Ammonia Gas Sensing Characteristic of P3HT-rGO-MWCNT Composite Films
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Graphene Solution
2.2. Preparation of the P3HT-rGO-MWCNT Blends
2.3. Fabrication of Gas Sensors NH3 by the Drop-Casting Method
2.4. Characterization Techniques
3. Results and Discussion
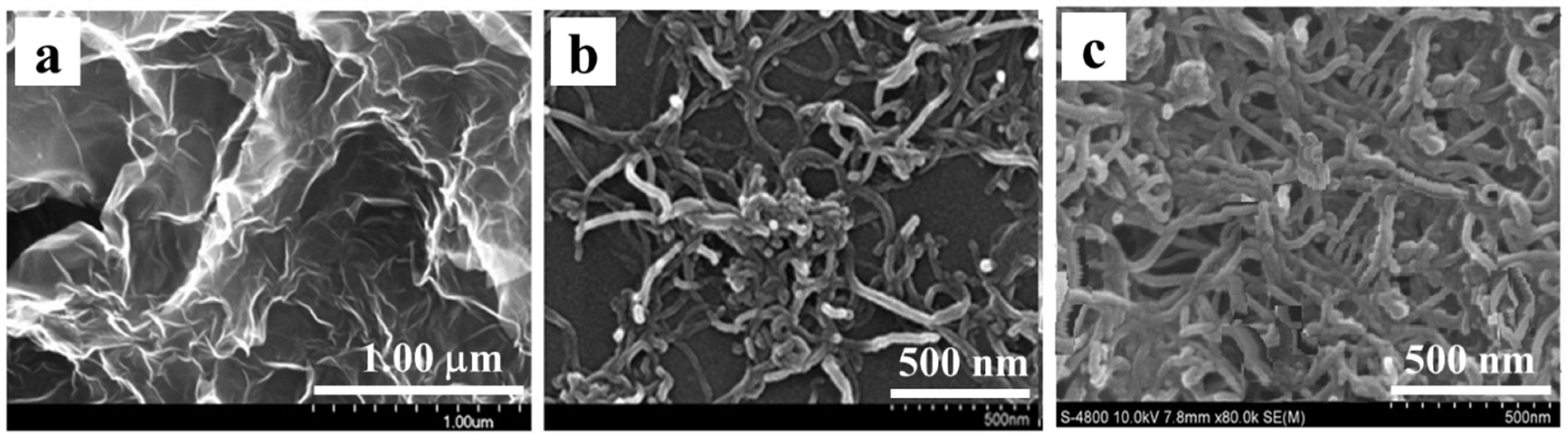
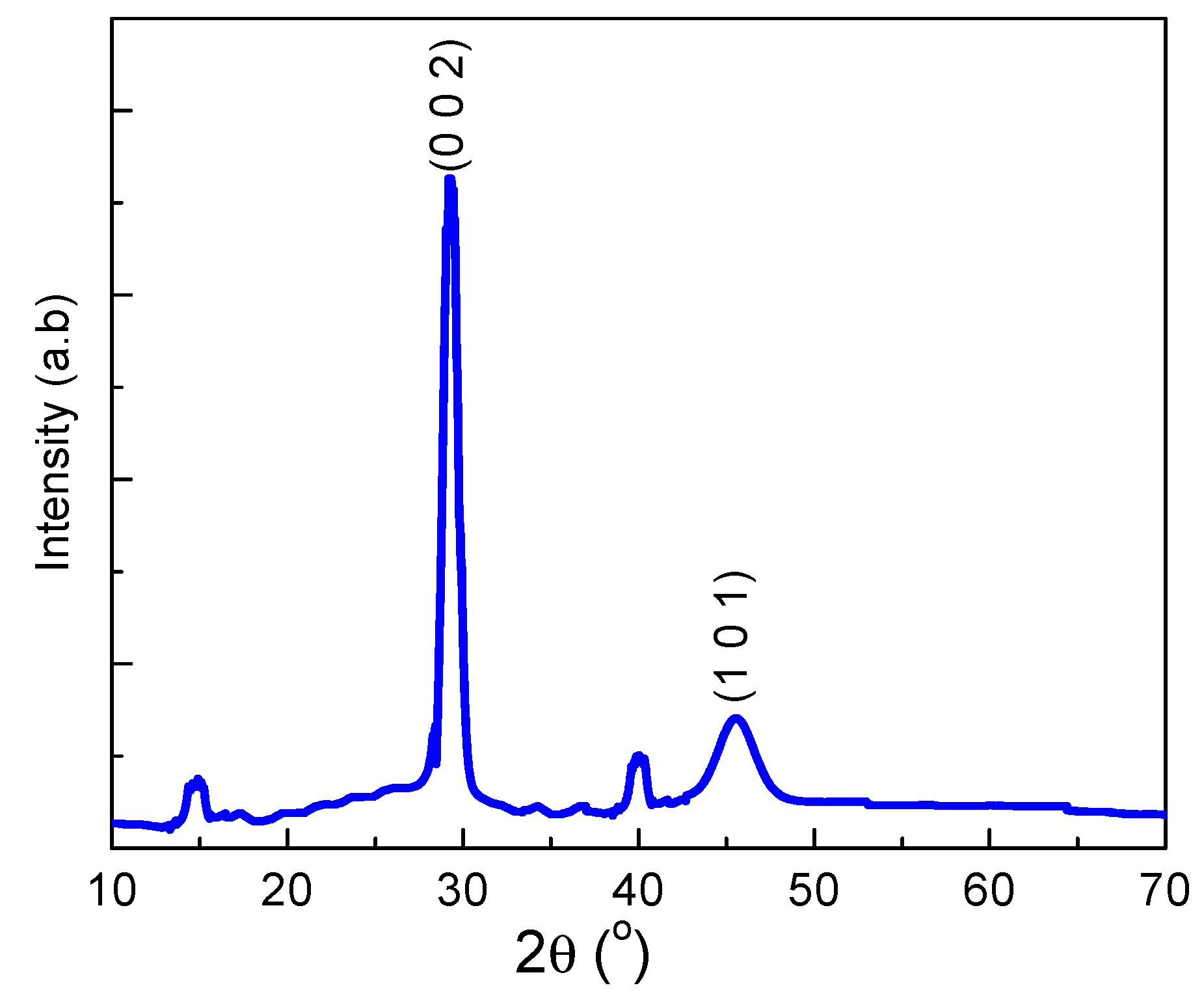
3.1. Morphology and Structure of the Films
3.2. UV-Vis Absorption Properties
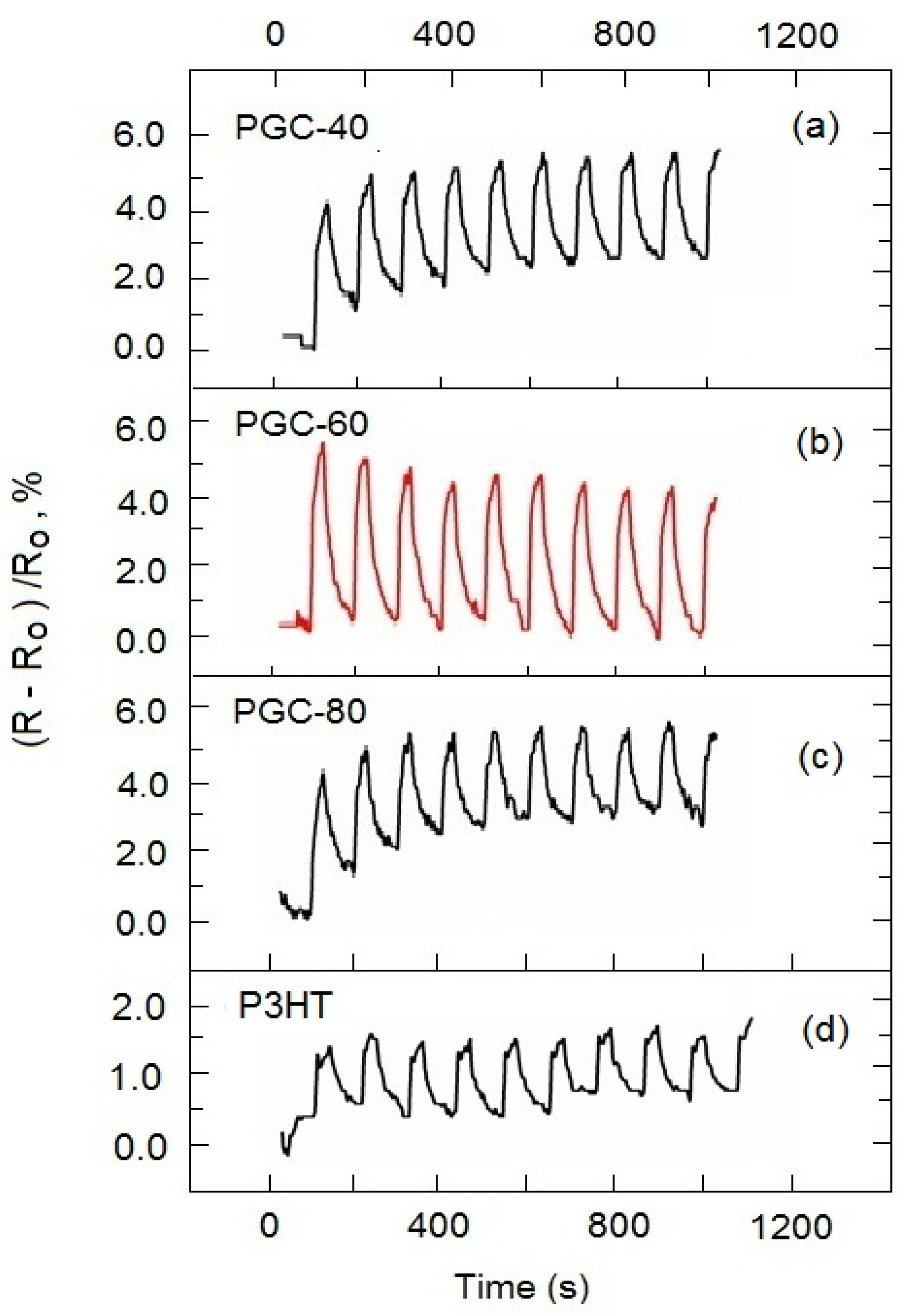
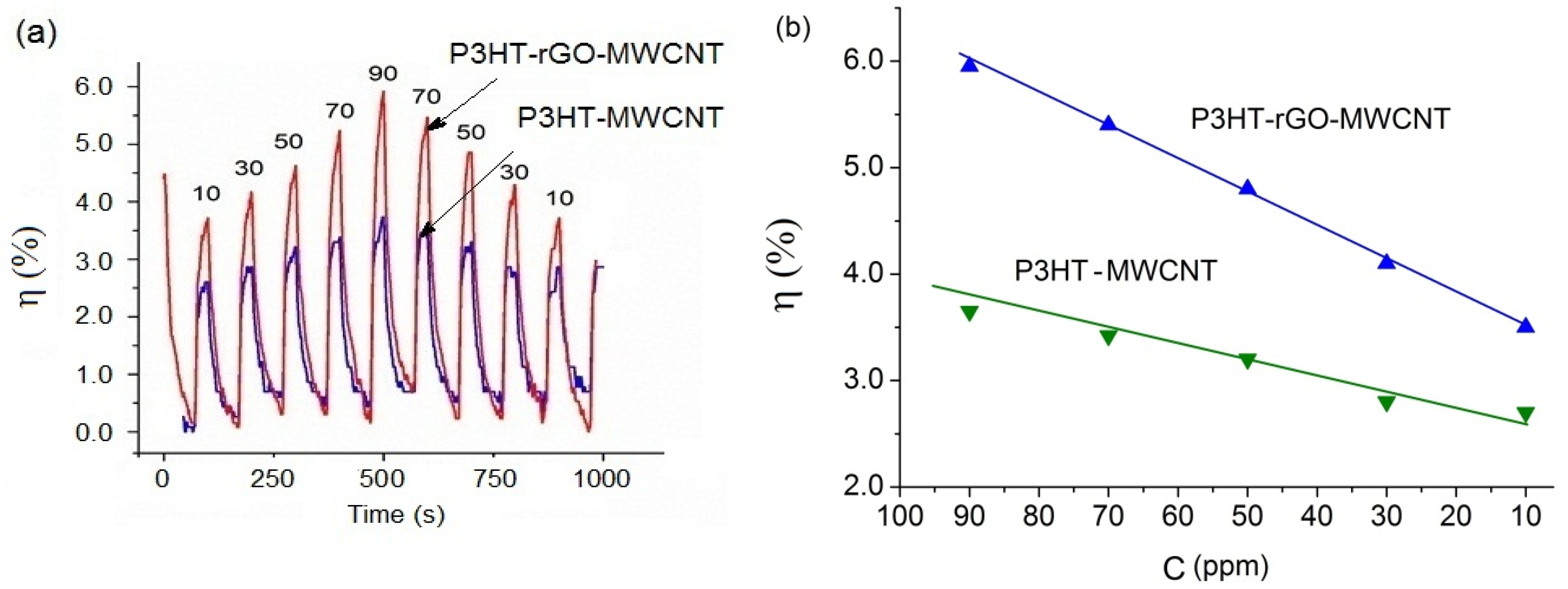
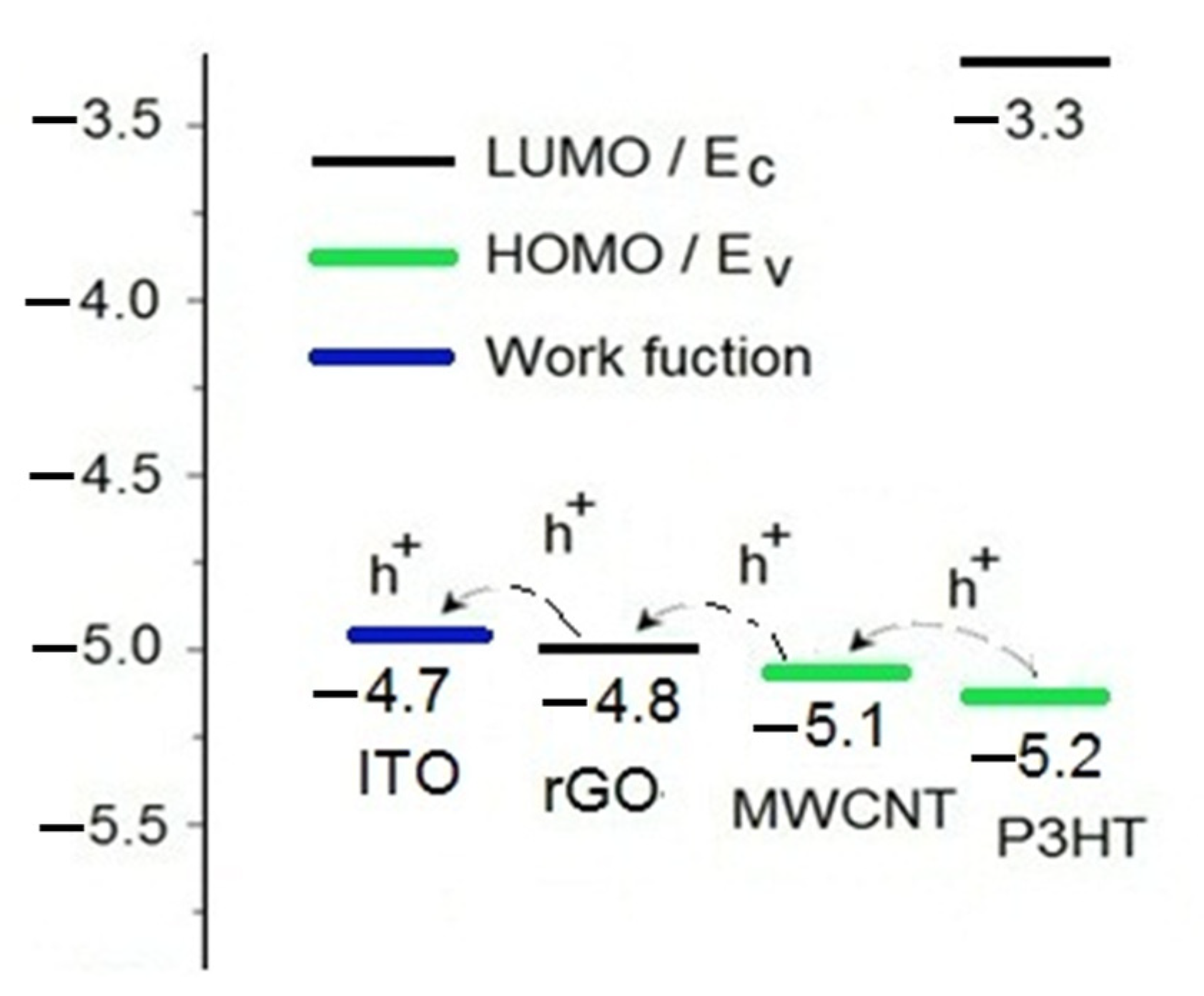
3.3. Ammonia Gas Sensing of P3HT-Based Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hibbard, T.; Crowley, K.; Killard, A.J. Direct measurement of ammonia in simulated human breath using an inkjet-printed polyaniline nanoparticle sensor. Anal. Chim. Acta 2013, 779, 6–63. [Google Scholar] [CrossRef]
- Gardon, M.; Guilemany, J.M. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci. 2013, 24, 1410–1421. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas sensors based on conducting polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Patois, T.; Sanchez, J.-B.; Bergeretal, F. Elaborationofammo-nia gas sensors based on electrodeposited polypyrrole-Cobalt phthalocyanine hybrid films. Talanta 2013, 17, 45–54. [Google Scholar] [CrossRef]
- Mkhize, N.; Murugappan, K.; Castell, M.R.; Bhaskaran, H. Electrohydrodynamic jet printed conducting polymer for enhanced chemiresistive gas sensors. J. Mater. Chem. C 2021, 9, 4591–4596. [Google Scholar] [CrossRef]
