A Multi-Nuclear MAS-NMR Study on the Structural Properties of Silicalite-1 Zeolite Synthesized Using N- and P-Based Organic Structure Directing Agents
Abstract
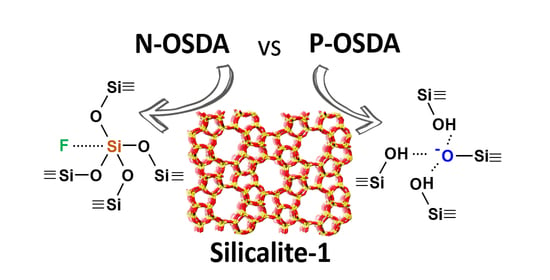
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Silicalite-1 Zeolites
2.2. Characterization Techniques
3. Results and Discussion
3.1. Synthesis of Silicalite-1 Zeolites
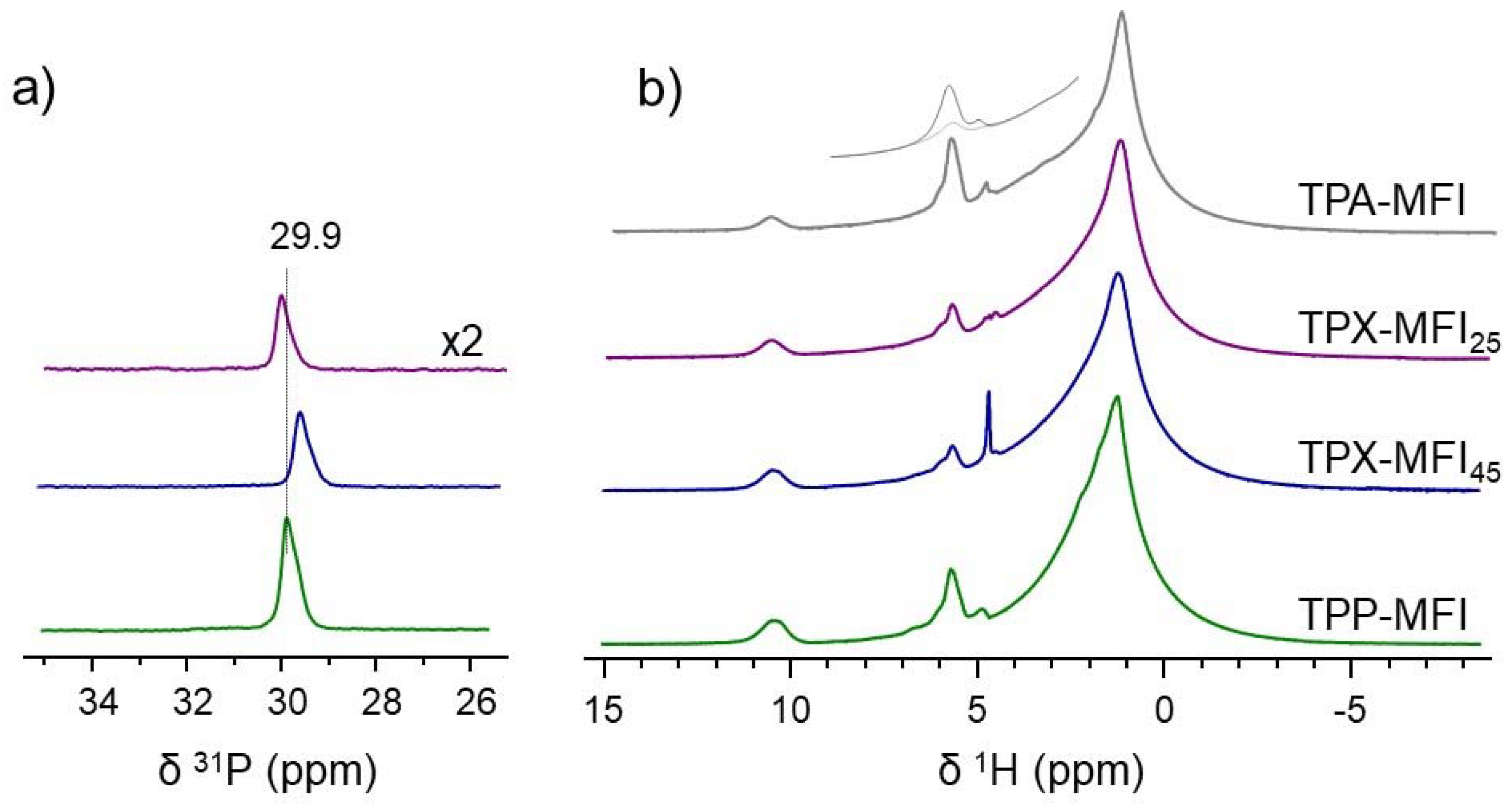
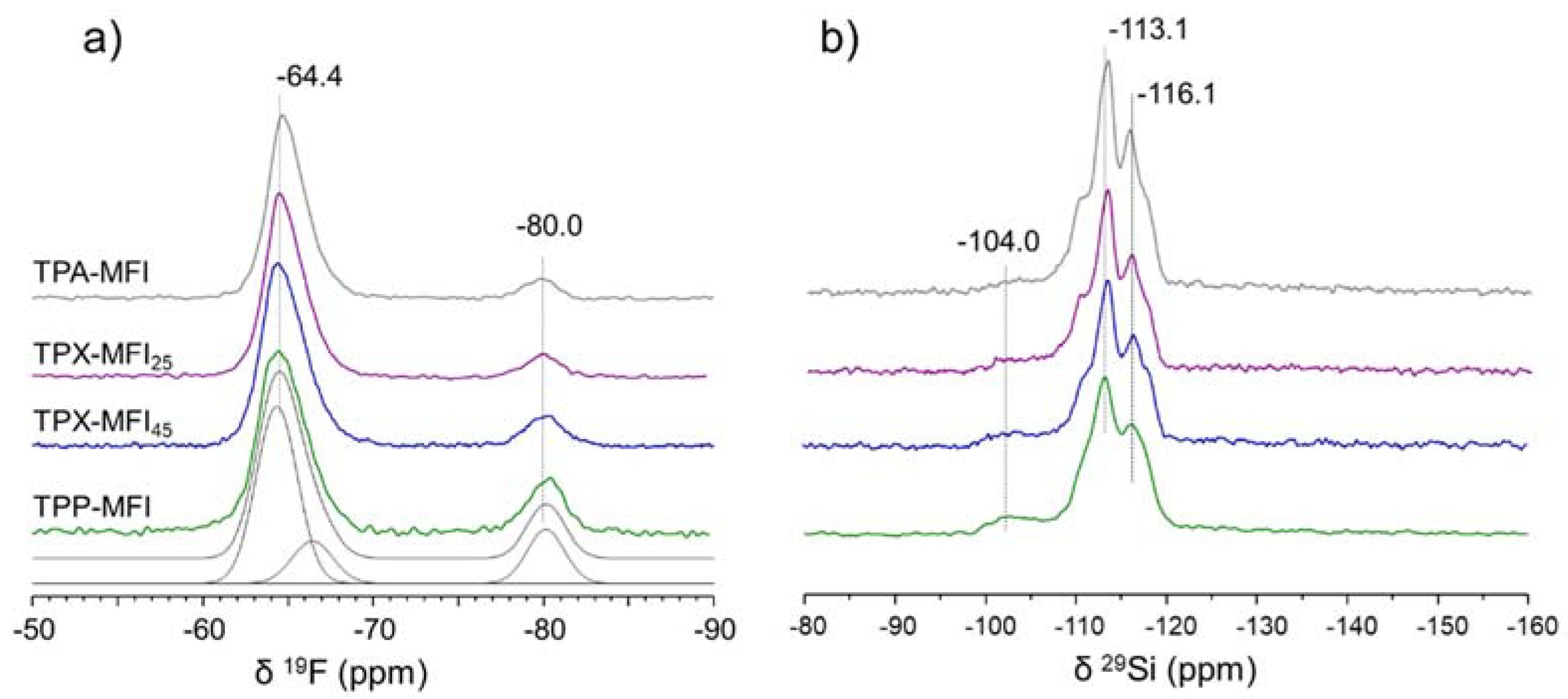
3.2. Structural Characterization of Silicalite-1 Zeolites by MAS NMR Spectroscopy
3.2.1. Characterization of the Framework and Guest Species
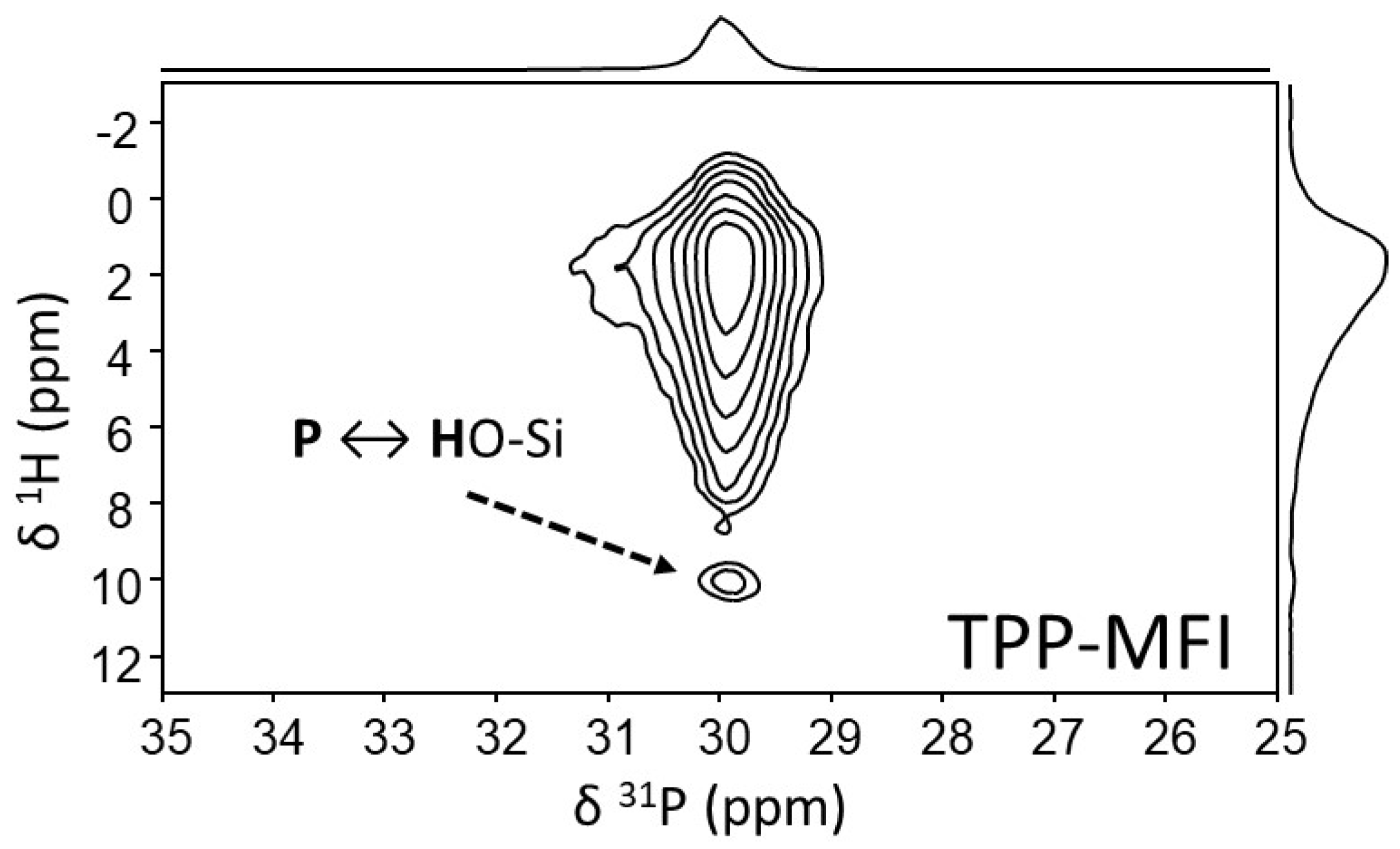
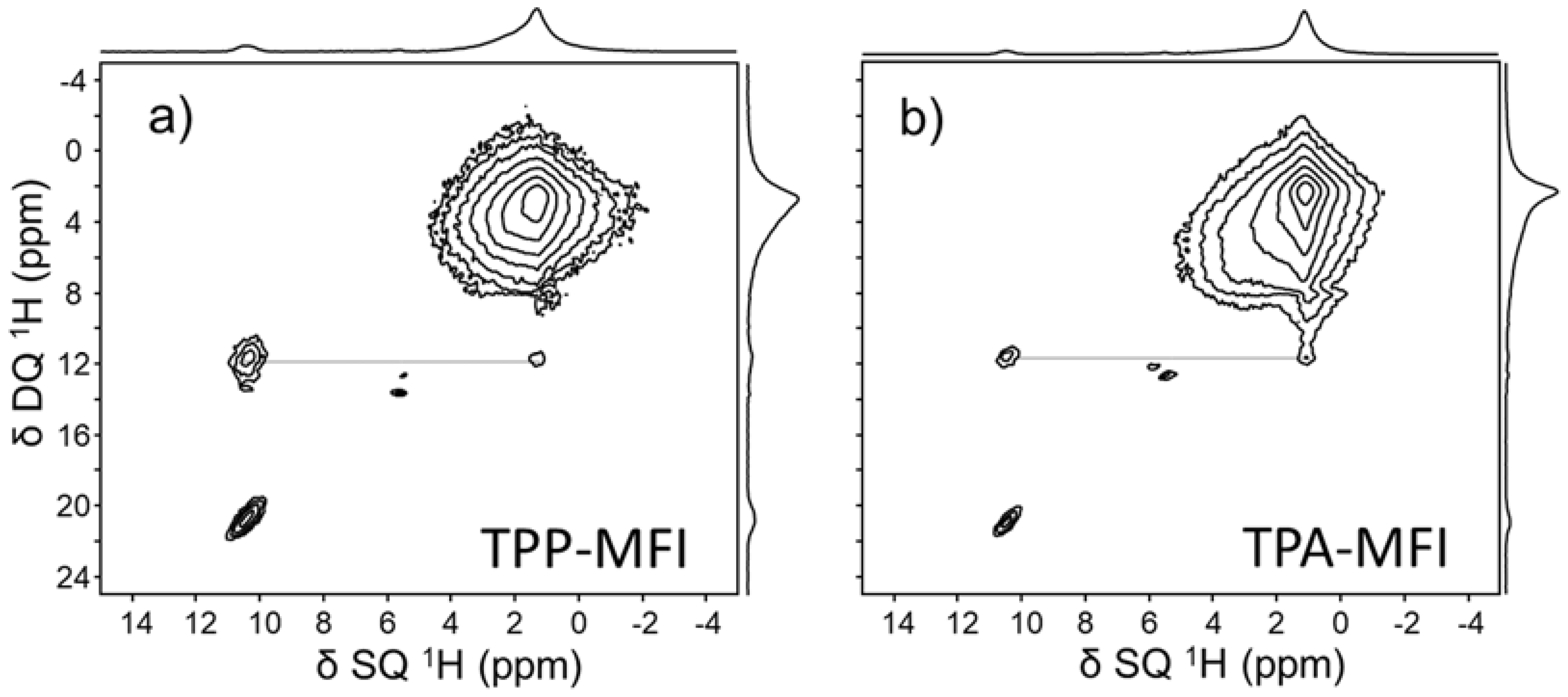
3.2.2. Characterization of the Interactions OSDA-Zeolite by Bidimensional NMR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrer, R.M. Zeolites and Clay Minerals as Sorbents and Molecular Sieves; Academic Press: London, UK; New York, NY, USA, 1978. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; Wiley: New York, NY, USA; London, UK, 1973. [Google Scholar]
- Flanigen, E.M. Zeolites and molecular sieves: An historical perspective. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001; Chapter 2; Volume 137, pp. 11–35. [Google Scholar]
- Cejka, J.; Corma, A.; Zones, S. Zeolites and Catalysis: Synthesis, Reactions and Applications; John Wiley & Sons: Weinheim, Germany, 2010. [Google Scholar]
