In Vitro Evaluation of Cytotoxicity and Proliferative Effects of Lyophilized Porcine Liver Tissue on HepG2 Hepatoma Cells and Adipose-Tissue-Derived Mesenchymal Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Porcine Liver Lyophilized Powder
2.2. Solubilization of the Porcine Liver Lyophilized Powder
2.3. In-Vitro Cell Culture
2.4. Cell Cytotoxicity and Proliferation Assay
2.5. MTT Assay
2.6. Bromo-Deoxyuridine Incorporation Assay
2.7. Direct Cell Count
2.8. Statistical Analysis
3. Results
3.1. Protein Content of the Solution of LLP
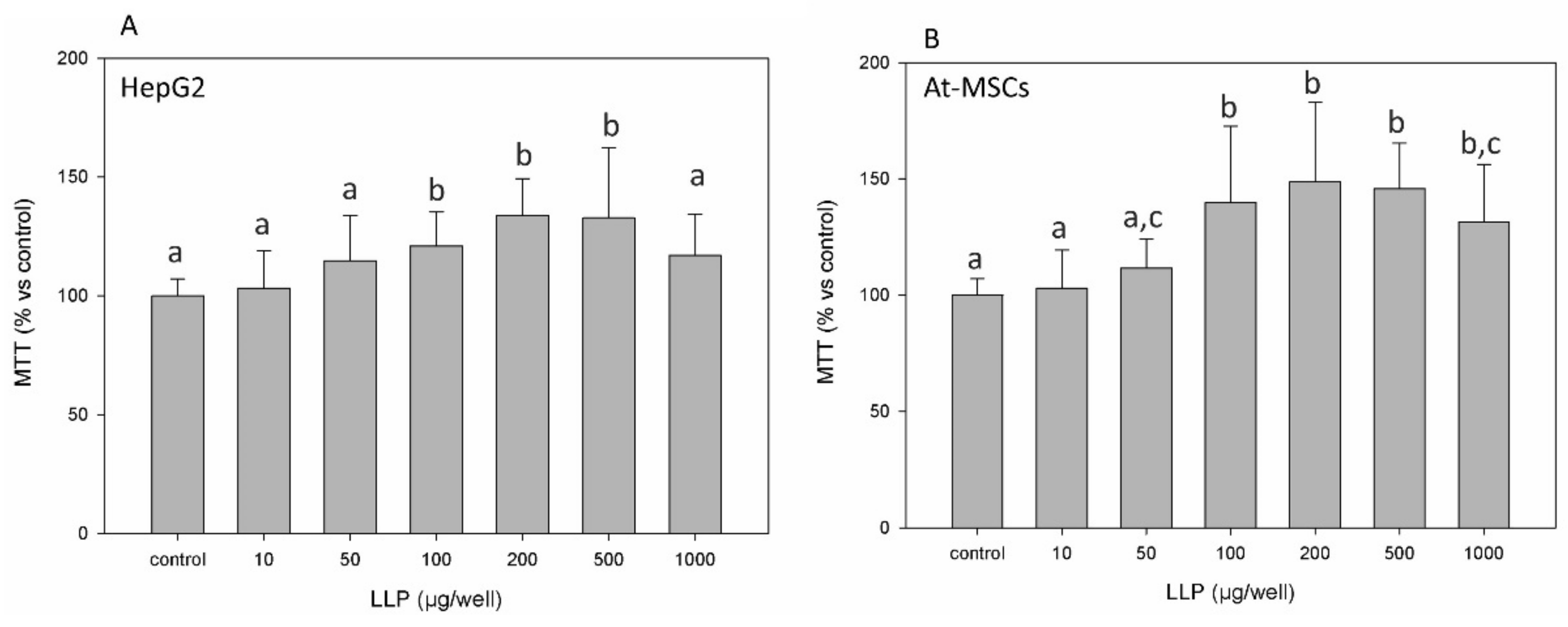
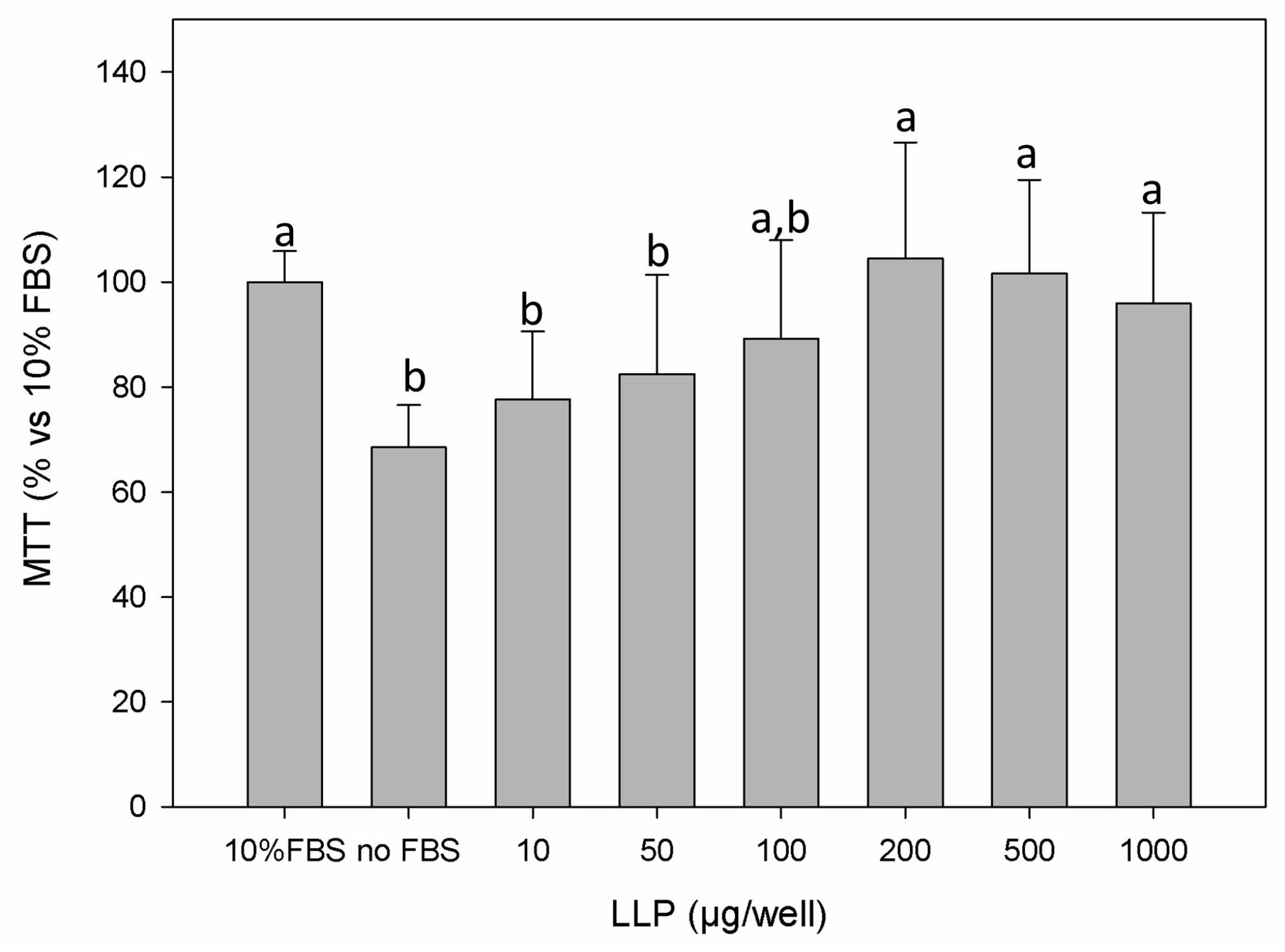
3.2. Cell Cytotoxicity
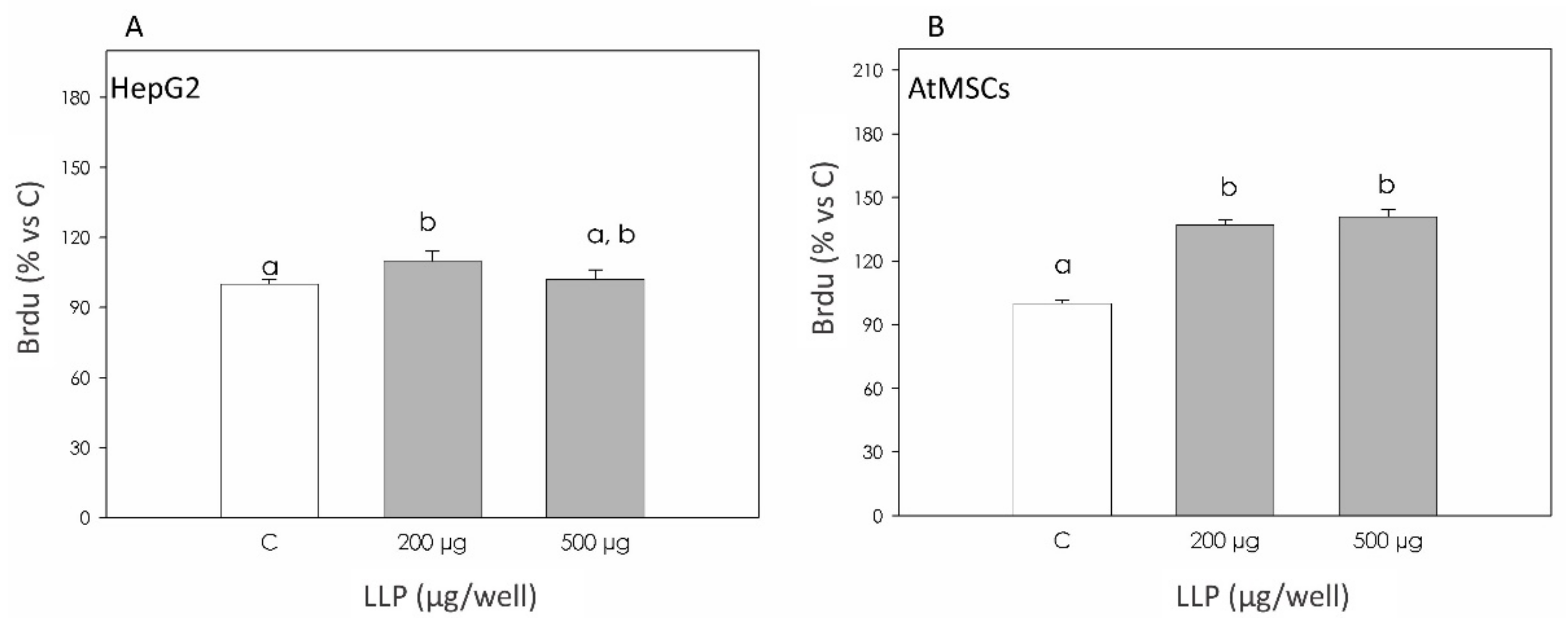
3.3. Cell Proliferation: Brdu Incorporation Assay
3.4. Cell Proliferation: Direct Cell Count
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mokdad, A.A.; Lopez, A.D.; Shahraz, S.; Lozano, R.; Stanaway, J.; Murray, C.J.L.; Naghavi, M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med. 2014, 12, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.; Webber, L.; Sheron, N. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Marchesini, G.; Pinto-Cortez, H.; Petta, S. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis: Implications for Liver Transplantation. Transplantation 2019, 103, 22–27. [Google Scholar] [CrossRef]
- Lemoinne, S.; Friedman, S.L. New and emerging anti-fibrotic therapeutics entering oralready in clinical trials in chronic liver diseases. Curr. Opin. Pharmacol. 2019, 49, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Rowe, I.A. Lessons from Epidemiology: The Burden of Liver Disease. Dig. Dis. 2017, 35, 304–309. [Google Scholar] [CrossRef]
- Hanje, A.J.; Fortune, B.; Song, M.; Hill, D.; McClain, C. The Use of Selected Nutrition Supplements and Complementary and Alternative Medicine in Liver Disease. Nutr. Clin. Pract. 2006, 21, 255–272. [Google Scholar] [CrossRef] [Green Version]