- Pang, Z.; Yildirim, E.; Pasquinelli, M.A.; Wei, Q. Ammonia Sensing Performance of Polyaniline-Coated Polyamide 6 Nanofibers. ACS Omega 2021, 6, 8950–8957. [Google Scholar] [CrossRef]
- Murugappan, K.; Castell, M.R. Bridging electrode gaps with conducting polymers around the electrical percolation threshold. Electrochem. Commun. 2018, 87, 40–43. [Google Scholar] [CrossRef]
- Armitage, B.I.; Murugappan, K.; Lefferts, M.J.; Cowsik, A.; Castell, M.R. Conducting polymer percolation gas sensor on a flexible substrate. J. Mater. Chem. C 2020, 8, 12669–12676. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Joo, B.-S.; Choi, N.-J.; Lim, J.-O.; Huh, J.-S.; Lee, D.-D. Visible optical sensing of ammonia based on polyaniline film. Sens. Actuators B 2003, 93, 148–152. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, P. Recent dvelopment on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Yin, P.T.; Kim, T.H.; Choi, J.W.; Lee, K.B. Prospects for graphene-nanoparticle-based hibryd sensors. Phys. Chem. Chem. Phys. 2013, 15, 12785–12799. [Google Scholar] [CrossRef] [Green Version]
- Chu, B.H.; Nicolosi, J.; Lo, C.F.; Strupinski, W.; Peartom, S.J.; Ren, F. Effect of Coated Platinum Thickness on Hydrogen Detection Sensitivity of Graphene-Based Sensors. Electrochem. Solid State Lett. 2011, 14, K43–K46. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z. Nanostructured silver nanowires-graphene hybrids for enhanced electrochemical detection of hydrogen peroxide. Appl. Phys. Lett. 2013, 102, 213104–213106. [Google Scholar] [CrossRef]
