Extraction, Characterization, and Applications of Pectins from Plant By-Products
Abstract
:1. Introduction
2. Structure and Production of Pectins
2.1. Structure
2.1.1. Homogalacturonan
2.1.2. Rhamnogalacturonan I
2.1.3. Rhamnogalacturonan II
2.1.4. Xylogalacturonan and Apiogalacturonan
2.2. Structure Classification
2.2.1. Degree of Methylation
- High-methoxyl (HM) pectin (Figure 3A) with a DM > 50%, mostly present in nature as native pectin.
- Low-methoxyl (LM) pectin (Figure 3B) with a DM < 50%. This LM pectin is only obtained after demethylation by enzymatic (methylesterases) or alkaline treatments of HM pectin. There are also several unconventional sources of low-methoxy pectin.

2.2.2. Degrees of Acetylation and Amidation
3. Pectin Extraction Methods
3.1. Traditional Methods for the Pectin Extraction
| Pectin Sources | Extraction Conditions | Yield (%) | DM (%) | GalA (%) | Mw (kg/mol) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Solvents | Temperatures | pH | S/L | Time | ||||||
| Grapefruit peel | CHE | HCl | 80 °C | 1.5 | 1:50 | 90 min | 23.50 | 67.59 | 55.20 | 132.01 | [49] |
| Fresh watermelon rinds | CHE | HNO3 (0.1 M) | BT | - | 1:25 | 1 h | 19.3 | 63.0 | 74.2 | 34.510 | [6] |
| Passionfruit | CHE | HCI | 98.7 °C | 2 | 1:30 | 60 min | 14.8 | 9.57 | 88.2 | 802 | [27] |
| Beet pulp | CHE | HCl | 80 °C | 1 | 1:50 | 3 h | 20.0 | 58.92 | 66.18 | 116 | [50] |
| Citron peels | CHE | citric acid | 95 °C | 1.5 | 1:30 | 95 | 28.31 | 51.33 | - | - | [51] |
| Unripe banana | CHE | citricacid | 86 °C | 2.0 | 1:50 | 6 h | 11.63 | - | 11.21 | - | [52] |
| Eggplant peel waste | CHE | citric acid | 90 °C | 2.5 | 1:40 | 90 | 26.1 | 60.2 | 69.7 | - | [53] |
| Medlar fruit | CHE | HCI | 89 °C | 4.2 | 1:25 | 4.83 h | 62.9 | 71.4 | 198 | [54] | |
| Pomelo peels | CHE | HNO3 | 90 °C | 2 | 1:30 | 90 | 23.19 | 57.87 | 86.26 | 353 | [55] |
| Lyophilized watermelon rinds | CHE | HNO3 (0.1 M) | BT | - | 1:25 | 1 h | 14.2 | 61.5 | 68.7 | 40.390 | [6] |
| Cubiu fruit | CHE | HNO3 | BT | 1.5 | 1:25 | 2 h | 14.2 | 62% | 75.0% | 628 | [56] |
| Sweet prickly pear | CHE | EDTA | 70 °C | 4.0 | 1:3 | 2 h | - | 26.83 | 65.23 | 204.08 | [57] |
| Cocoa pod husks | CHE | ascorbic acid | 95 °C | 2.5 | 1:10 | 45 | 4.2 | 8.1 | 74.5 | - | [58] |
| Stems of E. arvense | CHE | ammonium oxalate | 70 °C | 1:40 | 8 hr | 5.9 | 16 | 85 | 360 | [59] | |
| Papaya peel | CHE | HCl | 80 °C | 2.0 | 1:50 | 60 min | 16 | 53.4 | 70.5 | - | [60] |
| Potato pulp | CHE | citric acid | 90 °C | 2.04 | 1:15 | 60 min | 14.34 | 37.45 | 24.3 | 320 | [39] |
| Carrot pomace | CHE | - | 90 °C | 1.3 | - | 79.8 min | 15.2 | 45.2 | 75.5 | - | [61] |
| Lime peel | CHE | HCl | 95 °C | - | 1:40 | 1 h | 15.91 | 78.49 | 89.8 | 794.7 | [40] |
| Ponkan peel | CHE | HNO3 | - | 1.6 | 1:36 | 100 min | 25.6 | 85.7 | 84.5 | 80.6 | [62] |
| Chicory | CHE | - | 80 °C | 1.5 | 1:20 | 1 h | 12.2 | 44.7 | 71.9 | 260 | [63] |
| Sugar beet pulp | CHE | - | 80 °C | 1.5 | 1:20 | 1 h | 7.1 | 46.4 | 66.2 | 651 | [63] |
| Green tea leaf | CHE | deionized water | 80 °C | - | - | 3 h | 5.3 | 26.5 | 32.4 | 276 | [64] |
| Green tea leaf | CHE | HCl | 60 °C | 2 | - | 3 h | 6.1 | 21.1 | 31.8 | 396 | [64] |
| Green tea leaf | CHE | NaoH | 60 °C | 8 | - | 3 h | 9.2 | 24.7 | 41.6 | 334 | [64] |
| Apple pomace | CHE | HNO3 | 90 °C | 1.5 | 1:25 | 70 | 25.3 | 41.7 | 84.5 | 142 | [65] |
| Pomegranate peel | CHE | HNO3 | 86 °C | 1.7 | 1:20 | 80 min. | 8.5 | 75 | 62.0 | 549 | [66] |
| Apple pomace | CHE | HNO3 | BT | - | 1:40 | 10 min | 15.04 | 72.29 | 57.28 | - | [67] |
| Grapefruit peel | CHE | HCl | 80 °C | 1.5 | 1:50 | 1.5 h | - | 69.03 | 68.36 | 385.5 | [68] |
| Jackfruit rinds | CHE | distilled water | 90 °C | - | 1:25 | 1 h | 14.59 | - | 72.62 | - | [69] |
| Watermelon peel | CHE | H2SO4 | 90 °C | 1.0 | 1:20 | 150 min | 17.6 | 41.2 | 78.3 | 119 | [70] |
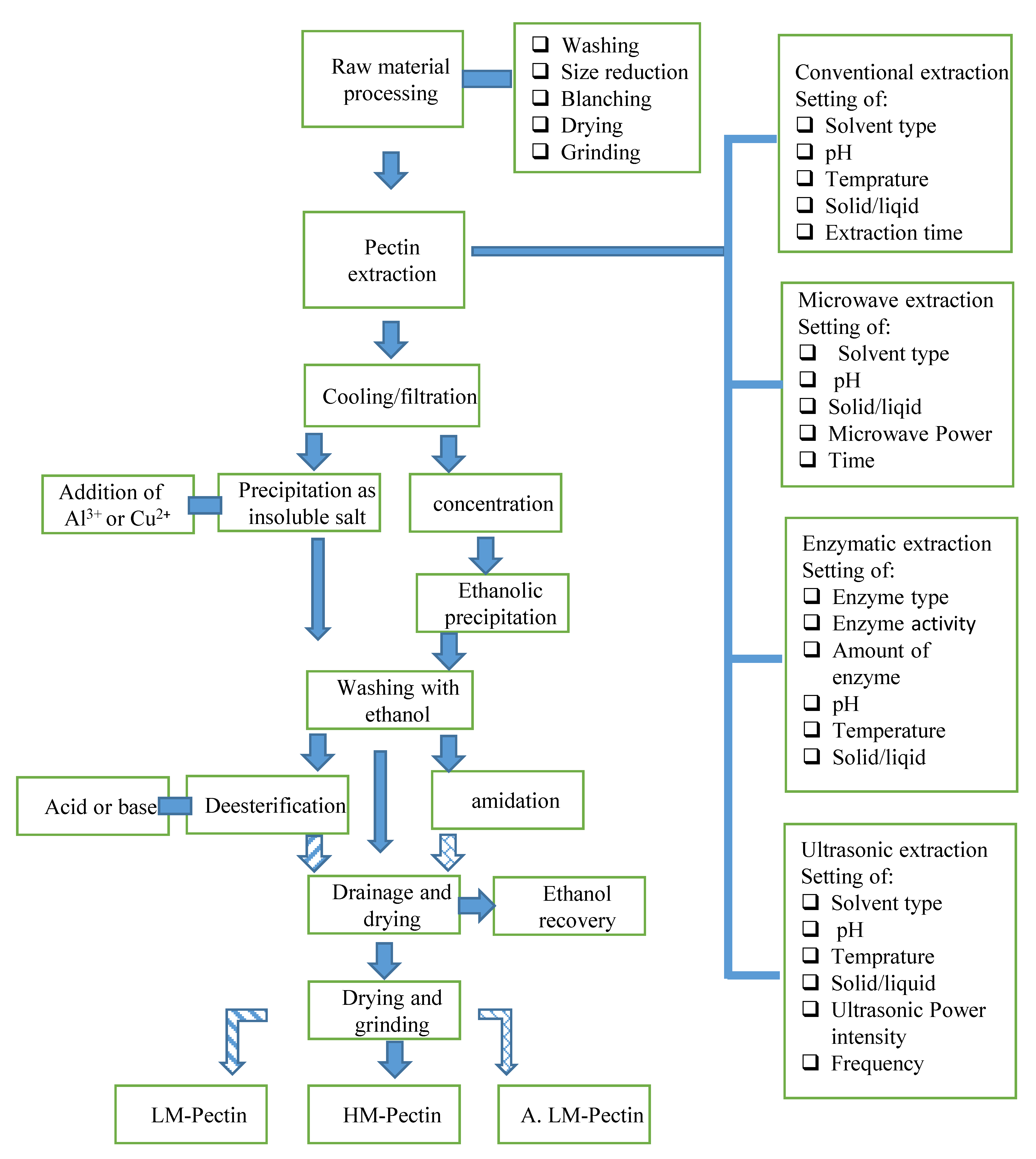
3.2. Green methods for Pectin Extraction
3.2.1. Microwave-Assisted Extraction
3.2.2. Enzymatic Extraction
3.2.3. Ultrasonic-Assisted Extraction
3.2.4. Dielectric Barrier Discharge Plasma Extraction (DBD)
4. Commercial Pectins
Pectin from Watermelon
| Pectin Sources | Extraction Conditions | Yields (%) | DM (%) | GalA (%) | Mw (kg/mol) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | Solvents | Temperatures (°C) | pH | S/L | Times | Power (w) or p. Intensity (w/cm) | Enzyme Treatments | ||||||
| Chicory root | EAE | sodium acetate buffer | 50 | 5.5 | 4 h | cellulase–protease | - | 52 | 55 | 250 | [94] | ||
| Lime peel | EAE | citrate buffer | 50 | 3.5 | 1:30 | 4 h | Laminex C2K | 22.5 | 82.2 | 115 | [94] | ||
| Lime peel | EAE | citrate buffer | 50 | 3.5 | 1:30 | 4 h | Validase TRL | 26.3 | 79.1 | 225.5 | [94] | ||
| Beetroot | EAE | citrate buffer | 30 | - | 1:100 | 20 h | cellulase | - | 80 | 55 | 1309 | [95] | |
| Butternut squash | EAE | citrate buffer | - | - | 1:100 | 20 h | cellulase | - | 2 | 54 | 136 | [95] | |
| Green tea leaf | EAE | HCl | 30 | 4.5 | - | 3 h | Viscozyme | 8.5 | 22.4 | 27.1 | [64] | ||
| Green tea leaf | EAE | HCl | 30 | 4.5 | - | 3 h | FoodPro® CBL | 5.1 | 40.9 | 26.6 | [64] | ||
| Prickly pear | UAE | - | 70 | 1.5 | 1:30 | 70 min | 330 W | 18.14 | 41.4 | 68.87 | [96] | ||