- Millini, R.; Zou, X.; Strohmaier, K.; Schwieger, W.; Eliasova, P.; Morris, R.E.; Weckhuysen, B.; Zhou, W.; Abdo, S.; Martinez, A. Zeolites in Catalysis: Properties and Applications; Royal Society of Chemistry: London, UK, 2017. [Google Scholar]
- Davis, M.E. Zeolites from a Materials Chemistry Perspective. Chem. Mater. 2014, 26, 239–245. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Palomino, M.; Valencia, S.; Rey, F. Zeolites and Other Adsorbents. In Nanoporous Materials for Gas Storage; Kaneko, K., Rodríguez-Reinoso, F., Eds.; Springer: Singapore, 2019; pp. 173–208. [Google Scholar]
- Zhang, S.; Pang, L.; Chen, Z.; Ming, S.; Dong, Y.; Liu, Q.; Liu, P.; Cai, W.; Li, T. Cu/SSZ-13 and Cu/SAPO-34 catalysts for deNOx in diesel exhaust: Current status, challenges, and future perspectives. Appl. Catal. A Gen. 2020, 607, 117855. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 30 April 2021).
- Corma, A. Inorganic Solid Acids and Their Use in Acid-Catalyzed Hydrocarbon Reactions. Chem. Rev. 1995, 95, 559–614. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Martínez, A. Zeolites in refining and petrochemistry. Stud. Surf. Sci. Catal. 2005, 157, 337–366. [Google Scholar]
- Vermeiren, W.; Gilson, J.-P. Impact of Zeolites on the Petroleum and Petrochemical Industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Saab, R.; Polychronopoulou, K.; Zheng, L.; Kumar, S.; Schiffer, A. Synthesis and performance evaluation of hydrocracking catalysts: A review. J. Ind. Eng. Chem. 2020, 89, 83–103. [Google Scholar] [CrossRef]