- Mattson, E.C.; Johns, J.E.; Pande, K.; Bosch, R.A.; Cui, S.; Josifovska, G.; Weinert, M.; Chen, J.H.; Hersam, M.C.; Hirschmugl, C.J. Vibrational excitations and low-energy electronic structure of epoxide-decorated graphene. J. Phys. Chem. Lett. 2013, 5, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Liang, H. Mid-infrared response of reduced graphene oxide and its high-temperature coefficient of resistance. AIP Adv. 2014, 4, 107131. [Google Scholar] [CrossRef] [Green Version]
- Mueller, T.; Xia, F.; Avouris, P. Graphene photodetectors for high-speed optical communications. Nat. Photonics 2010, 4, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Sun, Z.; Saito, M.; Yuan, Q.; Zhang, H.; Li, J.; Wang, Z.; Fujita, T.; Ding, F.; Zheng, Z.; et al. Regulating infrared photoresponses in reduced graphene oxide phototransistors by defect and atomic structure control. ACS Nano 2013, 7, 6310–6320. [Google Scholar] [CrossRef] [PubMed]
- Abid, P.S.; Islam, S.S.; Mishra, P.; Ahmad, S. Reduced graphene oxide (rGO) based wideband optical sensor and the role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 3537, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Ultrahigh Selective Room-Temperature Ammonia Gas Sensor Based on Tin–Titanium Dioxide/reduced Graphene/Carbon Nanotube Nanocomposites by the Solvothermal Method. ACS Omega 2019, 16, 16916–16924. [Google Scholar] [CrossRef] [Green Version]
- Su, P.-G.; Yang, L.-Y. NH3 gas sensor based on Pd/SnO2/RGO ternary composite operated at room-temperature. Chemical 2016, 223, 202–208. [Google Scholar]
- Zhang, L.; Tan, Q.; Kou, H.; Wu, D.; Zhang, W.; Xiong, J. Highly Sensitive NH3 Wireless Sensor Based on Ag-RGO Composite Operated at Room-temperature. Sci. Rep. 2019, 9, 9942. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Electrical conductivity and ammonia sensing studies on polythiophene/MWCNTs nanocomposites. Materialia 2020, 14, 100868. [Google Scholar] [CrossRef]
