Impact of Dietary Supplementation of Flaxseed Meal on Intestinal Morphology, Specific Enzymatic Activity, and Cecal Microbiome in Broiler Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Proximate Chemical Composition and Fatty Acid Profiles
2.2. Poultry and Experimental Treatments
2.3. Histology
2.4. Enzymatic Analysis
2.5. Microbiome Characterization
2.6. Statistical Analysis
3. Results
3.1. Composition Analysis of Flaxseed Meal
3.2. Performance Parameters
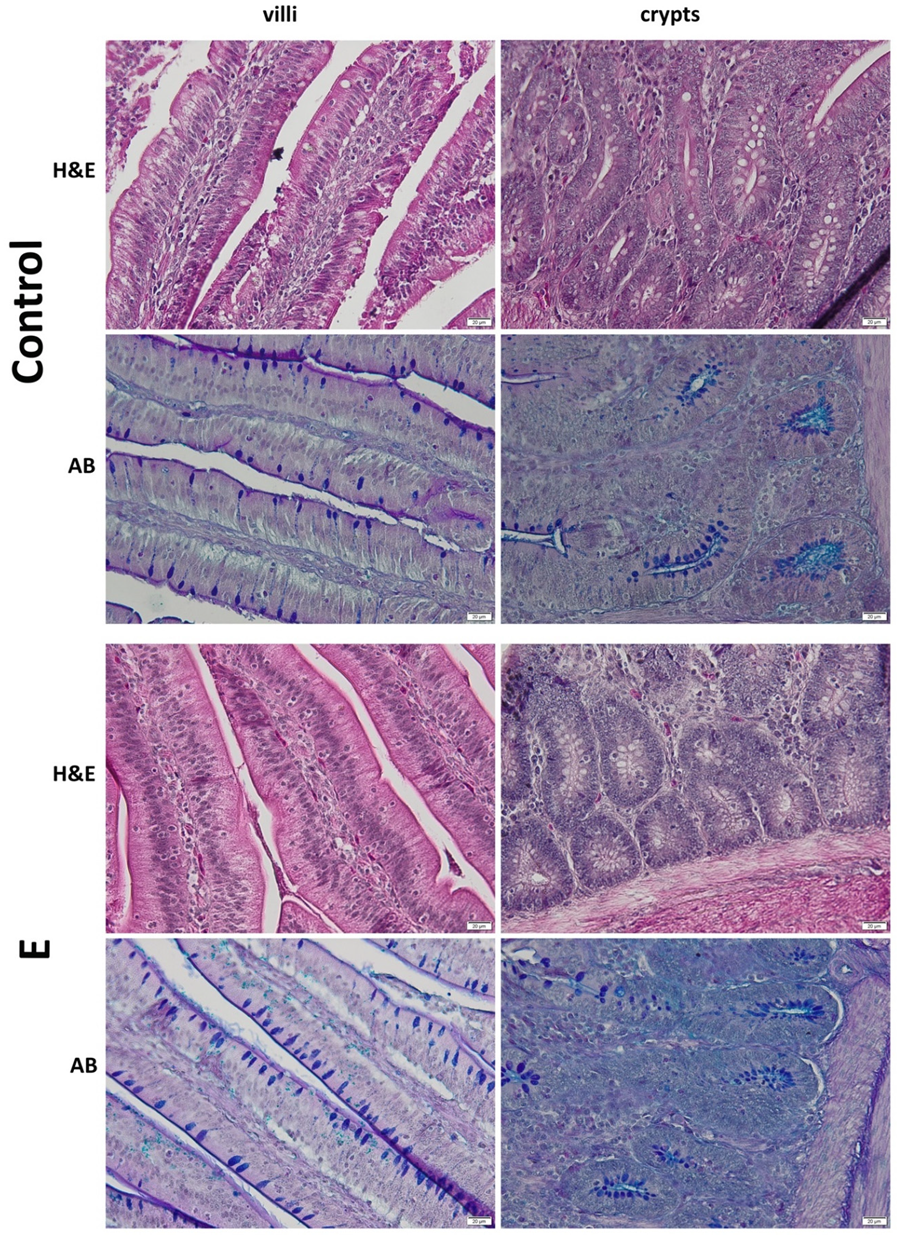
3.3. Histology of the Duodenum
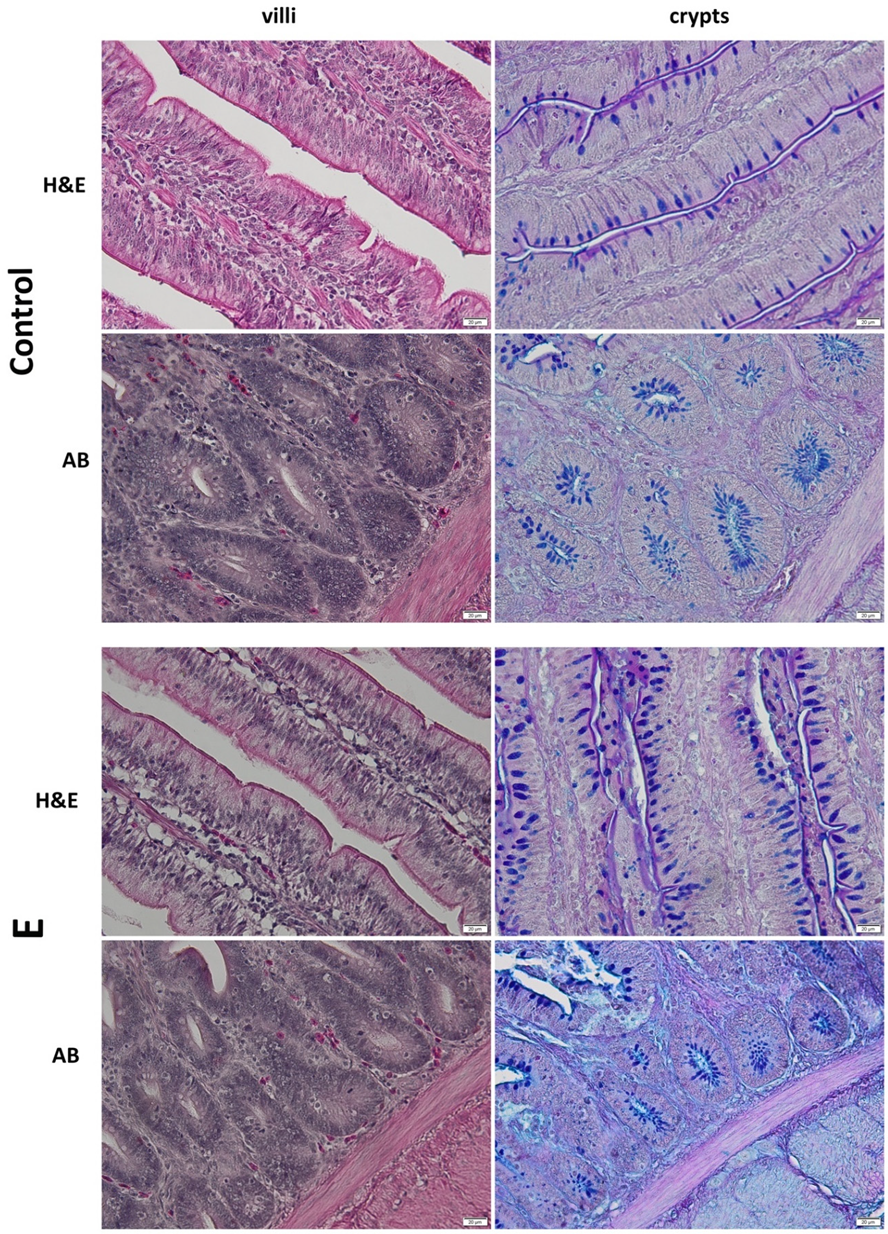
3.4. Histology of the Jejunum
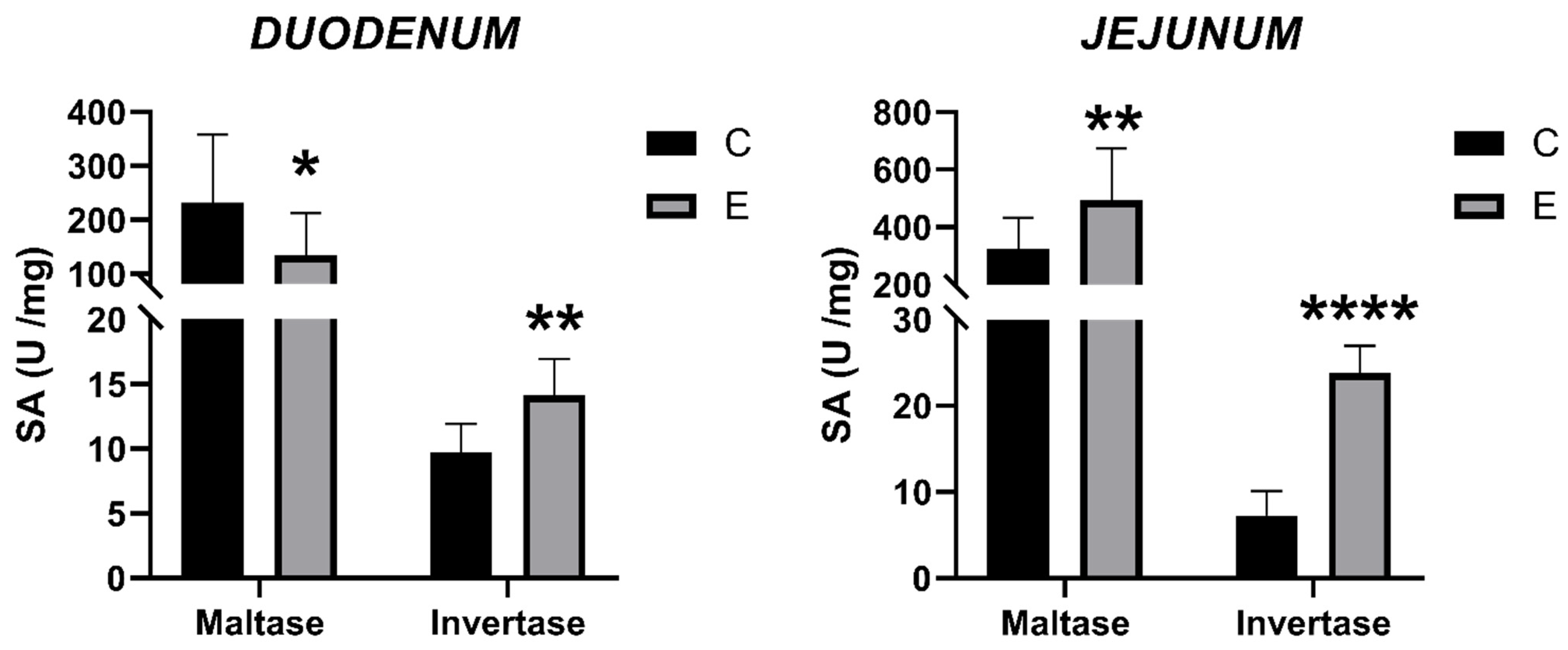
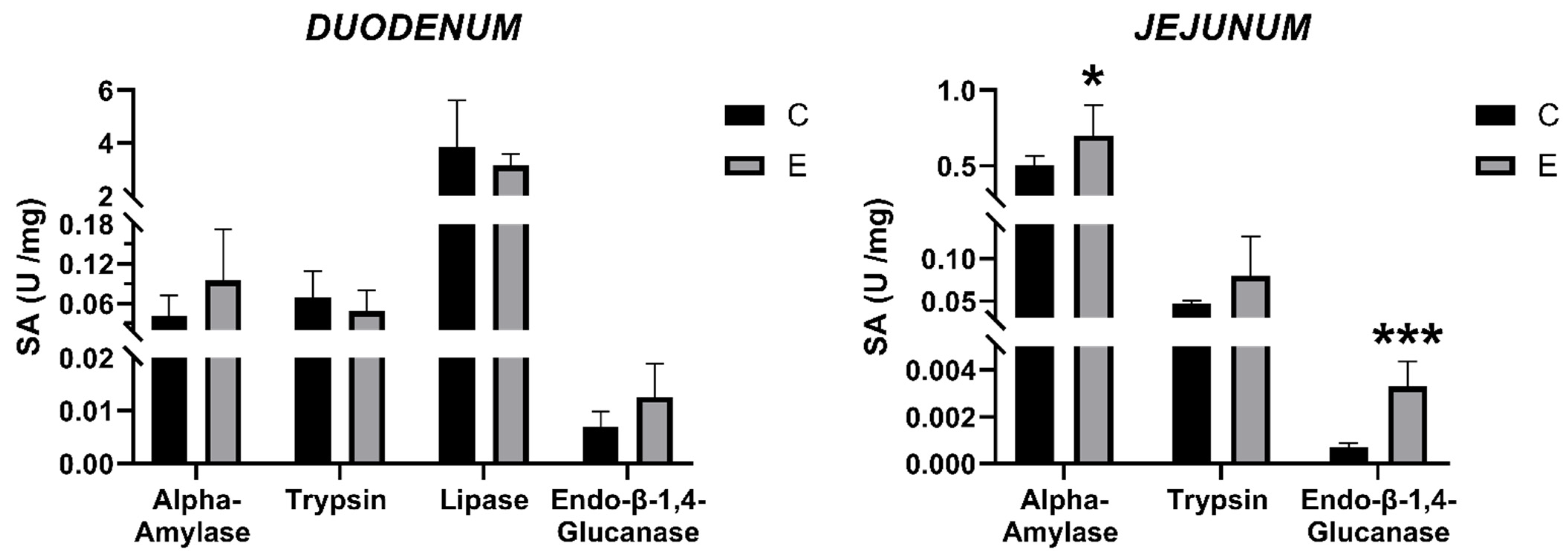
3.5. Intestinal Enzyme Activities
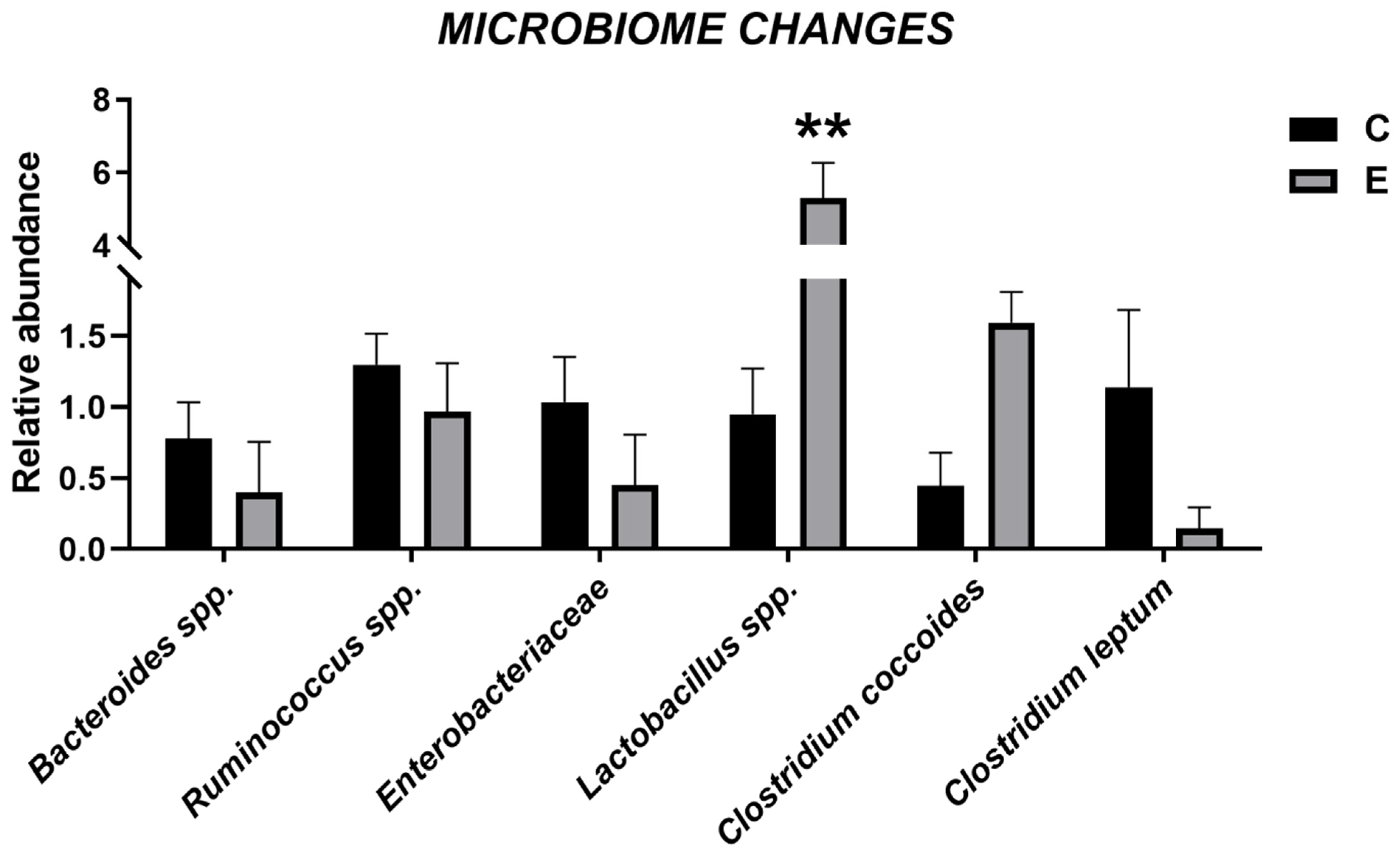
3.6. Intestinal Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Available online: https://www.statista.com/statistics/237637/production-of-poultry-meat-worldwide-since-1990/ (accessed on 26 March 2021).
- Wong, J.T.; Bruyn, J.; De Bagnol, B.; Grieve, H.; Li, M.; Pym, R.; Alders, R.G. Small-scale poultry and food security in resource-poor settings: A review. Glob. Food Sec. 2017, 15, 43–52. [Google Scholar] [CrossRef]
- Barton, M.D. Antibiotic use in animal feed and its impact on human health. Nutr. Res. Rev. 2021, 61, 279–299. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, Y.; Zhang, L.; Wu, Z.; Huang, Y.; Yan, H.; Zhong, J.; Wang, L.J.; Abdullah, H.M.; Wang, H.H. Antibiotic Administration Routes and Oral Exposure to Antibiotic Resistant Bacteria as Key Drivers for Gut Microbiota Disruption and Resistome in Poultry. Front. Microbiol. 2020, 11, 1319. [Google Scholar] [CrossRef]
- Hassan, M.A.; Masud, I.A. An overview of the hepatoprotective potentials of Phyllanthus amarus. J. Pharmacogn. Phytochem. 2018, 7, 2777–2782. [Google Scholar]
- Svihus, B. Function of the digestive system. In Informal Nutrition Symposium. J. Appl. Poult. Res. 2014, 23, 306–314. [Google Scholar] [CrossRef]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Breeding for efficiency in the broiler chicken: A review. Agron. Sustain. Dev. 2016, 36, 66. [Google Scholar] [CrossRef] [Green Version]
- Shafey, T.M.; Mahmoud, A.H.; Hussein, E.; Suliman, G.; Shafey, T.M.; Mahmoud, A.H.; Hussein, E.; Suliman, G. The Performance and Characteristics of Carcass and Breast Meat of Broiler Chickens Fed Diets Containing Flaxseed Meal chickens fed diets containing flaxseed meal. Ital. J. Anim. Sci. 2016, 13, 3514. [Google Scholar] [CrossRef] [Green Version]