- Barrer, R.M. Hydrothermal Chemistry of Zeolites; Academic Press: London, UK; New York, NY, USA, 1982. [Google Scholar]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater. 2005, 82, 1–78. [Google Scholar] [CrossRef]
- Grand, J.; Awala, H.; Mintova, S. Mechanism of zeolites crystal growth: New findings and open questions. CrystEngComm 2016, 18, 650–664. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Patton, R.L. Silica Polymorph and Process for Preparing Same. U.S. Patent No. 4,073,865, 14 February 1978. [Google Scholar]
- Guth, J.L.; Kessler, H.; Wey, R. New Route to Pentasil-Type Zeolites Using a Non Alkaline Medium in the Presence of Fluoride Ions. In Studies in Surface Science and Catalysis; Murakami, Y., Iijima, A., Ward, J.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 121–128. [Google Scholar]
- Camblor, M.A.; Corma, A.; Valencia, S. Spontaneous nucleation and growth of pure silica zeolite-β free of connectivity defects. Chem. Commun. 1996, 20, 2365–2366. [Google Scholar] [CrossRef]
- Camblor, M.Á.; Villaescusa, L.A.; Díaz-Cabañas, M.J. Synthesis of all-silica and high-silica molecular sieves in fluoride media. Top. Catal. 1999, 9, 59–76. [Google Scholar] [CrossRef] [Green Version]