- Chopra, S.; McGuire, K.; Gothard, N.; Rao, A.M. Selective gas detection using a carbon nanotube sensor. Appl. Phys. Lett. 2003, 83, 2280. [Google Scholar] [CrossRef]
- Long, L.M.; Dinh, N.N.; Thu, H.T.; Phong, H.T.; Trung, T.Q. Characterization of Humidity Sensing of Polymeric Graphene-Quantum-Dots composites incorporated with silver nanowires. VNU J. Sci. Math. Phys. 2017, 33, 52–60. [Google Scholar]
- Long, L.M.; Dinh, N.N.; Trung, T.Q. Synthesis and Characterization of Polymeric Graphene-Quantum-Dots based Nanocomposites for Humidity Sensing. J. Nanomater. 2015, 2016, 5849018. [Google Scholar] [CrossRef] [Green Version]
- Thao, T.T.; Trung, T.Q.; Truong, V.; Dinh, N.N. Enhancement of Power Efficiency and Stability of P3HT-based Organic Solar Cells under Elevated Operating-Temperatures by using a Nanocomposite Photoactive Layer. J. Nanomater. 2015, 2015, 463565. [Google Scholar] [CrossRef] [Green Version]
- Trung, T.Q.; Hoa, H.T.M.; Yoo, D.; Cuong, T.V.; Hur, S.H.; Chung, J.S.; Kim, E.J.; Kohl, P.A. Reduced graphene oxide as an over-coating layer on silver nanostructures for detecting NH3 gas at room temperature. Sens. Actuators B 2014, 194, 45–50. [Google Scholar]
- Cuong, T.V.; Pham, V.H.; Shin, E.W.; Chung, J.S.; Hủ, S.H.; Kim, E.J.; Tran, Q.T.; Nguyen, H.H.H.; Hoil, P.A. Temperature-dependent photoluminescence from chemically and thermally reduced graphene oxide. Appl. Phys. Lett. 2011, 99, 041905. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.H.; Tran, Q.T.; Chung, J.S.; Shin, E.W.; Kim, J.S.; Kim, E.J. Optoelectronic properties of graphene thin films prepared by thermal reduction of graphene oxide. Mater. Lett. 2010, 64, 765–767. [Google Scholar] [CrossRef]