- Sugiharto, S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 2016, 15, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Adedokun, S.A.; Olojede, O.C. Optimizing Gastrointestinal Integrity in Poultry: The Role of Nutrients and Feed Additives. Front. Vet. Sci. 2019, 5, 348. [Google Scholar] [CrossRef] [Green Version]
- Popescu, R.G.; Voicu, S.N.; Pircalabioru, G.G.; Gharbia, S.; Hermenean, A.; Georgescu, S.E.; Panaite, T.D. Effects of Dietary Inclusion of Bilberry and Walnut Leaves Powder on the Digestive Performances and Health of Tetra SL Laying Hens. Animals 2020, 10, 823. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R. The role of exogenous enzymes in promoting growth and improving nutrient digestibility in poultry. Iran. J. Vet. Res. 2018, 19, 157–164. [Google Scholar]
- Recoules, E.; Lessire, M.; Labas, V.; Duclos, M.J.; Lardic, L. Digestion dynamics in broilers fed rapeseed meal. Sci. Rep. 2019, 28, 3052. [Google Scholar] [CrossRef] [Green Version]
- Alshamy, Z.; Richardson, K.C.; Hu, H.; Hafez, H.M. Comparison of the gastrointestinal tract of a dual-purpose to a broiler chicken line: A qualitative and quantitative macroscopic and microscopic study. PLoS ONE 2018, 13, e0204921. [Google Scholar] [CrossRef] [Green Version]
- Lan, Y.; Verstegen, M.W.A.; Tamminga, S.; Williams, B.A. The role of the commensal gut microbial. Worlds Poult. Sci. J. 2005, 61, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cencic, A.; Chingwaru, W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Lutful Kabir, S.M. The role of probiotics in the poultry industry. Int. J. Mol. Sci. 2009, 10, 3531–3546. [Google Scholar] [CrossRef]
- Král, M.; Angelovi, M.; Mrázová, Ľ. Application of Probiotics in Poultry Production. Anim. Sci. Biotechnol. 2012, 45, 55–57, ISSN 2344-4576. [Google Scholar]
- Jha, R.; Das, R.; Oak, S.; Mishra, P. Probiotics (Direct-Fed Microbials) in Poultry Nutrition and Their Effects on Nutrient Utilization, Growth and Laying Performance, and Gut Health: A Systematic Review. Animals 2020, 10, 1863. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Stevanović, Z.D.; Bošnjak-Neumüller, J.; Pajić-Lijaković, I.; Raj, J.; Vasiljević, M. Essential Oils as Feed Additives-Future Perspectives. Molecules 2018, 23, 1717. [Google Scholar] [CrossRef] [Green Version]