- Sastre, G.; Vidal-Moya, J.A.; Blasco, T.; Rius, J.; Jorda, J.L.; Navarro, M.T.; Rey, F.; Corma, A. Preferential Location of Ge Atoms in Polymorph C of Beta Zeolite (ITQ-17) and Their Structure-Directing Effect: A Computational, XRD, and NMR Spectroscopic Study. Angew. Chem. Int. Ed. 2002, 41, 4722–4726. [Google Scholar] [CrossRef]
- Lobo, R.F.; Zones, S.I.; Davis, M.E. Structure-Direction in Zeolite Synthesis. Top. Incl. Sci. 1995, 21, 47–78. [Google Scholar] [CrossRef]
- Burton, A.W.; Zones, S.I. Organic Molecules in Zeolite Synthesis: Their Preparation and Structure-Directing Effects. In Introduction to Zeolite Science and Practice, 3rd ed.; Cejka, J., van Bekkum, H., Corma, A., Schüth, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 137–179. [Google Scholar]
- Moliner, M.; Rey, F.; Corma, A. Towards the Rational Design of Efficient Organic Structure-Directing Agents for Zeolite Synthesis. Angew. Chem. Int. Ed. 2013, 52, 13880–13889. [Google Scholar] [CrossRef]
- Rey, F.; Simancas, J. Beyond Nitrogen OSDAs. In Insights into the Chemistry of Organic Structure-Directing Agents in the Synthesis of Zeolitic Materials; Gómez-Hortigüela, L., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 103–138. [Google Scholar]
- Burton, A. Recent trends in the synthesis of high-silica zeolites. Catal. Rev. 2018, 60, 132–175. [Google Scholar] [CrossRef]
- Corma, A.; Diaz-Cabanas, M.J.; Jorda, J.L.; Rey, F.; Sastre, G.; Strohmaier, K.G. A Zeolitic Structure (ITQ-34) with Connected 9- and 10-Ring Channels Obtained with Phosphonium Cations as Structure Directing Agents. J. Am. Chem. Soc. 2008, 130, 16482–16483. [Google Scholar] [CrossRef] [PubMed]
