Non-Ionizing Millimeter Waves Non-Thermal Radiation of Saccharomyces cerevisiae—Insights and Interactions
Abstract
:1. Introduction
2. Results
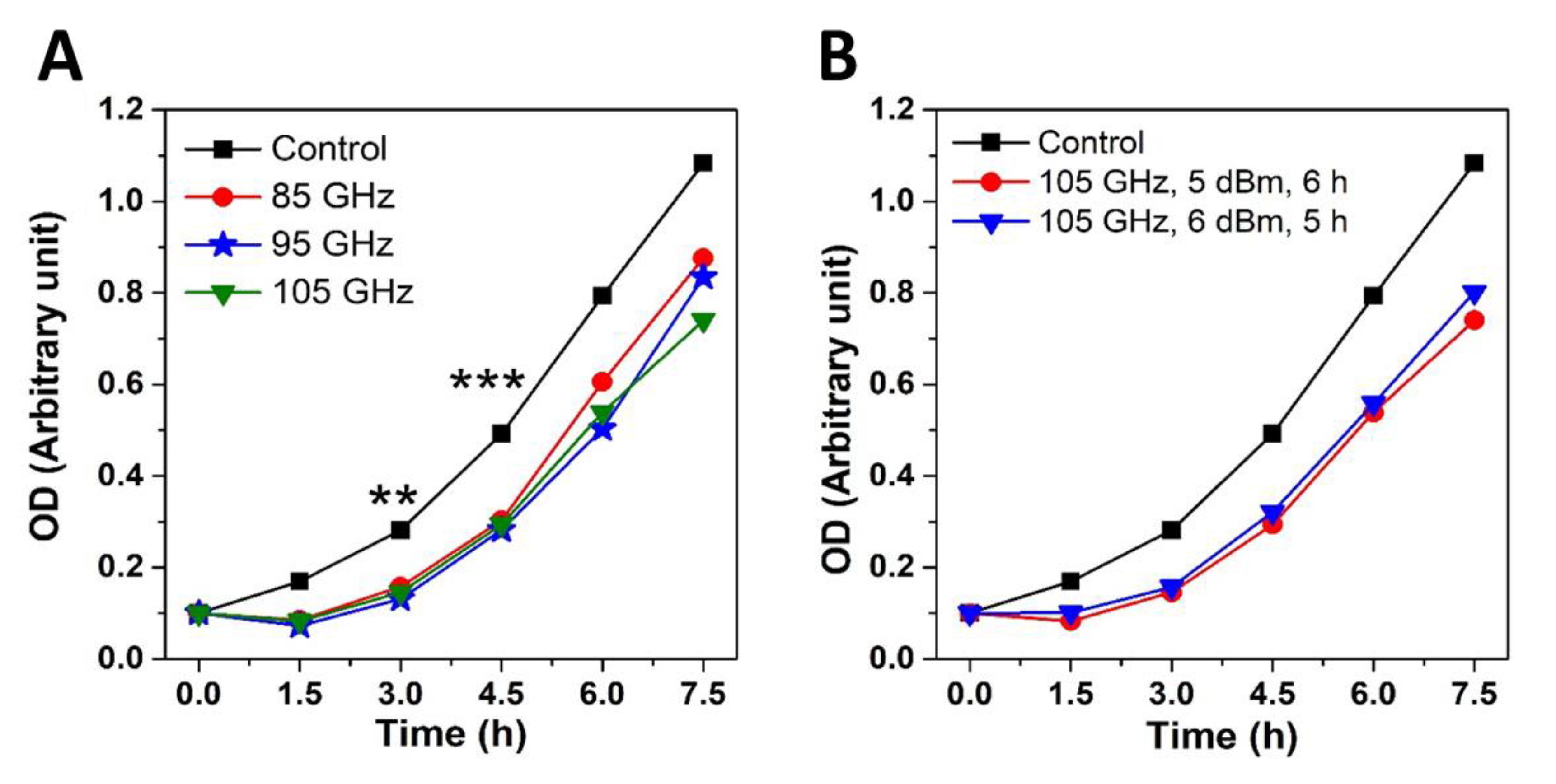
2.1. MMW Irradiation Effects Are Frequency, Energy Dose and Cell Density Dependent
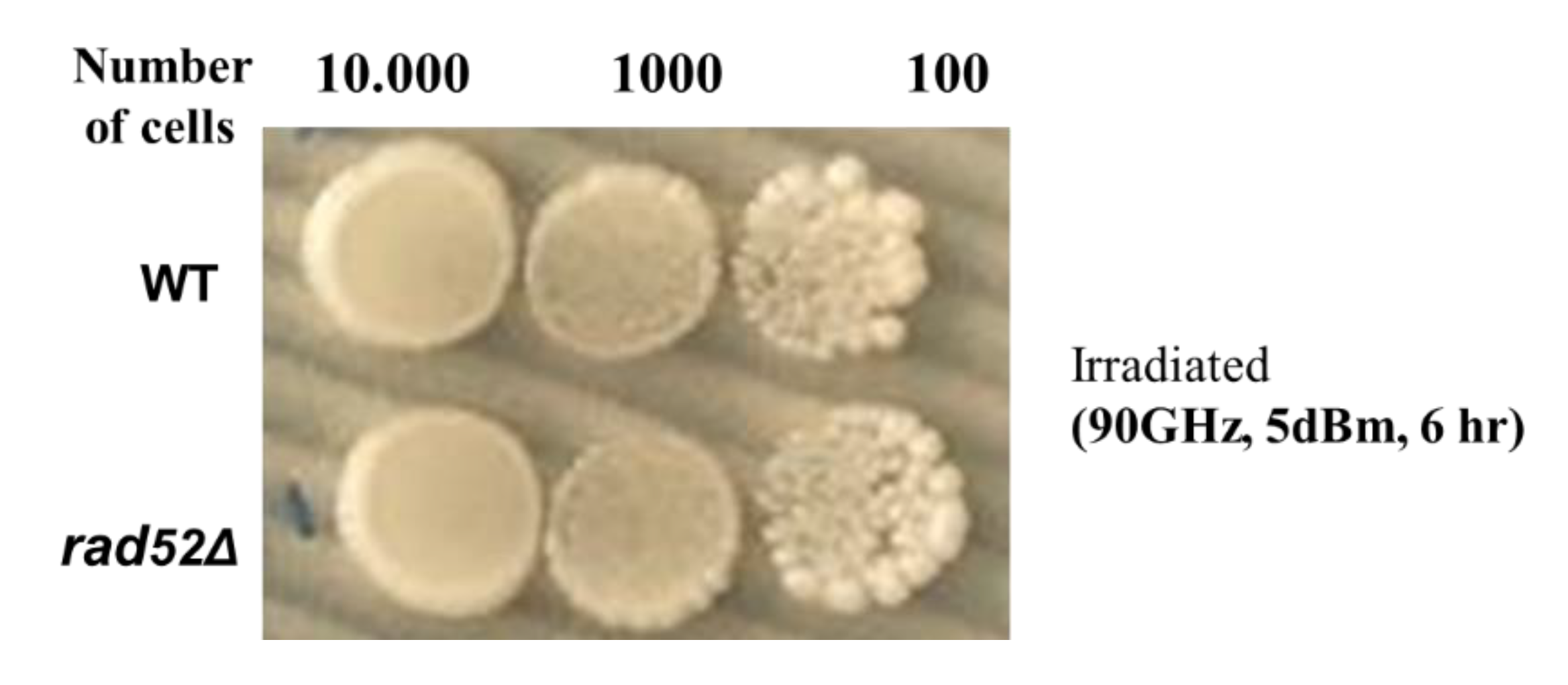
2.2. Non-Thermal MMW Wave Irradiation Does Not Cause Genomic DNA Double-Strand Alterations
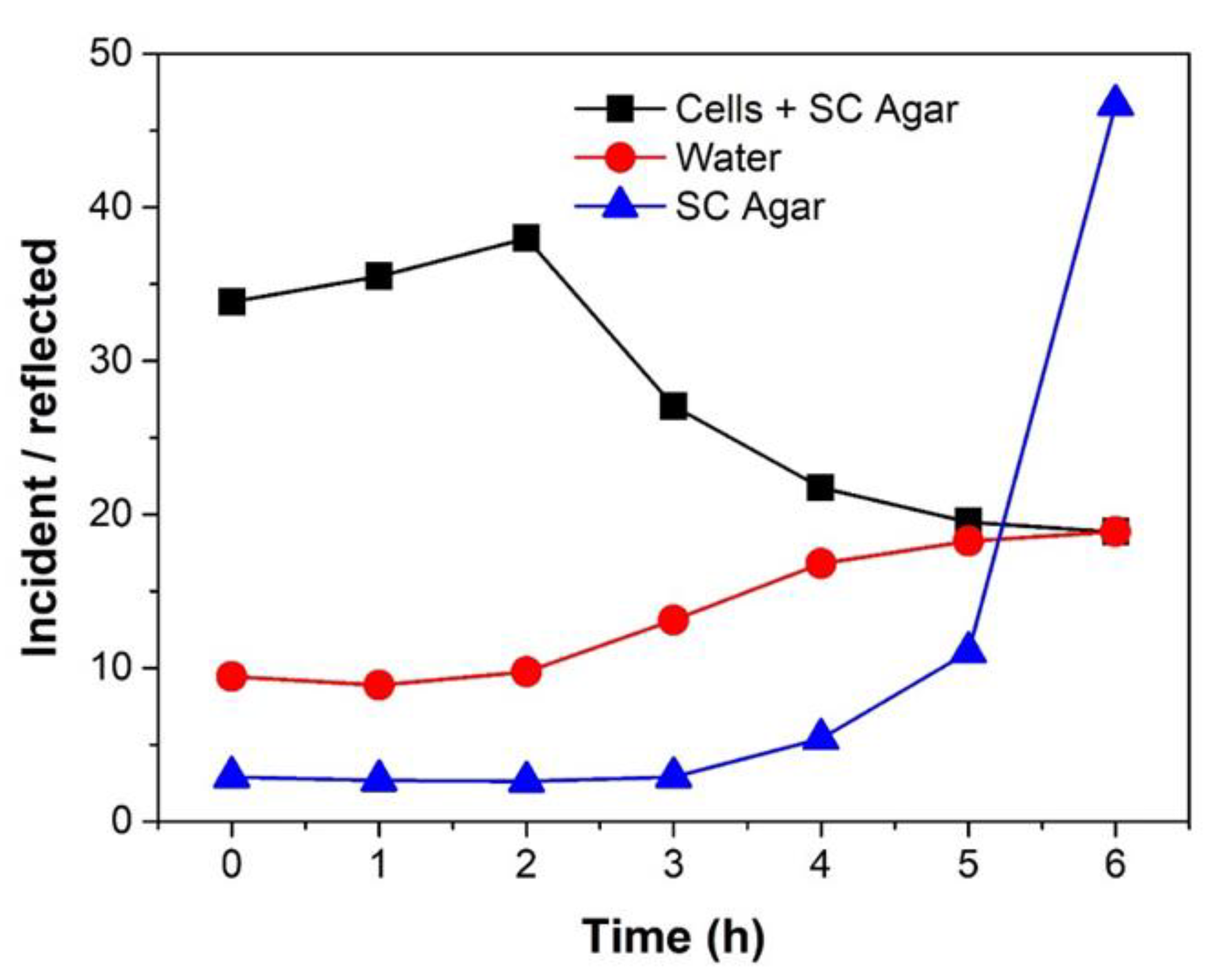
2.3. MMW Interaction with Water as a Factor for Reduced Cell Growth
3. Discussion
4. Materials and Methods
4.1. Conditions of Cell Culture
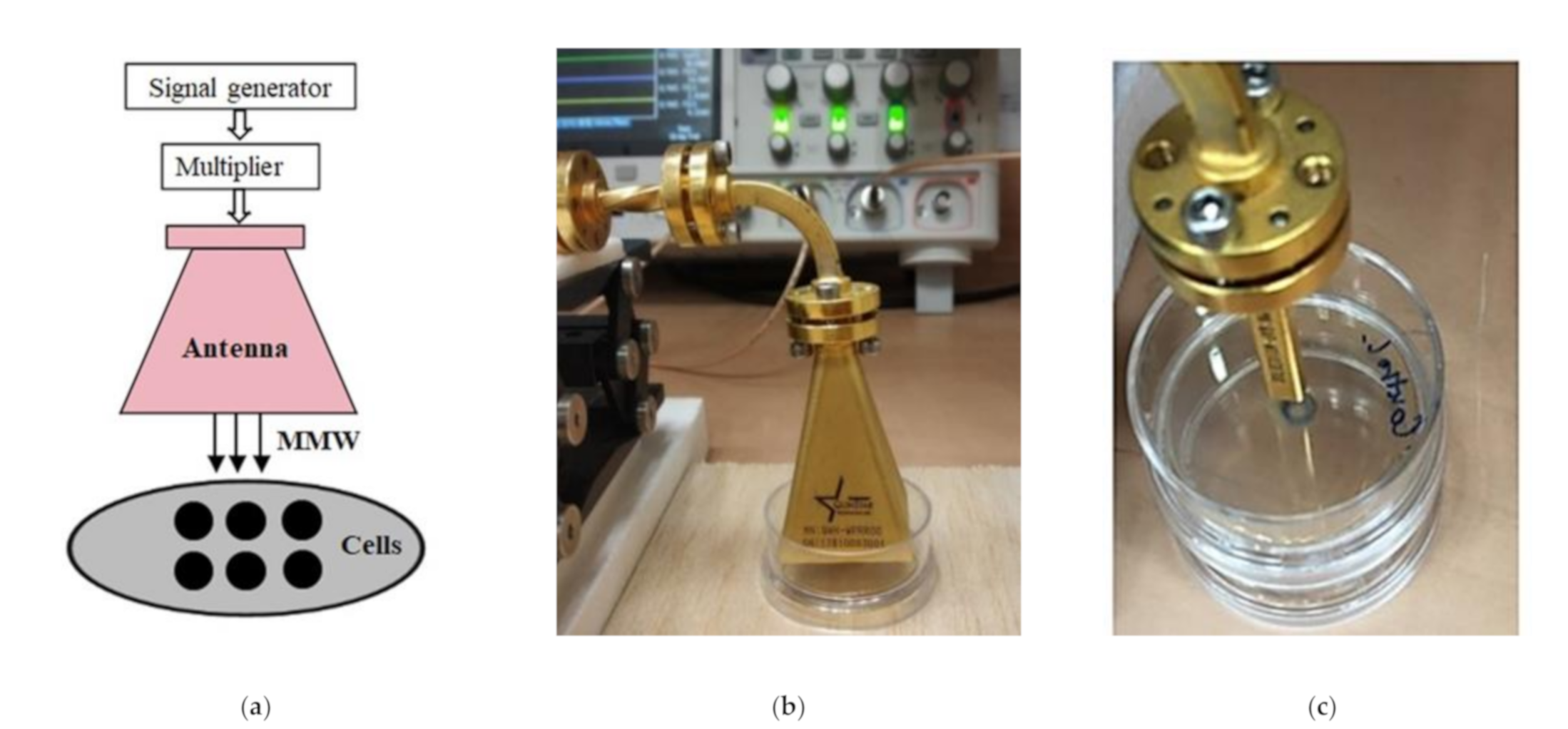
4.2. Conditions of Irradiation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koo, M.M.; Swann, R.; Mcphail, S.; Abel, G.A.; Elliss-Brookes, L.; Rubin, G.P.; Lyratzopoulos, G. Presenting Symptoms of Cancer and Stage at Diagnosis: Evidence from a Cross-Sectional, Population-Based Study. Lancet Oncol. 2020, 21, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.L. Ionizing Radiation: The Good, the Bad, and the Ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelis, C.D.; Salvo, N.; Barnes, E.; Draanen, J.V.; Stacey, E.; Mitera, G.; Breen, D.; Giotis, A.; Czarnota, G.; Pang, J. Prophylaxis and Management of Acute Radiation-Induced Skin Reactions: A Systematic Review of the Literature. Curr. Oncol. 2010, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komoshvili, K.; Becker, T.; Levitan, J.; Yahalom, A.; Barbora, A.; Liberman-Aronov, S. Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation. Appl. Sci. 2020, 10, 3187. [Google Scholar] [CrossRef]
- Komoshvili, K.; Israel, K.; Levitan, J.; Yahalom, A.; Barbora, A.; Liberman-Aronov, S. W-Band Millimeter Waves Targeted Mortality of H1299 Human Lung Cancer Cells without Affecting Non-Tumorigenic MCF-10A Human Epithelial Cells In Vitro. Appl. Sci. 2020, 10, 4813. [Google Scholar] [CrossRef]