- Gheisar, M.M.; Kim, I.H. Phytobiotics in poultry and swine nutrition—A review. Ital. J. Anim. Sci. 2018, 17, 92–99. [Google Scholar] [CrossRef]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed-a potential functional food source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Cherian, G.; Quezada, N. Egg quality, fatty acid composition and immunoglobulin Y content in eggs from laying hens fed full fat camelina or flax seed. J. Anim. Sci. Biotechnol. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirmohammadi, A.; Khalaji, S.; Yari, M. Effects of Linseed Expansion on its Dietary Molecular Structures, and on Broiler Chicks Digestive Enzymes Activity, Serum Metabolites, and Ileal Morphology. J. Appl. Poult. Res. 2019, 28, 997–1012. [Google Scholar] [CrossRef]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Wiesenfeld, P.W.; Babu, U.S.; Bryant, M.; Flynn, T.J.; Ruggles, D.I. Effects of flaxseed and defatted flaxseed meal on reproduction and development in rats. Food Chem. Toxicol. 2003, 41, 819–834. [Google Scholar] [CrossRef]
- Conforti, F.D.; Cachaper, K.F. Effects of selected antioxidants on physical and sensory characteristics of yeast bread containing flaxseed meal. Int. J. Consum. Stud. 2009, 33, 89–93. [Google Scholar] [CrossRef]
- Aziza, A.E.; Panda, A.K.; Quezada, N.; Cherian, G. Nutrient digestibility, egg quality, and fatty acid composition of brown laying hens fed camelina or flaxseed meal. J. Appl. Poult. Res. 2013, 22, 832–841. [Google Scholar] [CrossRef]
- Shahid, M.S.; Raza, T.; Wu, Y.; Hussain Mangi, M.; Nie, W.; Yuan, J. Comparative Effects of Flaxseed Sources on the Egg ALA Deposition and Hepatic Gene Expression in Hy-Line Brown Hens. Foods 2020, 9, 1663. [Google Scholar] [CrossRef]
- Beheshti Moghadam, M.; Cherian, G. Use of flaxseed in poultry feeds to meet the human need for n-3 fatty acids. Worlds Poult. Sci. J. 2017, 73, 803–812. [Google Scholar] [CrossRef]
- Zuidhof, M.J.; Betti, M.; Korver, D.R.; Hernandez, F.I.; Schneider, B.L.; Carney, V.L.; Renema, R.A. Omega-3-enriched broiler meat: 1. Optimization of a production system. Poult. Sci. 2009, 88, 1108–1120. [Google Scholar] [CrossRef]
- Apperson, K.D.; Cherian, G. Effect of whole flax seed and carbohydrase enzymes on gastrointestinal morphology, muscle fatty acids, and production performance in broiler chickens. Poult. Sci. 2017, 96, 1228–1234. [Google Scholar] [CrossRef]
- Raza, A.; Bashir, S.; Tabassum, R. An update on carbohydrases: Growth performance and intestinal health of poultry. Heliyon 2019, e01437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Gesquiere, I.; Greupink, R.; Keszthelyi, D.; Koskinen, M.; et al. Impact of gastrointestinal physiology on drug absorption in special populations--An UNGAP review. Eur. J. Pharm. Sci. 2020, 147, 105280. [Google Scholar] [CrossRef]