| Suaeda fruticosaleaves | UAE | citric acid | 90 | 2.9 | 1:30 | 37 min | 140 W | 34.0 | 33 | 47.5 | 229 | [97] | |
| Pomegranate peels | UAE | citrate buffer | - | 5 | 1:15 | 20 min | 150 W | 24.8 | 68.5 | 72 | 146.5 | [98] | |
| Grapefruit | UAE | HCl | 70 | 1.5 | 1:50 | 25 min | - | 17.92 | 75.1 | 68.21 | 68.3 | [75] | |
| Grapefruit peel | UAE | - | 67 | - | 1:50 | 28 min | 800 W | - | 58.7 | 56.39 | 279.47 | [68] | |
| Grapefruit peel | UAE | HCl | 66.7 | 1.5 | 1:50 | 27.9 min | 12.56 W/cm2 | 27.46 | 65.5 | 50.03 | 109.5 | [49] | |
| Musa balbisiana | UAE | citric acid | 3.2 | 1:15 | 27 min | 323 w | 8.99 | [99] | |||||
| Eggplant peel | UAE | citric acid | - | 1.5 | 1:20 | 30 min | 50 W | 33.64 | 61.2 | 66.08 | [100] | ||
| Passionfruit peel | UAE | HNO3 | 85 | 2.0 | 1:30 | 10 min | 644 W/cm2 | 12.67 | 60.3 | 66.65 | [101] | ||
| Papaya powder | UAE | HCl | 60 | 2.0 | 1:4 | 56 min | 320 | 2.61 | - | - | [102] | ||
| Apple peel waste | UAE | HCl | 63 | 2.36 | 1:23 | 18 min | 90 W | 8.93 | 70 | 70.24 | 198.65 | [103] | |
| Apple pomace | MAE | HCl | - | 1.01 | 1:14 | 20.8 min | 499.4 W | 15.75 | - | - | - | [104] | |
| Dragon fruit | MAE | citric acid | 75 | 2.9 | 1:56 | 12 min | 183 W | 17.01 | 45.0 | 60.10 | [105] | ||
| Jackfruit rinds | MAE | distilled water | - | - | 1:25 | 10 min | 600 W | 17.63 | - | 70.29 | - | [69] | |
| Sweet lemon peel | MAE | citric acid | - | 1.5 | - | 3 min | 700 W | 25.31 | 5.80 | 87.2 | 615.8 | [106] | |
| Grapefruit | MAE | HCl | - | - | 1:50 | 6 min | 900 W | 27.81 | 80 | 75 | 50 | [75] | |
| Pumpkin | MAE | HCl | 80 | 1.0 | - | 10 min | 1200 W | 11.3 | 56.3 | 58.9 | - | [107] | |
| Watermelon rinds | MAE | - | - | 1.52 | 1:20 | 128 s | 477 W | 25.79 | - | - | - | [76] | |
| Watermelon peel | MAE | sulfuric acid | - | 1.5 | 1:20 | 7 min | 500 W | 19.6 | 48.7 | 74.9 | 149.9 | [70] | |
| Watermelon rinds | MAE | acetic acid | - | 2 | 1:100 | 12 min | 279 W | 5.76 | 56.8 | - | - | [8] | |
| Watermelon rind | MAE | sulfuric acid | - | - | 1:10 | 15 min | 39,9 W | 18 | - | - | - | [108] | |
| Banana peels | MAE | HCl | - | 3.00 | 1:50 | 100 s | 900 W | 2.18 | - | - | - | [109] | |
| Sour orange peel | MAE | citric acid | - | 1.50 | 1:15 | 3 min | 700 W | 28.8 | 1.5 | 71.0 | - | [110] | |
| Pomelo peel | MAE | NaOH | - | - | 1:30 | 2 min | 1100 W | 24.2 | - | 85.7 | 142 | [26] | |
| Pomelo peel | MAE | HCl | - | - | 1:30 | 2 min | 1100 W | 20.5 | 71.2 | 85.0 | 327 | [26] | |
| Pistachio green hull | MAE | - | 1.5 | 1:15 | 165 s | 700 W | - | 18.13 | 12.1 | 66.0 | 1659 | [111] | |
| Lime peel | MAE | HCl | 1:40 | 700 W | - | 23.32 | 70.8 | 91.00 | 635.63 | [40] | |||
5. Techno-Functional Properties and Application of Pectin
5.1. Pectin Gel
5.2. Water/Oil-Holding Capacity
| Pectin Sources | Measurement Conditions | WHC | OHC | References |
|---|---|---|---|---|
| Sunflower stalk pith | 0.1 g powder/6.0 g deionized water mixing for 1 min incubating at room temperature for 30 min centrifuging at 9000× g for 30 min | 40.2 g water/g powder | 40.4 g oil/g powder | [130] |
| Tomato pomace | 1 g powder/20 mL deionized water mixing for 1 min incubating at room temperature for 60 min centrifuging at 4000× g for 30 min | 3.57 g water/g powder | 2.65 g oil/g powder | [131] |
| Eggplant | 0.5 g powder/50 mL deionized water incubating at room temperature for 60 min centrifuging at 5000× g for 20 min | 6.02 g water/g powder | 2.6 g oil/g powder | [100] |
| Walnut | 1 g powder/10 mL deionized water mixing for 1 min centrifuging at 3000× g for 20 min | 5.84 g water/g powder | 2.22 g oil/g powder | [132] |
| Commercial apple pectin | 1 g powder/60 mL deionized water incubating at room temperature for 24 h centrifuging at 14,000× g for 1 h | 2.00 g water/g powder | 2.22 g oil/g powder | [133] |
| Commercial citrus pectin | 1 g powder/60 mL deionized water incubating at room temperature for 24 h centrifuging at 14,000× g for 1 h | 10.00 g water/g powder | 2.59 g oil/g powder | [133] |
| By-product from olive oil production | 1 g powder/60 mL deionized water incubating at room temperature for 24 h centrifuging at 14,000× g for 1 h | 1.87 g water/g powder | 6.17 g oil/g powder | [133] |
| Pistachio green hull | 1 g powder/10 mL deionized water mixing for 1 min centrifuging at 3000× g for 30 min | 4.11 g water/g powder | 2.02 g oil/g powder | [111] |
| Opuntia ficus indica | 0.5 g powder/50 mL deionized water incubating at room temperature for 60 min centrifuging at 5000× g for 20 min | 4.84 g water/g powder | 1.01 g oil/g powder | [96] |
| Watermelon rind | 0.5 g powder/50 mL deionized water incubating at room temperature for 60 min centrifuging at 15,025× g for 20 min | 2 g water/g powder | 4 g oil/g powder | [129] |
5.3. Emulsion
| Sources | Process Conditions | Emulsion-Based Characteristics | References |
|---|---|---|---|
| Beet pectin | pH 7.0 oil phase: 10 wt% homogenization pressures (9–19) kpsi | the reduction in surface tension reported is as follows: gum arabic ≥ beet pectin emulsions could not be prepared using beet pectin at concentrations greater than 2 wt% the minimum D[4,3] at 1% beet pectin is lower than that obtained with 3% gum arabic | [143] |