- Mirbeik-Sabzevari, A.; Tavassolian, N. Ultrawideband, Stable Normal and Cancer Skin Tissue Phantoms for Millimeter-Wave Skin Cancer Imaging. IEEE Trans. Biomed. Eng. 2019, 66, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Coln, E.; Schoenbach, K.; Gellerman, M.; Fox, P.; Rec, L.; Beebe, S.; Liu, S. A Study on Biological Effects of Low-Intensity Millimeter Waves. IEEE Trans. Plasma Sci. 2002, 30, 1489–1496. [Google Scholar]
- Ziskin, M.C. Physiological Mechanisms Underlying Millimeter Wave Therapy. Bioelectromagnet. Curr. Concepts 2006, 241–251. [Google Scholar]
- Altmann, K.; Dürr, M.; Westermann, B. Saccharomyces cerevisiae as a Model Organism to Study Mitochondrial Biology. Methods Mol. Biol. Mitochondria 2007, 372, 81–90. [Google Scholar]
- Beck, H.; Dobritzsch, D.; Piå¡kur, J. Saccharomyces Kluyverias a Model Organism to Study Pyrimidine Degradation. FEMS Yeast Res. 2008, 8, 1209–1213. [Google Scholar] [CrossRef] [Green Version]
- Kachroo, A.H.; Laurent, J.M.; Yellman, C.M.; Meyer, A.G.; Wilke, C.O.; Marcotte, E.M. Systematic Humanization of Yeast Genes Reveals Conserved Functions and Genetic Modularity. Science 2015, 348, 921–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundler, W. Intensity- and Frequency-Dependent Effects of Microwaves on Cell Growth Rates. J. Electroanal. Chem. 1992, 342, 361–365. [Google Scholar] [CrossRef]
- Grundler, W.; Keilmann, F.; Fröhlich, H. Resonant Growth Rate Response of Yeast Cells Irradiated by Weak Microwaves. Phys. Lett. A 1977, 62, 463–466. [Google Scholar] [CrossRef]