- Mir, N.A.; Tyagi, P.K.; Biswas, A.K.; Tyagi, P.K.; Mandal, A.B.; Wani, M.A.; Deo, C.; Biswas, A.; Verma, A. Performance and meat quality of broiler chicken fed a ration containing flaxseed meal and higher dietary lysine levels. J. Agric. Sci. 2018, 156, 291–299. [Google Scholar] [CrossRef]
- Untea, A.E.; Varzaru, I.; Panaite, T.D.; Gavris, T.; Lupu, A.; Ropota, M. The Effects of Dietary Inclusion of Bilberry and Walnut Leaves in Laying Hens’ Diets on the Antioxidant Properties of Eggs. Animals 2020, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Panaite, T.D.; Criste, R.D.; Olteanu, M.; Untea, A.E.; Ropota, M.; Varzaru, I.; Lupu, A. Feeding value of local phyto-additives, potential ingredients in poultry diets. Sci. Papers Ser. D Anim. Sci. 2020, LXII, 1–8, ISSN 2393-2260. [Google Scholar]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Șoica, C.; Iuga, M.; Mironeasa, S. Effects of Supplementing Grape Pomace to Broilers Fed Polyunsaturated Fatty Acids Enriched Diets on Meat Quality. Animals 2020, 10, 947. [Google Scholar] [CrossRef]
- NRC, National Research Council. Nutrient Requirements for Poultry, 9th ed.; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Bernfeld, P. Amylases: Alpha and beta methods. Enzymology 1995, 1, 149–158. [Google Scholar]
- Hummel, B.C.W. A modified spectrophotometric determination of chymotrypsin, trypsin and thrombin. Can. J. Biochem. Physiol. 1959, 37, 1393–1399. [Google Scholar] [CrossRef]
- Ahmed, I.; Zia, M.; Iqbal, H. Bioprocessing of Proximally Analyzed Wheat Straw for Enhanced Cellulase Production through Process Optimization with Trichodermaviride under SSF. IJBSE 2010, 4, 119–125. [Google Scholar]
- Lokapirnasari, W.P.; Nazar, D.S.; Nurhajati, T. Supranianondo, K.; Yulianto, A.B. Production and assay of cellulolytic enzyme activity of Enterobacter cloacae WPL 214 isolated from bovine rumen fluid waste of Surabaya abbatoir, Indonesia. Vet. World 2015, 8, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Panaite, T.D.; Criste, R.D.; Ropota, M.; Criste, V.; Vasile, G.; Olteanu, M.; Vlaicu, P.A.; Socoliuc, R.P. Determination of the feeding value of food industry by-products. Sci. Pap. Anim. Sci. Ser. Lucrări Ştiinţifice Seria Zooteh. 2016, 66, 106–111. [Google Scholar]
- Akhtar, M.N.; Mushtaq, Z.; Ahmad, N.; Khan, M.K.; Ahmad, M.H.; Hussain, A.I.; Imran, M. Optimal Ultrasound-Assisted Process Extraction, Characterization, and Functional Product Development from Flaxseed Meal Derived Polysaccharide Gum. Processes 2019, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Vlaicu, P.A.; Panaite, T.D.; Dragotoiu, D.; Ropota, M.; Bobe, E.; Olteanu, M.; Criste, R.D. Feeding quality of the meat from broilers fed with dietary food industry by-products (flaxseed, rapeseeds and buckthorn meal, grape pomace). Sci. Pap. Ser. D Anim. Sci. 2017, 60, 123–130. [Google Scholar]