- Dorset, D.L.; Kennedy, G.J.; Strohmaier, K.G.; Diaz-Cabañas, M.J.; Rey, F.; Corma, A. P-Derived Organic Cations as Structure-Directing Agents: Synthesis of a High-Silica Zeolite (ITQ-27) with a Two-Dimensional 12-Ring Channel System. J. Am. Chem. Soc. 2006, 128, 8862–8867. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.H.; Jorda, J.L.; Rey, F.; Corma, A. Synthesis and Structure Determination of a New Microporous Zeolite with Large Cavities Connected by Small Pores. J. Am. Chem. Soc. 2012, 134, 13232–13235. [Google Scholar] [CrossRef] [PubMed]
- Simancas, R.; Dari, D.; Velamazán, N.; Navarro, M.T.; Cantín, A.; Jordá, J.L.; Sastre, G.; Corma, A.; Rey, F. Modular Organic Structure-Directing Agents for the Synthesis of Zeolites. Science 2010, 330, 1219. [Google Scholar] [CrossRef] [PubMed]
- Simancas, R.; Jordá, J.L.; Rey, F.; Corma, A.; Cantín, A.; Peral, I.; Popescu, C. A New Microporous Zeolitic Silicoborate (ITQ-52) with Interconnected Small and Medium Pores. J. Am. Chem. Soc. 2014, 136, 3342–3345. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Hernández, M.; Wan, W.; Zou, X.; Jordá, J.L.; Cantín, A.; Rey, F.; Corma, A. The first zeolite with a tri-directional extra-large 14-ring pore system derived using a phosphonium-based organic molecule. Chem. Commun. 2015, 51, 7602–7605. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Yamanaka, N.; Tsunoji, N.; Sadakane, M.; Sano, T. Facile Synthesis of AEI Zeolites by Hydrothermal Conversion of FAU Zeolites in the Presence of Tetraethylphosphonium Cations. Chem. Lett. 2014, 43, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Degnan, T.; Chitnis, G.; Schipper, P.H. History of ZSM-5 fluid catalytic cracking additive development at Mobil. Microporous Mesoporous Mater. 2000, 35, 245–252. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [Green Version]
- Blasco, T.; Corma, A.; Martínez-Triguero, J. Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition. J. Catal. 2006, 237, 267–277. [Google Scholar] [CrossRef]
- Bleken, F.L.; Chavan, S.; Olsbye, U.; Boltz, M.; Ocampo, F.; Louis, B. Conversion of methanol into light olefins over ZSM-5 zeolite: Strategy to enhance propene selectivity. Appl. Catal. A Gen. 2012, 447–448, 178–185. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, S.; Wei, Y.; Li, J.; Wang, J.; Zhang, W.; Gao, S.; Liu, Z. Changing the balance of the MTO reaction dual-cycle mechanism: Reactions over ZSM-5 with varying contact times. Chin. J. Catal. 2016, 37, 1413–1422. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Control of the Al Distribution in the Framework of ZSM-5 Zeolite and Its Evaluation by Solid-State NMR Technique and Catalytic Properties. J. Phys. Chem. C 2015, 119, 15303–15315. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Biligetu, T.; Wang, Y.; Tatsumi, T. Unique Al Distribution in the MFI Framework and Its Impact on Catalytic Properties. Chem. Lett. 2017, 46, 798–800. [Google Scholar] [CrossRef]