- Furia, L.; Hill, D.W.; Gandhi, O.P. Effect of Millimeter-Wave Irradiation on Growth of Saccharomyces cerevisiae. IEEE Trans. Biomed. Eng. 1986, BME-33, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Vojisavljevic, V.; Alsuhaim, H.S.; Pirogova, E. Low power microwave exposures at 968MHz increase the growth rate of Breanomyces bruxellensis yeast cells. In Proceedings of the 2016 IEEE International Conference on Microwave and Millimeter Wave Technology (ICMMT), Beijing, China, 5–8 June 2016; pp. 1061–1063. [Google Scholar]
- Smolyanskaya, A.Z.; Vilenskaya, R.L. Effects of Millimeter-Band Electromagnetic Radiation on the Functional Activity of Certain Genetic Elements of Bacterial Cells. Sov. Phys. Uspekhi 1974, 16, 571–572. [Google Scholar] [CrossRef]
- Komoshvili, K.; Levitan, J.; Aronov, S.; Kapilevich, B.; Yahalom, A. Millimeter waves non-thermal effect on human lung cancer cells. In Proceedings of the 2011 IEEE International Conference on Microwaves, Communications, Antennas and Electronic Systems (COMCAS 2011), Tel Aviv, Israel, 7–9 November 2011. [Google Scholar]
- Feng, Z.; Scott, S.P.; Bussen, W.; Sharma, G.; Guo, G.; Pandita, T.; Powell, S. Rad52 inactivation is synthetically lethal with BRCA2 deficiency. Proc. Natl. Acad. Sci. USA 2011, 108, 686–691. [Google Scholar] [CrossRef] [Green Version]
- Bhowmick, R.; Minocherhomji, S.; Hickson, I.D. RAD52 Facilitates Mitotic DNA Synthesis Following Replication Stress. Mol. Cell. 2016, 64, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Yasuhara, T.; Kato, R.; Hagiwara, Y.; Shiotani, B.; Yamauchi, M.; Nakada, S.; Shibata, A.; Miyagawa, K. Human Rad52 Promotes XPG-Mediated R-loop Processing to Initiate Transcription-Associated Homologous Recombination Repair. Cell 2018, 175, 558–570.e11. [Google Scholar] [CrossRef] [Green Version]
- Game, J.C. Radiation-Sensitive Mutants and Repair in Yeast. Yeast Genet. 1983, 109–137. [Google Scholar]