- Konieczka, P.; Czauderna, M.; Smulikowska, S. The enrichment of chicken meat with omega-3 fatty acids by dietary fish oil or its mixture with rapeseed or flaxseed-Effect of feeding duration: Dietary fish oil, flaxseed, and rapeseed and n-3 enriched broiler meat. Anim. Feed Sci. Technol. 2017, 223, 42–52. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Magowan, E.; Hollmann, M.; Ball, M.E.E.; Molnar, A.; Witter, K.; Ertl, R.; Hawken, R.J.; Lawlor, P.G.; O’Connell, N.E.; et al. Differences in intestinal size, structure and function contributing to feed efficiency in broiler chickens reared at geographically distant locations. Poultry Sci. 2018, 97, 578–591. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best practice & research. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Adabi, S.G.; Hajibabaei, A.; Casey, N.H.; Bayraktaglu, A.G. The effect of various dietary vegetable oil sources on villi morphology and liver aldehydes in young layers. S. Afr. J. Anim. Sci. 2016, 46, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Barboza, P.S.; Parker, K.L.; Hume, I.D. Chapter 5. Digestive Function. In Integrative Wildlife Nutrition, 1st ed.; Barboza, P.S., Parker, K.L., Hume, I.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 73–93. [Google Scholar] [CrossRef]
- Ciminari, E.; Chediack, J.G. Activity of Digestive Enzymes in Chicken’s Small Intestine and Caeca: Effect of Dietary Protein and Carbohydrate Content. Asian J. Poult. Sci. 2014, 8, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Hooton, D.; Lentle, R.; Monro, J.; Wickham, M.; Simpson, R. The Secretion and Action of Brush Border Enzymes in the Mammalian Small Intestine. Rev. Physiol. Biochem. Pharmacol. 2015, 168, 59–118. [Google Scholar] [CrossRef] [PubMed]
- Dida, M.F. Review Paper on Enzyme Supplementation in Poultry Ration. IJBC 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Wooten, M.W.; Wrenn, R.W. Linoleic Acid is a potent activator of protein kinase C type III-α isoforms in pancreatic acinar cells; its role in amylase secretion. Biochem. Biophys. Res. Commun. 1998, 153, 67–73. [Google Scholar] [CrossRef]
- Su, C.-H.; Hsu, C.-H.; Ng, L.-T. Inhibitory potential of fatty acids on key enzymes to types 2 diabetes. Biofactors 2013, 39, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Al-Harthi, M.A.; Abo El-Maaty, H.M. The effects of Different Oil sources on Performance, Digestive Enzymes, Immunological, Antioxidant and Morphometric Responses of Broiler Chicks. Front. Vet. Sci. 2020, 7, 181. [Google Scholar] [CrossRef]