| Pomegranate peel | oil phase: 50 wt% homogenization at 20,000 rpm for 90 s. | pomegranate pectin cannot effectively reduce surface tension after incubation of the fresh emulsion at 80 °C for 1 h, the emulsion stability was 96.7%, at pectin concentration of 2.0% | [66] |
| Cauliflower | pH 3.8 oil phase: 10 wt% homogenization: 800,000 rpm at room temperature 1 min and followed by ultrasonic wave probe (3 min) | cauliflower pectin was able to reduce surface tension from 28 mN/m (citrate buffer, pH 3.8) to 12 mN/m (0.5 wt% pectin solution) the presence of acetyl groups and proteins in the sample was most likely responsible for its emulsifying properties | [144] |
| Chicory root pulp | pH 3.5 oil phase: 15 wt% homogenization: 20,000 rpm for 2 min and followed by ultrasonic wave at 300 W and 2 min | the minimum D[3,2] values for chicory root pulp pectin (CRP) (0.58 μm) and sugar beet pectin (0.54 μm) were obtained at critical concentrations of 1.5% and 2%, respectively CRP was shown to behave like SBP in the fabrication of emulsions of small droplet sizes CRP reduced interfacial tensions (19 from 42 mN/cm) CRP may reach its maximum emulsification capacity at a concentration of 1.5% | [63] |
| Beetroot | oil phase: 20 wt% homogenization at 24,000 rpm | beetroot pectin (BRP) gave a lower surface tension than gum arabic the minimum D[3,2] of emulsion was obtained with a BRP concentration of 4% (w/w) BRP had smaller droplets than emulsion in the first 10 days, and no difference in final droplet size was reported between BRP and gum arabic | [145] |
| Watermelon rind | oil phase: 10 wt% homogenization at 24,000 rpm for 4 min | D[3,2] and D[4,3] obtained for emulsions prepared with pectin of watermelon rind were similar to those obtained with gum arabic pectin of watermelon rind gave rise to a lower dynamic surface tension than gum arabic | [6] |
| Potato pulp | oil phase: 50 wt% - 0.5% w/w of pectin solution homogenization at 10,000 rpm for 3 min | the emulsifying activity of potato pulp (PP) pectins extracted by HCl (47.71%) is higher than those of commercial citrus (44.87%) and apple (45.34%) pectins | [39] |
| Pistachio green hull | oil phase: 50 wt% - 0.5% w/w of pectin solution homogenization at 10,000 g for 4 min | the emulsifying activity of pistachio green hull pectin (58.3%) was higher than pectins from Citrus medica peel (46.5%) and sour orange peel (40.7%) After 30 days, the emulsion stability was 87.9% and 88.6% at 4 °C and 24 °C, respectively surface tension of pistachio green hull pectin solutions was 46.23 ± 0.32 mN/m at concentration of 0.5% w/v; these values were lower than the data obtained from sugar beet pulp | [111] |
6. Pectin Applications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Brnčić, S.R. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: A review. Food Rev. Int. 2020, 1–31. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.; Rather, S.A.; Wani, S.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Riadh, I.; Jebari, H.; Dalessandro, G. Bioactive compounds and antioxidant activities of different watermelon (Citrullus lanatus (Thunb.) Mansfeld) cultivars as affected by fruit sampling area. J. Food Compos. Anal. 2011, 24, 307–314. [Google Scholar] [CrossRef]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef]
- Petkowicz, C.; Vriesmann, L.; Williams, P. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Ahmed, A.R. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Sari, A.; Ishartani, D.; Dewanty, P. Effects of microwave power and irradiation time on pectin extraction from watermelon rinds (Citrullus lanatus) with acetic acid using microwave assisted extraction method. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Semarang, Indonesia, 26–27 September 2017; p. 012085. [Google Scholar]
- Jarvis, M.C. Structure and properties of pectin gels in plant cell walls. Plant Cell Environ. 1984, 7, 153–164. [Google Scholar]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Williams, P.A. Renewable Resources for Functional Polymers and Biomaterials: Polysaccharides, Proteins and Polyesters; The Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Olano-Martin, E.; Gibson, G.R.; Rastall, R. Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J. Appl. Microbiol. 2002, 93, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- do Nascimento Oliveira, A.; de Almeida Paula, D.; de Oliveira, E.B.; Saraiva, S.H.; Stringheta, P.C.; Ramos, A.M. Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int. J. Biol. Macromol. 2018, 113, 395–402. [Google Scholar] [CrossRef]
- Capel, F.; Nicolai, T.; Durand, D.; Boulenguer, P.; Langendorff, V. Influence of chain length and polymer concentration on the gelation of (amidated) low-methoxyl pectin induced by calcium. Biomacromolecules 2005, 6, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