- Game, J.C. DNA double-strand breaks and the RAD50-RAD57 genes in Saccharomyces. Semin. Cancer Biol. 1993, 4, 73–83. [Google Scholar]
- Lunkenheimer, P.; Emmert, S.; Gulich, R.; Köhler, M.; Wolf, M.; Schwab, M.; Loidl, A. Electromagnetic-Radiation Absorption by Water. Phys. Rev. E 2017, 96, 062607. [Google Scholar] [CrossRef] [Green Version]
- White, J. Variation in Water Content of Yeast Cells Caused BY Varying Temperatures of Growth and by Other Cultural Conditions. J. Inst. Brew. 1952, 58, 47–50. [Google Scholar] [CrossRef]
- Edwards, G.S.; Davis, C.C.; Saffer, J.D.; Swicord, M.L. Resonant Microwave Absorption of Selected DNA Molecules. Phys. Rev. Lett. 1984, 53, 2060. [Google Scholar] [CrossRef]
- Belyaev, I.Y.; Alipov, Y.D.; Polunin, V.A.; Shcheglov, V.S. Evidence for Dependence of Resonant Frequency of Millimeter Wave Interaction with Escherichia coliK12 Cells on Haploid Genome Length. Electro. Magn. 1993, 12, 39–49. [Google Scholar]
- Koyama, S.; Narita, E.; Shimizu, Y.; Suzuki, Y.; Shiina, T.; Taki, M.; Shinohara, N.; Miyakoshi, J. Effects of Long-Term Exposure to 60 GHz Millimeter-Wavelength Radiation on the Genotoxicity and Heat Shock Protein (Hsp) Expression of Cells Derived from Human Eye. Int. J. Environ. Res. Public Health 2016, 13, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svetlana, M.; Rogacheva, M.; Babaeva, I. The effect of millimeter waves at the yeast Saccharomyces cerevisiae during heliogeophysical disturbances. In Proceedings of the SPIE 8699 Saratov Fall Meeting and Workshop on Laser Physics and Photonics, Optical Technologies in Biophysics and Medicine XIV; and Laser Physics and Photonics XIV, 86990N, Saratov, Russian Federation, 25–28 September 2012. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; Spoel, D.V.D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef]