- Hu, Y.D.; Lan, D.; Zhu, Y.; Pang, H.Z.; Mu, X.P.; Hu, X.F. Effect of diets with different energy and lipase levels on performance, digestibility and carcass trait in broilers. Asian-Australas. J. Anim. Sci. 2018, 31, 1275–1284. [Google Scholar] [CrossRef] [Green Version]
- Baião, N.C.; Lara, L.J.C. Oil and Fat in Broiler Nutrition. Braz. J. Poultry Sci. 2005, 7, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Couëdelo, L.; Boué-Vaysse, C.; Fonseca, L.; Montesinos, E.; Djoukitch, S.; Combe, N.; Cansell, M. Lymphatic absorption of α-linolenic acid in rats fed flaxseed oil-based emulsion. Br. J. Nutr. 2011, 105, 1026–1035. [Google Scholar] [CrossRef] [Green Version]
- Polycarpo, G.V.; Cruz, V.C.; Alexandre, N.C.; Fascina, V.B.; Souza, I.M.G.P.; Cravo, J.C.M.; Albuquerque, R.; Sartori, J.R.; Pezzato, A.C. Effect of lipid sources and inclusion levels in diets for broiler chickens. Arq. Bras. Med. Vet. Zootec. 2014, 66, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Rajarammohan, S.; Thakur, R.; Hassan, M. Linear and branched β-Glucans degrading enzymes from versatile Bacteroides uniformis JCM 13288T and their roles in cooperation with gut bacteria. Gut Microbes 2020, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.M. Review: The structure and function of cellulase (endo-β-1,4-glucanase) and hemicellulase (β-1,3-glucanase and endo-β-1,4-mannase) enzymes in invertebrates that consume materials ranging from microbes, algae to leaf litter. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2020, 240, 110354. [Google Scholar] [CrossRef]
- Knaus, U.G.; Hertzberger, R.; Pircalabioru, G.G.; Yousefi, S.P.; Branco Dos Santos, F. Pathogen control at the intestinal mucosa—H2O2 to the rescue. Gut Microbes 2017, 8, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Growth of probiotic lactobacilli in the presence of oleic acid enhances subsequent survival in gastric juice. Microbiology 2007, 153, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamar, G.; Santamarina, A.B.; Dias, G.C.; Masquio, D.C.L.; de Rosso, V.V.; Pisani, L.P. Relationship between fatty acids intake and Clostridium coccoides in obese individuals with metabolic syndrome. Food Res. Int. 2018, 113, 86–92. [Google Scholar] [CrossRef]