- Khedmat, L.; Izadi, A.; Mofid, V.; Mojtahedi, S.Y. Recent advances in extracting pectin by single and combined ultrasound techniques: A review of techno-functional and bioactive health-promoting aspects. Carbohydr. Polym. 2020, 229, 115474. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [Green Version]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Jackson, C.L.; Dreaden, T.M.; Theobald, L.K.; Tran, N.M.; Beal, T.L.; Eid, M.; Gao, M.Y.; Shirley, R.B.; Stoffel, M.T.; Kumar, M.V. Pectin induces apoptosis in human prostate cancer cells: Correlation of apoptotic function with pectin structure. Glycobiol. 2007, 17, 805–819. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Cabrera, J.C.; Bonnin, E.; Quéméner, B.; Hellìn, P.; Thibault, J.-F. Mapping sugar beet pectin acetylation pattern. Phytochemistry 2005, 66, 1832–1843. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Zou, M.; Lv, R.; Wang, D.; Hou, F.; Feng, H.; Ma, X.; Zhong, J.; Ding, T. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Bonnin, E.; Saulnier, L.; Brunel, M.; Marot, C.; Lesage-Meessen, L.; Asther, M.; Thibault, J.-F. Release of ferulic acid from agroindustrial by-products by the cell wall-degrading enzymes produced by Aspergillus niger I-1472. Enzym. Microb. Technol. 2002, 31, 1000–1005. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Castillo-Israel, K.; Baguio, S.; Diasanta, M.; Lizardo, R.; Dizon, E.; Mejico, M. Extraction and characterization of pectin from Saba banana [Musa‘saba’(Musa acuminata x Musa balbisiana)] peel wastes: A preliminary study. Int. Food Res. J. 2015, 22. [Google Scholar]
- Rosenbohm, C.; Lundt, I.; Christensen, T.I.; Young, N.G. Chemically methylated and reduced pectins: Preparation, characterisation by 1H NMR spectroscopy, enzymatic degradation, and gelling properties. Carbohydr. Res. 2003, 338, 637–649. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Mischnick, P. Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocoll. 2019, 87, 237–244. [Google Scholar] [CrossRef]
- Kulkarni, S.; Vijayanand, P. Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. flavicarpa L.). LWT-Food Sci. Technol. 2010, 43, 1026–1031. [Google Scholar] [CrossRef]
- Stephen, A.M. Food Polysaccharides and Their Applications; CRC Press: Florida, FL, USA, 1995; Volume 67. [Google Scholar]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Garti, N.; Leser, M.E. Emulsification properties of hydrocolloids. Polym. Adv. Technol. 2001, 12, 123–135. [Google Scholar] [CrossRef]
- Matia-Merino, L.; Lau, K.; Dickinson, E. Effects of low-methoxyl amidated pectin and ionic calcium on rheology and microstructure of acid-induced sodium caseinate gels. Food Hydrocoll. 2004, 18, 271–281. [Google Scholar] [CrossRef]
- Chen, J.; Niu, X.; Dai, T.; Hua, H.; Feng, S.; Liu, C.; McClements, D.J.; Liang, R. Amino acid-amidated pectin: Preparation and characterization. Food Chem. 2020, 309, 125768. [Google Scholar] [CrossRef]
- Turakhozhaev, M.; Khodzhaev, M. Plant pectin substances. Methods of isolating pectin substances. Chem. Nat. Compd. 1993, 29, 558–565. [Google Scholar] [CrossRef]
- Freitas, C.; Sousa, R.; Dias, M.; Coimbra, J. Extraction of Pectin from Passion Fruit Peel. Food Eng. Rev. 2020, 12, 460–472. [Google Scholar] [CrossRef]
- Li, D.-Q.; Jia, X.; Wei, Z.; Liu, Z.-Y. Box–Behnken experimental design for investigation of microwave-assisted extracted sugar beet pulp pectin. Carbohydr. Polym. 2012, 88, 342–346. [Google Scholar] [CrossRef]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering conventional and innovative methods for pectin extraction from fruit and vegetable waste: Targeting rhamnogalacturonan I. Trends Food Sci. Technol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Dranca, F.; Oroian, M. Extraction, purification and characterization of pectin from alternative sources with potential technological applications. Food Res. Int. 2018, 113, 327–350. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Microwave heating extraction of pectin from lime peel: Characterization and properties compared with the conventional heating method. Food Chem. 2019, 278, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Fishman, M.L.; Chau, H.K.; Hoagland, P.D.; Hotchkiss, A.T. Microwave-assisted extraction of lime pectin. Food Hydrocoll. 2006, 20, 1170–1177. [Google Scholar] [CrossRef]
- Canteri-Schemin, M.H.; Fertonani, H.C.R.; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Braz. Arch. Biol. Technol. 2005, 48, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, A.M. Effect of different acids, heating time and particle size on pectin extraction from watermelon rinds. J. Kerbala Univ. 2008, 6, 234–243. [Google Scholar]
- Abidin, S.A.S.Z.; Badarudin, N.S.A.; Ab Mutalib, S.R. Ultrasound-Assisted Extraction Increases Pectin Yield from Watermelon (Citrullus Lanatus) Rind. In Proceedings of the 2020 11th IEEE Control and System Graduate Research Colloquium (ICSGRC), Shah Alam, Malaysia, 8 August 2020; pp. 291–294. [Google Scholar]
- Hu, W.; Zhao, Y.; Yang, Y.; Zhang, H.; Ding, C.; Hu, C.; Zhou, L.; Zhang, Z.; Yuan, S.; Chen, Y. Microwave-assisted extraction, physicochemical characterization and bioactivity of polysaccharides from Camptotheca acuminata fruits. Int. J. Biol. Macromol. 2019, 133, 127–136. [Google Scholar] [CrossRef]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Rodi Kandri, Y.; Ouazzani Chahdi, F.; Touzani, H.; Errachidi, F. Yield, esterification degree and molecular weight evaluation of pectins isolated from orange and grapefruit peels under different conditions. PLoS One 2016, 11, e0161751. [Google Scholar] [CrossRef] [Green Version]