- O’Brien, E.P.; Brooks, B.R.; Thirumalai, D. Effects of PH on Proteins: Predictions for Ensemble and Single-Molecule Pulling Experiments. J. Am. Chem. Soc. 2011, 134, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, N.V.D.; Estrin, D.A.; Martí, M.A.; Roitberg, A.E. PH-Dependent Conformational Changes in Proteins and Their Effect on Experimental PKas: The Case of Nitrophorin 4. PLoS Comput. Biol. 2012, 8, e1002761. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-M.; Chen, H.-P.; Wang, L.-T.; Wang, J.-R.; Luo, T.-N.; Chen, Y.-J.; Liu, S.-I.; Sun, C.-K. Microwave Resonant Absorption of Viruses through Dipolar Coupling with Confined Acoustic Vibrations. Appl. Phys. Lett. 2009, 94, 043902. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, H.-C.; Liu, T.-M.; Lu, J.-T.; Hung, W.-T.; Huang, Y.-R.; Tsai, Y.-C.; Kao, C.-L.; Chen, S.-Y.; Sun, C.-K. Efficient Structure Resonance Energy Transfer from Microwaves to Confined Acoustic Vibrations in Viruses. Sci. Rep. 2015, 5, 18030. [Google Scholar] [CrossRef] [Green Version]
- Havens, C.; Ho, A.; Yoshioka, N.; Dowdy, S. Regulation of Late G1/S Phase Transition and APCCdh1 by Reactive Oxygen Species. Mol. Cell. Biol. 2006, 26, 4701–4711. [Google Scholar] [CrossRef] [Green Version]
- Veenhuis, M.; Mateblowski, M.; Kunau, W.H.; Harder, W. Proliferation of microbodies in Saccharomyces cerevisiae. Yeast 1987, 3, 77–84. [Google Scholar] [CrossRef]
- Yofe, I.; Soliman, K.; Chuartzman, S.G.; Morgan, B.; Weill, U.; Yifrach, E.; Dick, T.P.; Cooper, S.J.; Ejsing, C.S.; Schuldiner, M.; et al. Pex35 is a regulator of peroxisome abundance. J. Cell Sci. 2017, 130, 791–804. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Deng, T.; Ni, Y.; Zhan, Y.; Huang, W.; Liu, J.; Liao, C. Cytochrome c modulates the mitochondrial signaling pathway and polymorphonuclear neutrophil apoptosis in bile duct-ligated rats. Exp. Ther. Med. 2016, 12, 333–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Liu, Y.; Liu, S.; Luo, T.; Zhong, G.; Liu, A.; Zeng, Q.; Xin, X. Millimeter wave exposure induces apoptosis in human melanoma A375 cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2019, 39, 76–81. [Google Scholar] [PubMed]
- Sorolla, A.; Romanenko, S.; Siegel, P.H.; Wallace, V. Utilisation of MMW Radiation to Facilitate Apoptosis in Triple Negative Breast Cancer Cell Lines via TRPV1 Receptor Sensitization. In Proceedings of the 2019 44th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz), Paris, France, 1–6 September 2019; pp. 1–2. [Google Scholar]
- ICNIRP. Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Cowen, L.E.; Steinbach, W.J. Stress, drugs, and evolution: The role of cellular signaling in fungal drug resistance. Eukaryot. Cell 2008, 7, 747–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, I.; Rocha, R.; Santos, M.C.; Mateus, D.D.; Moura, G.R.; Carreto, L.; Santos, M.A. A genetic code alteration is a phenotype diversity generator in the human pathogen Candida albicans. PLoS ONE 2007, 2, e996. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.M.; Paredes, J.A.; Moura, G.R.; Manadas, B.; Lima-Costa, T.; Rocha, R.; Miranda, I.; Gomes, A.C.; Koerkamp, M.J.; Perrot, M.; et al. Critical roles for a genetic code alteration in the evolution of the genus Candida. EMBO J. 2007, 26, 4555–4565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-González, D.; Perusquía-Ortiz, A.M.; Hundeiker, M.; Bonifaz, A. Opportunistic yeast infections: Candidiasis, cryptococcosis, trichosporonosis