- Navarro, V.J.; Khan, I.; Björnsson, E.; Seeff, L.B.; Serrano, J.; Hoofnagle, J.H. Liver injury from herbal and dietary supplements. Hepatology 2017, 65, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Feizi, A.; Gatto, F.; Uhlen, M.; Nielsen, J. Human protein secretory pathway genes are expressed in a tissue-specific pattern to match processing demands of the secretome. NPJ Syst. Biol. Appl. 2017, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Chan, H.F.; Wang, H.; Shao, D.; Tao, Y.; Li, M. Stem cell therapy and tissue engineering strategies using cell aggregates and decellularized scaffolds for the rescue of liver failure. J. Tissue Eng. 2021, 12. [Google Scholar] [CrossRef]
- Sellaro, T.L.; Ranade, A.; Faulk, D.M.; McCabe, G.P.; Dorko, K.; Badylak, S.F.; Strom, S. Maintenance of Human Hepatocyte Function In Vitro by Liver-Derived Extracellular Matrix Gels. Tissue Eng. Part A 2010, 16, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Soto-Gutierrez, A.; Zhang, L.; Medberry, C.; Fukumitsu, K.; Faulk, D.; Jiang, H.; Reing, J.; Gramignoli, R.; Komori, J.; Ross, M.; et al. A Whole-Organ Regenerative Medicine Approach for Liver Replacement. Tissue Eng. Part C Methods 2011, 17, 677–686. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Chen, E.; Li, L. Liver-derived human mesenchymal stem cells: A novel therapeutic source for liver diseases. Stem Cell Res. Ther. 2016, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Beer, L.; Mildner, M.; Ankersmit, H.J. Cell secretome based drug substances in regenerative medicine: When regulatory affairs meet basic science. Ann. Transl. Med. 2017, 5, 170. [Google Scholar] [CrossRef] [Green Version]
- Maguire, G. Stem cell therapy without the cells. Commun. Integr. Biol. 2013, 6, e26631. [Google Scholar] [CrossRef]
- Vishnubhatla, I.; Corteling, R.; Stevanato, L.; Hicks, C.; Sinden, J. The Development of Stem Cell-Derived Exosomes as a Cell-Free Regenerative Medicine. J. Circ. Biomark. 2014, 3. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [Green Version]