- Parolini, C. Effects of Fish n-3 PUFAs on Intestinal Microbiota and Immune System. Mar. Drugs. 2019, 17, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Immunoregulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Braz. J. Med. Biol. Res. 1998, 31, 467–490. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M. A Review of the Role of Gut microbiome in Obesity. E3S Web Conf. 2020, 218, 03010. [Google Scholar] [CrossRef]
- Respondek, F.; Gerard, P.; Bossis, M.; Boschat, L.; Bruneau, A.; Rabot, S.; Wagner, A.; Martin, J.C. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic paramet;rs of humanized gnotobiotic diet induced obesity mice. PLoS ONE 2013, 8, e71026. [Google Scholar] [CrossRef] [PubMed]
| Taxonomic Target | Primer Sequence |
|---|---|
| Eubacteria | UniF340 ACT CCT ACG GGA GGC AGC AGT UniR514 ATT ACC GCG GCT GCT GGC |
| Ruminococcus | Fwd ACTGAGAGGTTGAACGGCCA Rev CCTTTACACCCAGTAATTCCGGA |
| Enterobacteriaceae | Uni515F GTG CCA GCM GCC GCG GTAA Ent826R GCC TCA AGG GCA CAA CCT CCA AG |
| Clostridium leptum | Fwd GCACAAGCAGTGGAGT Rev CTTCCTCCGTTTTGTCAA |
| Clostridium cocoides | Fwd GAC GCC GCG TGA AGG A Rev AGC CCC AGC CTT TCA CAT C |
| Bacteroides | Fwd CCT ACG ATG GAT AGG GGT T Rev CAC GCT ACT TGG CTG GTT CAG |
| Lactobacilli | LabF362 ACG AGT AGG GAA ATC TTC CA LabR677 CAC CGC TAC ACA TGG AG |
| Specification | Flaxseed Meal |
|---|---|
| Proximate chemical composition (%) | |
| Dry matter, % | 91.06 ± 0.438 |
| Organic matter, % | 85.92 ± 0.354 |
| Crude protein, % | 33.29 ± 1.570 |
| Gross fat, % | 13.90 ± 0.438 |
| Fiber, % | 9.62 ± 0.028 |
| Ash, % | 5.15 ± 0.092 |
| Fatty acid profoiles (g % of total fat) | |
| Miristic acid, (C14:0) | 0.08 ± 0.007 |
| Pentadecanoic acid, (C15:0) | 0.17 ± 0.028 |
| Palmitic acid, (C16:0) | 4.69 ± 0.403 |
| Palmitoleic acid, (C16:1) | 0.10 ± 0.021 |
| Heptadecanoic acid, (C17:0) | 0.36 ± 0.035 |
| Stearic acid, (C18:0) | 2.49 ± 0.265 |
| Oleic acid, (C18:1) | 18.29 ± 0.537 |
| Linoleic acid, (C18:2n6) | 16.83 ± 0.576 |
| Arachidic acid, (C20:0) | 0.14 ± 0.028 |
| Linolenic γ acid, (C 18:3n6) | 0.21 ± 0.014 |
| Linolenic acid, (C18:3n3) | 55.93 ± 0.539 |
| Octadecatetraenoic acid, (C18:4n3) | 0.14 ± 0.010 |
| Eicosatrienoic acid, (C20:2n6) | 0.13 ± 0.027 |
| Eicosatrienoic acid, (C20:3n6) | 0.06 ± 0.021 |
| Eicosatrienoic acid, (C20:3n3) | 0.05 ± 0.014 |
| Arachidonic acid, (C20:4n6) | 0.11 ± 0.010 |
| Docosadienoic acid, (C22:2n6) | 0.09 ± 0.014 |
| Docosatetraenoic acid, (C22:4n6) | 0.10 ± 0.014 |
| Other fatty acids | 0.24 ± 0.069 |
| SFAs | 7.92 ± 0.120 |
| MUFAs | 18.39 ± 0.516 |
| PUFAs | 73.65 ± 1.089 |
| n3 | 56.12 ± 0.559 |
| n6 | 17.53 ± 0.940 |
| n6/n3 | 0.31 ± 0.028 |
| Parameter | Experimental Diet 1 | p-Value | |
|---|---|---|---|
| C | E | ||