- Hamidon, N.H.; Zaidel, D.N.A. Effect of extraction conditions on pectin yield extracted from sweet potato peels residues using hydrochloric acid. Chem. Eng. Trans. 2017, 56, 979–984. [Google Scholar]
- Andersen, N.M.; Cognet, T.; Santacoloma, P.; Larsen, J.; Armagan, I.; Larsen, F.; Gernaey, K.; Abildskov, J.; Huusom, J.K. Dynamic modelling of pectin extraction describing yield and functional characteristics. J. Food Eng. 2017, 192, 61–71. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Xu, Y.; Cao, Y.; Jiang, Z.; Ding, T.; Ye, X.; Liu, D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food Chem. 2015, 178, 106–114. [Google Scholar] [CrossRef]
- Mesbahi, G.; Jamalian, J.; Farahnaky, A. A comparative study on functional properties of beet and citrus pectins in food systems. Food Hydrocoll. 2005, 19, 731–738. [Google Scholar] [CrossRef]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.; Hosseini, S.S. Pectin extraction from citron peel: Optimization by Box–Behnken response surface design. Food Sci. Biotechnol. 2018, 27, 997–1005. [Google Scholar] [CrossRef]
- Marenda, F.R.B.; Colodel, C.; Canteri, M.H.G.; de Olivera Müller, C.M.; Amante, E.R.; de Oliveira Petkowicz, C.L.; de Mello Castanho Amboni, R.D. Investigation of cell wall polysaccharides from flour made with waste peel from unripe banana (Musa sapientum) biomass. J. Sci. Food Agric. 2019, 99, 4363–4372. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S.; Najari, Z. An integrated valorization of industrial waste of eggplant: Simultaneous recovery of pectin, phenolics and sequential production of pullulan. Waste Manag. 2019, 100, 101–111. [Google Scholar] [CrossRef]
- Al-Amoudi, R.H.; Taylan, O.; Kutlu, G.; Can, A.M.; Sagdic, O.; Dertli, E.; Yilmaz, M.T. Characterization of chemical, molecular, thermal and rheological properties of medlar pectin extracted at optimum conditions as determined by Box-Behnken and ANFIS models. Food Chem. 2019, 271, 650–662. [Google Scholar] [CrossRef]
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters and its properties. Food Hydrocoll. 2014, 35, 383–391. [Google Scholar] [CrossRef]
- Colodel, C.; de Oliveira Petkowicz, C.L. Acid extraction and physicochemical characterization of pectin from cubiu (Solanum sessiliflorum D.) fruit peel. Food Hydrocoll. 2019, 86, 193–200. [Google Scholar] [CrossRef]
- Morales-Martínez, Y.; del Rocío López-Cuellar, M.; Chavarría-Hernández, N.; Rodríguez-Hernández, A.I. Rheological behaviour of acetylated pectins from cactus pear fruits (Opuntia albicarpa and O. matudae). Food Hydrocoll. 2018, 85, 110–119. [Google Scholar] [CrossRef]
- Priyangini, F.; Walde, S.G.; Chidambaram, R. Extraction optimization of pectin from cocoa pod husks (Theobroma cacao L.) with ascorbic acid using response surface methodology. Carbohydr. Polym. 2018, 202, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Patova, O.; Smirnov, V.; Golovchenko, V.; Vityazev, F.; Shashkov, A.; Popov, S. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydr. Polym. 2019, 209, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Altaf, U.; Immanuel, G.; Iftikhar, F. Extraction and characterization of pectin derived from papaya (Carica papaya Linn.) peel. Int. J. Sci. Eng. Technol. 2015, 3, 970–974. [Google Scholar]
- Jafari, F.; Khodaiyan, F.; Kiani, H.; Hosseini, S.S. Pectin from carrot pomace: Optimization of extraction and physicochemical properties. Carbohydr. Polym. 2017, 157, 1315–1322. [Google Scholar] [CrossRef]
- Colodel, C.; Vriesmann, L.C.; de Oliveira Petkowicz, C.L. Rheological characterization of a pectin extracted from ponkan (Citrus reticulata blanco cv. ponkan) peel. Food Hydrocoll. 2019, 94, 326–332. [Google Scholar] [CrossRef]
- Pi, F.; Liu, Z.; Guo, X.; Guo, X.; Meng, H. Chicory root pulp pectin as an emulsifier as compared to sugar beet pectin. Part 1: Influence of structure, concentration, counterion concentration. Food Hydrocoll. 2019, 89, 792–801. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Zhang, F.; Yang, X.; Ni, L.; Zhang, W.; Liu, Z.; Zhang, Y. Improving viscosity and gelling properties of leaf pectin by comparing five pectin extraction methods using green tea leaf as a model material. Food Hydrocoll. 2020, 98, 105246. [Google Scholar] [CrossRef]
- Yapo, B.; Besson, V.; Grah, A.; Kouassi, K.; Dago, G. Macromolecular and viscoelastic properties of low methoxy pectin from cashew apple pomace. Univ. J. Food Nutr. Sci 2014, 2, 1–6. [Google Scholar]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Sato, M.d.F.; Rigoni, D.C.; Canteri, M.H.G.; Petkowicz, C.L.d.O.; Nogueira, A.; Wosiacki, G. Chemical and instrumental characterization of pectin from dried pomace of eleven apple cultivars. Acta Scientiarum. Agron. 2011, 33, 383–389. [Google Scholar]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of pectin from grapefruit peel: A comparison of ultrasound-assisted and conventional heating extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Koh, P.; Leong, C.; Noranizan, M. Microwave-assisted extraction of pectin from jackfruit rinds using different power levels. Int. Food Res. J. 2014, 21, 2091. [Google Scholar]