- Fierabracci, A.; Del Fattore, A.; Luciano, R.; Muraca, M.; Teti, A.M.; Muraca, M. Recent Advances in Mesenchymal Stem Cell Immunomodulation: The Role of Microvesicles. Cell Transplant. 2015, 24, 133–149. [Google Scholar] [CrossRef]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Fouraschen, S.M.; Pan, Q.; de Ruiter, P.; Farid, W.R.; Kazemier, G.; Kwekkeboom, J.; Ijzermans, J.N.; Metselaar, H.J.; Tilanus, H.W.; De Jonge, J.; et al. Secreted Factors of Human Liver-Derived Mesenchymal Stem Cells Promote Liver Regeneration Early after Partial Hepatectomy. Stem Cells Dev. 2012, 21, 2410–2419. [Google Scholar] [CrossRef]
- Chiabotto, G.; Pasquino, C.; Camussi, G.; Bruno, S. Molecular Pathways Modulated by Mesenchymal Stromal Cells and Their Extracellular Vesicles in Experimental Models of Liver Fibrosis. Front. Cell Dev. Biol. 2020, 8, 594794. [Google Scholar] [CrossRef]
- Bruno, S.; Pasquino, C.; Sanchez, M.B.H.; Tapparo, M.; Figliolini, F.; Grange, C.; Chiabotto, G.; Cedrino, M.; Deregibus, M.C.; Tetta, C.; et al. HLSC-Derived Extracellular Vesicles Attenuate Liver Fibrosis and Inflammation in a Murine Model of Non-alcoholic Steatohepatitis. Mol. Ther. 2020, 28, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Nojima, H.; Freeman, C.M.; Schuster, R.M.; Japtok, L.; Kleuser, B.; Edwards, M.J.; Gulbins, E.; Lentsch, A.B. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J. Hepatol. 2016, 64, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Ferroni, O.; Tassinari, R.; Cavallini, C.; Taglioli, V.; Olivi, E.; Ventura, C.E. Il liofilizzato di fegato di suino Neorland® è fonte di esosomi ben conservati e biologicamente attivi su cellule staminali umane. La Med. Biol. 2019, 1, 3–9. [Google Scholar]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [PubMed]
- Gugjoo, M.B.; Amarpal, A.; Sharma, G.T. Mesenchymal stem cell basic research and applications in dog medicine. J. Cell. Physiol. 2019, 234, 16779–16811. [Google Scholar] [CrossRef]