- Long, L.M.; Dinh, N.N.; Trung, T.Q. Characterization of NH3 sensing properties of P3HT+rGO+CNT composite films made by spin-coating. Commun. Phys. 2018, 28, 369–377. [Google Scholar] [CrossRef] [Green Version]
- An, G.; Yu, P.; Xiao, M.; Liu, Z.; Miao, Z.; Ding, K.; Mao, L. Low-temperature synthesis of Mn3O4 nanoparticles loaded on multi-walled carbon nanotubes and their application in electrochemical capacitors. Nanotechnology 2008, 19, 275709. [Google Scholar] [CrossRef]
- Chen, T.; Wu, X.; Rieke, R.D. Regiocontrolled Synthesis of Poly(3-alkylthiophenes) Mediated by Rieke Zinc: Their Characterization and Solid-State Properties. J. Am. Chem. Soc. 1995, 117, 233–244. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.-W.; Eppakayala, V.; Kim, J.-H. Green synthesis of graphene and its cytotoxic, effects in human breast cancer cells. Int. J. Nanomed. 2013, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boukhvalov, D.W.; Katsnelson, M.I. Modeling of graphite oxide. J. Am. Chem. Soc. 2008, 130, 10698–10701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.; Chang, L.Y.; Lim, S.K.; Zhao, J.; Smith, M.; Zhao, N.; Bulovic, V.; Bawendi, M.; Gradecak, S. Inorganic-organic hybrid solar cell: Bridging quantum dot to conjugated polymer nanowires. Nano Lett. 2011, 11, 3998–4002. [Google Scholar] [CrossRef]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
