX, MHeatX |
| Units | Case 1 | Case 2 | |
|---|---|---|---|
| CH3OH Production | kg/s | 29.97 | 37.91 |
| H2 Production | kg/s | 4.22 | 3.87 |
| Total Fuel Produced (CH3OH+H2) | kg/s | 34.19 | 41.78 |
| Energy of the Produced Fuel (H2 and CH3OH) | kg/GJ | 8.893 | 11.501 |
| Electricity Produced | MWe | 709 | 620 |
| Heating Duty Required | MWt | 1666.07 | 1680.49 |
| Cooling Duty Reuired | MWt | 280.17 | 303.00 |
| Energy Integaration | MWt | 956.22 | 1142.96 |
| Auxillary | MW | 413.42 | 543.60 |
| Efficiency (CH3OH+H2+Electricity) | 47.1% | 50.6% |
| Units | Case 1 | Case2 | |
|---|---|---|---|
| Selling Fuel Price (H2+CH3OH) | €/MT | 443.74 | 411.30 |
| Selling Price of Methanol | €/MT | 412.26 | 371.06 |
| Selling Price of H2 | €/MT | 31.49 | 40.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, U.; Zahid, U.; Onaizi, S.A.; Abdul Jameel, A.G.; Ahmad, N.; Ahmad, N.; AlMohamadi, H. Co-Production of Hydrogen and Methanol Using Fuel Mix Systems: Technical and Economic Assessment. Appl. Sci. 2021, 11, 6577. https://doi.org/10.3390/app11146577
Ahmed U, Zahid U, Onaizi SA, Abdul Jameel AG, Ahmad N, Ahmad N, AlMohamadi H. Co-Production of Hydrogen and Methanol Using Fuel Mix Systems: Technical and Economic Assessment. Applied Sciences. 2021; 11(14):6577. https://doi.org/10.3390/app11146577
Chicago/Turabian StyleAhmed, Usama, Umer Zahid, Sagheer A. Onaizi, Abdul Gani Abdul Jameel, Nauman Ahmad, Nabeel Ahmad, and Hamad AlMohamadi. 2021. "Co-Production of Hydrogen and Methanol Using Fuel Mix Systems: Technical and Economic Assessment" Applied Sciences 11, no. 14: 6577. https://doi.org/10.3390/app11146577