- Suelzu, C.M.; Conti, V.; Khalidy, Y.; Montagna, S.; Strusi, G.; Di Lecce, R.; Berni, P.; Basini, G.; Ramoni, R.; Grolli, S. Xenobiotic-Free Medium Guarantees Expansion of Adipose Tissue-Derived Canine Mesenchymal Stem Cells both in 3D Fibrin-Based Matrices and in 2D Plastic Surface Cultures. Cells 2020, 9, 2578. [Google Scholar] [CrossRef]
- Mora, S.; García-Román, J.; Gómez-Ñañez, I.; García-Román, R. Chronic liver diseases and the potential use of S-adenosyl-l-methionine as a hepatoprotector. Eur. J. Gastroen. Hepat. 2018, 30, 893–900. [Google Scholar] [CrossRef]
- Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl. Health Stat. Rep. 2015, 79, 1–16. [Google Scholar]
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary Supplement Use in the United States, 2003–2006. J. Nutr. 2010, 141, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Fulgoni, V.L.; Keast, D.R.; Bailey, R.; Dwyer, J. Foods, Fortificants, and Supplements: Where Do Americans Get Their Nutrients? J. Nutr. 2011, 141, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Lucena, M.I.; Navarro, V.J. Hepatotoxicity Induced by Herbal and Dietary Supplements. Semin. Liver Dis. 2014, 34, 172–193. [Google Scholar] [CrossRef] [Green Version]
- Au, J.S.; Navarro, V.J.; Rossi, S. Review article: Drug-induced liver injury—Its pathophysiology and evolving diagnostic tools. Aliment. Pharmacol. Ther. 2011, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.J.; López-Ortega, S.; López-Vega, M.C.; Robles, M.; Cueto, I.; Lucena, M.I. Idiosyncratic drug hepatotoxicity: A 2008 update. Expert Rev. Clin. Pharmacol. 2008, 1, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Björnsson, E. Risk Factors for Idiosyncratic Drug-Induced Liver Injury. Gastroenterology 2010, 138, 2246–2259. [Google Scholar] [CrossRef] [Green Version]
- Bunchorntavakul, C.; Reddy, K.R. Review article: Herbal and dietary supplement hepatotoxicity. Aliment. Pharmacol. Ther. 2013, 37, 3–17. [Google Scholar] [CrossRef]