- Jiang, L.N.; Shang, J.J.; He, L.B.; Dan, J.M. Comparisons of microwave-assisted and conventional heating extraction of pectin from seed watermelon peel. Adv. Mater. Res. 2012, 550–553, 1801–1806. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Hu, D.; Xiao, K.; Wu, J.-Y. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Hoagland, P.; Ayyad, K. Characterization of pectin, flash-extracted from orange albedo by microwave heating, under pressure. Carbohydr. Res. 1999, 323, 126–138. [Google Scholar] [CrossRef]
- Bagherian, H.; Ashtiani, F.Z.; Fouladitajar, A.; Mohtashamy, M. Comparisons between conventional, microwave-and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem. Eng. Process. Process. Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydr. Polym. 2014, 101, 786–791. [Google Scholar] [CrossRef]
- Hua, X.; Wang, K.; Yang, R.; Kang, J.; Zhang, J. Rheological properties of natural low-methoxyl pectin extracted from sunflower head. Food Hydrocoll. 2015, 44, 122–128. [Google Scholar] [CrossRef]
- Poojary, M.M.; Orlien, V.; Passamonti, P.; Olsen, K. Enzyme-assisted extraction enhancing the umami taste amino acids recovery from several cultivated mushrooms. Food Chem. 2017, 234, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ye, X.; Sun, Y.; Wu, D.; Wu, N.; Hu, Y.; Chen, S. Ultrasound effects on the degradation kinetics, structure, and antioxidant activity of sea cucumber fucoidan. J. Agric. Food Chem. 2014, 62, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Namieśnik, J.; Konieczka, P. Ultrasound-Assisted Extraction. In The Application of Green Solvents in Separation Processes; Pena-Pereira, F., Tobiszewski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 301–324. [Google Scholar]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the egg-box model in calcium− alginate gels with X-ray diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Ilghami, A.; Ghanbarzadeh, S.; Hamishehkar, H. Optimization of the ultrasonic-assisted extraction of phenolic compounds, ferric reducing activity and antioxidant activity of the beta vulgaris using response surface methodology. Pharm. Sci. 2015, 21, 46–50. [Google Scholar] [CrossRef]
- Llano, K.R.A.; Marsellés-Fontanet, A.R.; Martín-Belloso, O.; Soliva-Fortuny, R. Impact of pulsed light treatments on antioxidant characteristics and quality attributes of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2016, 33, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Gharibzahedi, S.M.T.; Smith, B.; Guo, Y. Pectin extraction from common fig skin by different methods: The physicochemical, rheological, functional, and structural evaluations. Int. J. Biol. Macromol. 2019, 136, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Tiwari, B.; Raghavarao, K.; Cullen, P. Nonthermal plasma inactivation of food-borne pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.; Martynenko, A.; Chemat, F.; Paniwnyk, L.; Barba, F.J.; Jambrak, A.R. Thermodynamics, transport phenomena, and electrochemistry of external field-assisted nonthermal food technologies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1832–1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, M.T.; Villamiel, M.; Moreno, R.; Moreno, F.J. Structural and rheological properties of pectins extracted from industrial sugar beet by-products. Molecules 2019, 24, 392. [Google Scholar] [CrossRef] [Green Version]
- Campbel, M. Watermelond rind pectin extraction. Submitted to the Faculty of the Graduate College of the Oklahoma State University, 2006. [Google Scholar]
- Hadkar, U.; Dhruv, N.; Malode, Y.; Chavan, B. Microwave assisted extraction of phytoconstituents. Asian J. Phytomedicine Clin. Res. 2013, 2, 73–86. [Google Scholar]
- Korish, M. Potential utilization of Citrullus lanatus var. Colocynthoides waste as a novel source of pectin. J. Food Sci. Technol. 2015, 52, 2401–2407. [Google Scholar] [CrossRef] [Green Version]
- Dominiak, M.; Søndergaard, K.M.; Wichmann, J.; Vidal-Melgosa, S.; Willats, W.G.; Meyer, A.S.; Mikkelsen, J.D. Application of enzymes for efficient extraction, modification, and development of functional properties of lime pectin. Food Hydrocoll. 2014, 40, 273–282. [Google Scholar] [CrossRef]
- Fissore, E.N.; Rojas, A.M.; Gerschenson, L.N.; Williams, P.A. Butternut and beetroot pectins: Characterization and functional properties. Food Hydrocoll. 2013, 31, 172–182. [Google Scholar] [CrossRef]
- Bayar, N.; Bouallegue, T.; Achour, M.; Kriaa, M.; Bougatef, A.; Kammoun, R. Ultrasonic extraction of pectin from Opuntia ficus indica cladodes after mucilage removal: Optimization of experimental conditions and evaluation of chemical and functional properties. Food Chem. 2017, 235, 275–282. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. Complete utilization of waste pomegranate peels to produce a hydrocolloid, punicalagin rich phenolics, and a hard carbon electrode. ACS Sustain. Chem. Eng. 2018, 6, 16363–16374. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Al-Dhabi, N.A.; Ponmurugan, K.; Moorthy, I.G.; Sivarajasekar, N. Ultrasound assisted citric acid mediated pectin extraction from industrial waste of Musa balbisiana. Ultrason. Sonochemistry 2017, 35, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Eggplant peel as a high potential source of high methylated pectin: Ultrasonic extraction optimization and characterization. LWT 2019, 105, 182–189. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT-Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Li, X.-S.; Liu, Z.-Y.; Lu, B.-S. Ultrasonic-assisted extraction of pectin from chaenomeles. J. South Univ. 2014, 21, 4115–4120. [Google Scholar] [CrossRef]