- Yang, S.H.; Nguyen, T.P.; Le Rendu, P.; Hsu, C.S. Optical and electrical properties of PPV/SiO2 and PPV/TiO2 composite materials. Compos. Part A Appl. Sci. Manufact. 2005, 36, 509–515. [Google Scholar] [CrossRef]
- Omer, B.M. Optical Properties of Poly (3-hexylthiophene-2,5-diyl) and Poly (3-hexylthiophene-2,5-diyl) /[6,6]-Phenyl C61-butyric Acid 3-ethylthiophene Ester Thin Films. J. Nano Electron. Phys. 2013, 5, 03010. [Google Scholar]
- Gavgani, J.N.; Dehsari, H.S.; Hasani, A.; Mahyari, M.; Shalamzari, E.K.; Salehi, A.; Taromi, F.A. A room temperature volatile organic compound sensor with enhanced performance, fast response and recovery based on N-doped graphene quantum dots and poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate) nanocomposite. Royal Soc. Chem. 2015, 5, 57559–57567. [Google Scholar] [CrossRef]
- Ye, Z.B.; Jiang, Y.D.; Tai, H.L.; Yuan, Z. The investigation of reduced graphene oxide/P3HT composite films for ammonia detection. Integr. Ferroelectr. 2014, 154, 81. [Google Scholar] [CrossRef]
- Majzlíková, P.; Sedlácek, J.; Prášek, J.; Pekárek, J.; Svatoš, V.; Bannov, A.G.; Jašek, O.; Synek, P.; Eliáš, M.; Zajícková, L.; et al. Sensing Properties of Multiwalled Carbon Nanotubes Grown in MW Plasma Torch: Electronic and Electrochemical Behavior, Gas Sensing, Field Emission, IR Absorption. Sensors 2015, 15, 2644–2661. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, Y.; Wang, X.; Wang, J.; Gaskov, A.M.; Akbar, S.A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators B Chem. 2016, 230, 330–336. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hu, N.; Wang, Y.; Zhang, Y.; Zhou, Z.; Liu, Y.; Shen, S.; Peng, C. Ammonia gas sensors based on chemically reduced graphene oxide sheets self-assembled on Au electrodes. Nanoscale Res. Lett. 2014, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varghese, O.K.; Kichambre, P.D.; Gong, D.; Ong, K.G.; Dickey, E.C.; Grimes, C.A. Gas sensing characteristics of multi-wall carbon nanotubes. Sens. Actuators B 2001, 81, 32–41. [Google Scholar] [CrossRef]
- Aba, L.; Yusuf, Y.; Mitrayana, L.; Triyana, K. Selectivity Improvement of Gas Sensor Based on Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Thin Film by Using Imprinting Method. J. Mod. Phys. 2012, 3, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Gao, C.; Li, Y.; Han, W.; Fu, W.; He, Y.; Xie, E. Enhanced charge separation and transfer through Fe2O3/ITO nanowire arrays wrapped with reduced graphene oxide for water-splitting. Nano Energy 2016, 30, 892–899. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanh, T.S.T.; Trung, T.Q.; Giang, L.T.T.; Nguyen, T.Q.; Lam, N.D.; Dinh, N.N. Ammonia Gas Sensing Characteristic of P3HT-rGO-MWCNT Composite Films. Appl. Sci. 2021, 11, 6675. https://doi.org/10.3390/app11156675
Khanh TST, Trung TQ, Giang LTT, Nguyen TQ, Lam ND, Dinh NN. Ammonia Gas Sensing Characteristic of P3HT-rGO-MWCNT Composite Films. Applied Sciences. 2021; 11(15):6675. https://doi.org/10.3390/app11156675
Chicago/Turabian StyleKhanh, Tran Si Trong, Tran Quang Trung, Le Thuy Thanh Giang, Tran Quang Nguyen, Nguyen Dinh Lam, and Nguyen Nang Dinh. 2021. "Ammonia Gas Sensing Characteristic of P3HT-rGO-MWCNT Composite Films" Applied Sciences 11, no. 15: 6675. https://doi.org/10.3390/app11156675