- Dan, Y.Y.; Yeoh, G.C. Liver stem cells: A scientific and clinical perspective. J. Gastroenterol. Hepatol. 2008, 23, 687–698. [Google Scholar] [CrossRef]
- Wan, T.-C.; Liu, Y.-T.; Duann, L.-T.; Yu, K.-H.; Chen, C.-M.; Lin, L.-C.; Sakata, R. Effects of animal liver and bile extracts on biochemical values of rat ethanol-induced fatty liver. Anim. Sci. J. 2013, 85, 75–80. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! STEM CELLS Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [Green Version]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Loneker, A.E.; Faulk, D.M.; Hussey, G.S.; D’Amore, A.; Badylak, S.F. Solubilized liver extracellular matrix maintains primary rat hepatocyte phenotype in-vitro. J. Biomed. Mater. Res. Part A 2016, 104, 1846–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nhung, T.H.; Nam, N.H.; Nguyen, N.T.K.; Nghia, H.; Van Thanh, N.; Ngoc, P.K.; Van Pham, P. A comparison of the chemical and liver extract-induced hepatic differentiation of adipose derived stem cells. Vitr. Cell. Dev. Biol. Anim. 2015, 51, 1085–1092. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. Methods Mol. Biol. 2014, 1250, 333–348. [Google Scholar] [CrossRef]
- Sung, S.; Kim, J.; Jung, Y. Liver-Derived Exosomes and Their Implications in Liver Pathobiology. Int. J. Mol. Sci. 2018, 19, 3715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. J. Hepatol. 2013, 59, 621–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, M.B.; Fonsato, V.; Gatti, S.; Deregibus, M.C.; Sordi, A.; Cantarella, D.; Calogero, R.; Bussolati, B.; Tetta, C.; Camussi, G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J. Cell. Mol. Med. 2010, 14, 1605–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| HepG2 | Treatment | |||
|---|---|---|---|---|
| Control | 100 µg | 200 µg | 500 µg | |
| Average Cell Number ± SD | 450,000 ± 82,585 | 495,000 ± 148,951 | 517,833 ± 53,504 | 421,185 ± 92,766 |