- Shivamathi, C.; Moorthy, I.G.; Kumar, R.V.; Soosai, M.R.; Maran, J.P.; Kumar, R.S.; Varalakshmi, P. Optimization of ultrasound assisted extraction of pectin from custard apple peel: Potential and new source. Carbohydr. Polym. 2019, 225, 115240. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Wu, J.; Wang, Z.; Liao, X.; Hu, X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007, 78, 693–700. [Google Scholar] [CrossRef]
- Dao, T.A.T.; Webb, H.K.; Malherbe, F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwave-assisted heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of microwave-assisted extraction and structural characterization of pectin from sweet lemon peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, B.H.; Lee, H.; Lee, S.; Bae, I.Y.; Lee, H.G.; Fishman, M.L.; Chau, H.K.; Savary, B.J.; Hotchkiss, A.T., Jr. Structural characteristics of pumpkin pectin extracted by microwave heating. J. Food Sci. 2012, 77, C1169–C1173. [Google Scholar] [CrossRef]
- Hartati, I.; Riwayati, I.; Subekti, E. Microwave Assisted Extraction of Watermelon Rind Pectin with Different Kind of Acid Solution. ICETIA2014 2014, 27–30. [Google Scholar]
- Swamy, G.J.; Muthukumarappan, K. Optimization of continuous and intermittent microwave extraction of pectin from banana peels. Food Chem. 2017, 220, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Khodaiyan, F.; Labbafi, M.; Hosseini, S.S.; Hojjati, M. Pistachio green hull pectin: Optimization of microwave-assisted extraction and evaluation of its physicochemical, structural and functional properties. Food Chem. 2019, 271, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Schols, H.A.; Bakx, E.J.; Schipper, D.; Voragen, A.G. A xylogalacturonan subunit present in the modified hairy regions of apple pectin. Carbohydr. Res. 1995, 279, 265–279. [Google Scholar] [CrossRef]
- Ross-Murphy, S.B. Rheological characterisation of gels 1. J. Texture Stud. 1995, 26, 391–400. [Google Scholar] [CrossRef]
- Sato, Y.; Miyawaki, O. Analysis of intermolecular interaction among pectin molecules in aqueous sugar solutions. Food Sci. Technol. Res. 2008, 14, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Oakenfull, D.; Scott, A. Hydrophobic interaction in the gelation of high methoxyl pectins. J. Food Sci. 1984, 49, 1093–1098. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Introduction to Food Hydrocolloids. In Handbook of Hydrocolloids, 3rd ed.; Williams, P.A., Phillips, G.O., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–26. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Morris, G.; Foster, T.; Harding, S. The effect of the degree of esterification on the hydrodynamic properties of citrus pectin. Food Hydrocoll. 2000, 14, 227–235. [Google Scholar] [CrossRef]
- Rouse, A.; Atkins, C.; Moore, E. The occurrence and evaluation of pectin in component parts of Valencia oranges during maturation. Proc. Fla. State Hortic. Soc. 1962, 75, 307–311. [Google Scholar]
- Günter, E.A.; Popeyko, O.V.; Markov, P.A.; Martinson, E.A.; Litvinets, S.G.; Durnev, E.A.; Popov, S.V.; Ovodov, Y.S. Swelling and morphology of calcium pectinate gel beads obtained from Silene vulgaris callus modified pectins. Carbohydr. Polym. 2014, 103, 550–557. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Brax, M.; Schaumann, G.E.; Diehl, D. Gel formation mechanism and gel properties controlled by Ca2+ in chia seed mucilage and model substances. J. Plant Nutr. Soil Sci. 2019, 182, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef]
- Celus, M.; Kyomugasho, C.; Salvia-Trujillo, L.; Van Audenhove, J.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Interactions between citrus pectin and Zn2+ or Ca2+ and associated in vitro Zn2+ bioaccessibility as affected by degree of methylesterification and blockiness. Food Hydrocoll. 2018, 79, 319–330. [Google Scholar] [CrossRef]
- Ngouémazong, D.E.; Jolie, R.P.; Cardinaels, R.; Fraeye, I.; Van Loey, A.; Moldenaers, P.; Hendrickx, M. Stiffness of Ca2+-pectin gels: Combined effects of degree and pattern of methylesterification for various Ca2+ concentrations. Carbohydr. Res. 2012, 348, 69–76. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Crépeau, M.-J.; Buchholt, H.-C.; Thibault, J.-F. Polyelectrolyte behaviour and calcium binding properties of sugar beet pectins differing in their degrees of methylation and acetylation. Biochem. Eng. J. 2003, 16, 191–201. [Google Scholar] [CrossRef]
- Han, W.; Meng, Y.; Hu, C.; Dong, G.; Qu, Y.; Deng, H.; Guo, Y. Mathematical model of Ca2+ concentration, pH, pectin concentration and soluble solids (sucrose) on the gelation of low methoxyl pectin. Food Hydrocoll. 2017, 66, 37–48. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Romdhane, M.B.; Haddar, A.; Ghazala, I.; Jeddou, K.B.; Helbert, C.B.; Ellouz-Chaabouni, S. Optimization of polysaccharides extraction from watermelon rinds: Structure, functional and biological activities. Food Chem. 2017, 216, 355–364. [Google Scholar] [CrossRef]