| Initial body weight (14 days), g | 506.71 ± 29.70 | 506.53 ± 51.73 | 0.9852 |
| Final body weight (42 day), g | 3163.45 ± 315.84 | 3136.67 ± 346.47 | 0.7534 |
| Body weight gain, g | 2656.75 ± 309.96 | 2630.12 ± 363.60 | 0.7166 |
| Average daily gain, g | 91.61 ± 10.69 | 90.69 ± 12.54 | 0.7189 |
| Averahe daily feed intake, g | 150.96 ± 34.19 | 148.54 ± 30.37 | 0.7767 |
| Feed/gain ratio | 1.65 ± 0.44 | 1.64 ± 0.40 | 0.9680 |
| Parameter | Dietary Treatment | ||
|---|---|---|---|
| Control | E | p-Value | |
| Villus height (µm) | 848 ± 33.2 | 1148 ± 29.1 *** | 1.01 × 10−12 |
| Crypt depth (µm) | 145 ± 7.4 | 164 ± 4.6 *** | 1.90 × 10−5 |
| Villus height/crypt depth | 5.9 ± 0.4 | 7.0 ± 0.3 *** | 1.18 × 10−5 |
| Parameter | Dietary Treatment | ||
|---|---|---|---|
| Control | E | p-Value | |
| Villus height (µm) | 935 ± 25.6 | 1251 ± 14.4 *** | 1.72 × 10−13 |
| Crypt depth (µm) | 152 ± 7.4 | 174 ± 3.5 *** | 5.45 × 10−6 |
| Villus height/crypt depth | 6.2 ± 0.3 | 7.2 ± 0.2 *** | 3.95 × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.G.; Voicu, S.N.; Gradisteanu Pircalabioru, G.; Gharbia, S.; Hermenean, A.; Georgescu, S.E.; Panaite, T.D.; Turcu, R.P.; Dinischiotu, A. Impact of Dietary Supplementation of Flaxseed Meal on Intestinal Morphology, Specific Enzymatic Activity, and Cecal Microbiome in Broiler Chickens. Appl. Sci. 2021, 11, 6714. https://doi.org/10.3390/app11156714
Popescu RG, Voicu SN, Gradisteanu Pircalabioru G, Gharbia S, Hermenean A, Georgescu SE, Panaite TD, Turcu RP, Dinischiotu A. Impact of Dietary Supplementation of Flaxseed Meal on Intestinal Morphology, Specific Enzymatic Activity, and Cecal Microbiome in Broiler Chickens. Applied Sciences. 2021; 11(15):6714. https://doi.org/10.3390/app11156714
Chicago/Turabian StylePopescu, Roua Gabriela, Sorina Nicoleta Voicu, Gratiela Gradisteanu Pircalabioru, Sami Gharbia, Anca Hermenean, Sergiu Emil Georgescu, Tatiana Dumitra Panaite, Raluca Paula Turcu, and Anca Dinischiotu. 2021. "Impact of Dietary Supplementation of Flaxseed Meal on Intestinal Morphology, Specific Enzymatic Activity, and Cecal Microbiome in Broiler Chickens" Applied Sciences 11, no. 15: 6714. https://doi.org/10.3390/app11156714
APA StylePopescu, R. G., Voicu, S. N., Gradisteanu Pircalabioru, G., Gharbia, S., Hermenean, A., Georgescu, S. E., Panaite, T. D., Turcu, R. P., & Dinischiotu, A. (2021). Impact of Dietary Supplementation of Flaxseed Meal on Intestinal Morphology, Specific Enzymatic Activity, and Cecal Microbiome in Broiler Chickens. Applied Sciences, 11(15), 6714. https://doi.org/10.3390/app11156714