and geotrichosis. J. Dtsch. Dermatol. Ges. 2013, 11, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Aubyn, G.B.; Tagoe, D.N.A. Prevalence of vaginal infections and associated lifestyles of students in the University of Cape Coast, Ghana. Asian Pac. J. Trop. Dis. 2013, 3, 267–270. [Google Scholar] [CrossRef]
- Hancock, C. WFS-1Advanced RF, Microwave and Millimeter Wave Energy Based Systems to Address a Range of Unmet and Growing Clinical Needs. In Proceedings of the The IEEE MTT International Microwave Symposium (IMS), Honolulu, HI, USA, 4–9 June 2017; Available online: https://zenodo.org/record/1156362 (accessed on 16 July 2021).


| Frequency (GHz) | Power Density (mW/cm2) | SAR (W/kg) | Time of Irradiation (h) |
|---|---|---|---|
| 85 | 1.39 ± 0.03 | 3.38 | 6 |
| 95 | 1.04 ± 0.02 | 2.61 | 6 |
| 105 | 0.83 ± 0.02 | 2.08 | 6 |
| 105 | 0.83 ± 0.02 | 2.08 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbora, A.; Rajput, S.; Komoshvili, K.; Levitan, J.; Yahalom, A.; Liberman-Aronov, S. Non-Ionizing Millimeter Waves Non-Thermal Radiation of Saccharomyces cerevisiae—Insights and Interactions. Appl. Sci. 2021, 11, 6635. https://doi.org/10.3390/app11146635
Barbora A, Rajput S, Komoshvili K, Levitan J, Yahalom A, Liberman-Aronov S. Non-Ionizing Millimeter Waves Non-Thermal Radiation of Saccharomyces cerevisiae—Insights and Interactions. Applied Sciences. 2021; 11(14):6635. https://doi.org/10.3390/app11146635
Chicago/Turabian StyleBarbora, Ayan, Shailendra Rajput, Konstantin Komoshvili, Jacob Levitan, Asher Yahalom, and Stella Liberman-Aronov. 2021. "Non-Ionizing Millimeter Waves Non-Thermal Radiation of Saccharomyces cerevisiae—Insights and Interactions" Applied Sciences 11, no. 14: 6635. https://doi.org/10.3390/app11146635