- Xu, M. Polysaccharides from Sunflower Stalk Pith: Chemical, Structural, and Partial Physicochemical Characterization. Food Hydrocoll. 2020, 100, 105082. [Google Scholar] [CrossRef]
- Namir, M.; Siliha, H.; Ramadan, M.F. Fiber pectin from tomato pomace: Characteristics, functional properties and application in low-fat beef burger. J. Food Meas. Charact. 2015, 9, 305–312. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-methylated pectin from walnut processing wastes as a potential resource: Ultrasound assisted extraction and physicochemical, structural and functional analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Pectin extracted from thermally treated olive oil by-products: Characterization, physico-chemical properties, in vitro bile acid and glucose binding. Food Hydrocoll. 2015, 43, 311–321. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Emulsion design to improve the delivery of functional lipophilic components. Annu. Rev. Food Sci. Technol. 2010, 1, 241–269. [Google Scholar] [CrossRef]
- Verkempinck, S.; Kyomugasho, C.; Salvia-Trujillo, L.; Denis, S.; Bourgeois, M.; Van Loey, A.; Hendrickx, M.; Grauwet, T. Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ak, M.M. Dynamic oscillatory shear testing of foods—selected applications. Trends Food Sci.Technol. 2000, 11, 115–127. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The emulsifying and emulsion-stabilizing properties of pectin: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D. Depletion flocculation of beverage emulsions by gum arabic and modified starch. J. Food Sci. 2001, 66, 457–463. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Liu, Z.; Pi, F.; Guo, X.; Guo, X.; Yu, S. Characterization of the structural and emulsifying properties of sugar beet pectins obtained by sequential extraction. Food Hydrocoll. 2019, 88, 31–42. [Google Scholar] [CrossRef]
- Siew, C.K.; Williams, P.A. Role of protein and ferulic acid in the emulsification properties of sugar beet pectin. J. Agric. Food Chem. 2008, 56, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Zykwinska, A.; Gaillard, C.; Boiffard, M.-H.; Thibault, J.-F.; Bonnin, E. “Green labelled” pectins with gelling and emulsifying properties can be extracted by enzymatic way from unexploited sources. Food Hydrocoll. 2009, 23, 2468–2477. [Google Scholar] [CrossRef]
- Fissore, E.N.; Rojas, A.M.; Gerschenson, L.N. Rheological performance of pectin-enriched products isolated from red beet (Beta vulgaris L. var. conditiva) through alkaline and enzymatic treatments. Food Hydrocoll. 2012, 26, 249–260. [Google Scholar] [CrossRef]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. Silpakorn Univ. Int. J. 2003, 3, 206–228. [Google Scholar]
- Linden, G.; Lorient, D. Biochimie Agro-lndustrielle: Valorisation alimentaire de la production agricole. Edition Masson, Paris. J. Biol. Chem. 1994, 193, 265–275. [Google Scholar]
- Ptichkina, N.; Markina, O.; Rumyantseva, G. Pectin extraction from pumpkin with the aid of microbial enzymes. Food Hydrocoll. 2008, 22, 192–195. [Google Scholar] [CrossRef]
- Min, B.; Lim, J.; Ko, S.; Lee, K.-G.; Lee, S.H.; Lee, S. Environmentally friendly preparation of pectins from agricultural byproducts and their structural/rheological characterization. Bioresour. Technol. 2011, 102, 3855–3860. [Google Scholar] [CrossRef]
- Kontogiorgos, V.; Margelou, I.; Georgiadis, N.; Ritzoulis, C. Rheological characterization of okra pectins. Food Hydrocoll. 2012, 29, 356–362. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Ding, T.; Sun, X.; Xu, Y.; Liu, D. Ultrasound effects on the degradation kinetics, structure and rheological properties of apple pectin. Ultrason. Sonochemistry 2013, 20, 222–231. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E.; Mazoyer, J.; Langendorff, V. Emulsion stabilizing properties of depolymerized pectin. Food Hydrocoll. 2002, 16, 249–256. [Google Scholar] [CrossRef]
- Cameron, R.G.; Savary, B.J.; Hotchkiss, A.T.; Fishman, M.L. Isolation, characterization, and pectin-modifying properties of a thermally tolerant pectin methylesterase from Citrus sinensis var. Valencia. J. Agric. Food Chem. 2005, 53, 2255–2260. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. https://doi.org/10.3390/app11146596
Belkheiri A, Forouhar A, Ursu AV, Dubessay P, Pierre G, Delattre C, Djelveh G, Abdelkafi S, Hamdami N, Michaud P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Applied Sciences. 2021; 11(14):6596. https://doi.org/10.3390/app11146596
Chicago/Turabian StyleBelkheiri, Anissa, Ali Forouhar, Alina Violeta Ursu, Pascal Dubessay, Guillaume Pierre, Cedric Delattre, Gholamreza Djelveh, Slim Abdelkafi, Nasser Hamdami, and Philippe Michaud. 2021. "Extraction, Characterization, and Applications of Pectins from Plant By-Products" Applied Sciences 11, no. 14: 6596. https://doi.org/10.3390/app11146596
APA StyleBelkheiri, A., Forouhar, A., Ursu, A. V., Dubessay, P., Pierre, G., Delattre, C., Djelveh, G., Abdelkafi, S., Hamdami, N., & Michaud, P. (2021). Extraction, Characterization, and Applications of Pectins from Plant By-Products. Applied Sciences, 11(14), 6596. https://doi.org/10.3390/app11146596