| Average Doubling Time ± SD | 22.51 ± 1.87 | 21.95 ± 3.02 | 21.03 ± 0.91 | 23.35 ± 2.63 |
| Average Cell-Doubling Number ± SD | 3.21 ± 0.27 | 3.31 ± 0.45 | 3.43 ± 0.15 | 3.10 ± 0.36 |
| At-MSCs | Treatment | |||
|---|---|---|---|---|
| Control | 100 µg | 200 µg | 500 µg | |
| Average Cell Number ± SD | 306,986 ± 43,236 | 713,373 ± 121,459 | 773,736 ± 154,696 | 848,833 ± 307,133 |
| Average Doubling Time ± SD | 27.09 ± 1.9 | 18.62 ± 1.15 | 18.14 ± 1.52 | 18.01 ± 2.82 |
| Average Cell-Doubling Number ± SD | 2.7 ± 0.19 | 3.86 ± 0.26 | 3.99 ± 0.33 | 4.1 ± 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berni, P.; Conti, V.; Ferroni, O.; Ramoni, R.; Basini, G.; Grolli, S. In Vitro Evaluation of Cytotoxicity and Proliferative Effects of Lyophilized Porcine Liver Tissue on HepG2 Hepatoma Cells and Adipose-Tissue-Derived Mesenchymal Stromal Cells. Appl. Sci. 2021, 11, 6691. https://doi.org/10.3390/app11156691
Berni P, Conti V, Ferroni O, Ramoni R, Basini G, Grolli S. In Vitro Evaluation of Cytotoxicity and Proliferative Effects of Lyophilized Porcine Liver Tissue on HepG2 Hepatoma Cells and Adipose-Tissue-Derived Mesenchymal Stromal Cells. Applied Sciences. 2021; 11(15):6691. https://doi.org/10.3390/app11156691
Chicago/Turabian StyleBerni, Priscilla, Virna Conti, Orlando Ferroni, Roberto Ramoni, Giuseppina Basini, and Stefano Grolli. 2021. "In Vitro Evaluation of Cytotoxicity and Proliferative Effects of Lyophilized Porcine Liver Tissue on HepG2 Hepatoma Cells and Adipose-Tissue-Derived Mesenchymal Stromal Cells" Applied Sciences 11, no. 15: 6691. https://doi.org/10.3390/app11156691
APA StyleBerni, P., Conti, V., Ferroni, O., Ramoni, R., Basini, G., & Grolli, S. (2021). In Vitro Evaluation of Cytotoxicity and Proliferative Effects of Lyophilized Porcine Liver Tissue on HepG2 Hepatoma Cells and Adipose-Tissue-Derived Mesenchymal Stromal Cells. Applied Sciences, 11(15), 6691. https://doi.org/10.3390/app11156691