- Biligetu, T.; Wang, Y.; Nishitoba, T.; Otomo, R.; Park, S.; Mochizuki, H.; Kondo, J.N.; Tatsumi, T.; Yokoi, T. Al distribution and catalytic performance of ZSM-5 zeolites synthesized with various alcohols. J. Catal. 2017, 353, 1–10. [Google Scholar] [CrossRef]
- Dedecek, J.; Balgová, V.; Pashkova, V.; Klein, P.; Wichterlova, B. Synthesis of ZSM-5 Zeolites with Defined Distribution of Al Atoms in the Framework and Multinuclear MAS NMR Analysis of the Control of Al Distribution. Chem. Mater. 2012, 24, 3231–3239. [Google Scholar] [CrossRef]
- Pashkova, V.; Sklenak, S.; Klein, P.; Urbanova, M.; Dědeček, J. Location of Framework Al Atoms in the Channels of ZSM-5: Effect of the (Hydrothermal) Synthesis. Chem. Eur. J. 2016, 22, 3937–3941. [Google Scholar] [CrossRef] [PubMed]
- Dědeček, J.; Tabor, E.; Sklenak, S. Tuning the Aluminum Distribution in Zeolites to Increase their Performance in Acid-Catalyzed Reactions. ChemSusChem 2019, 12, 556–576. [Google Scholar] [CrossRef]
- Kaarsholm, M.; Joensen, F.; Nerlov, J.; Cenni, R.; Chaouki, J.; Patience, G.S. Phosphorous modified ZSM-5: Deactivation and product distribution for MTO. Chem. Eng. Sci. 2007, 62, 5527–5532. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, L.-L.; Li, G.-D.; Shang, Y.-S.; Zhao, X.-M.; Ma, T.; Zhang, L.-M.; Zhai, Y.; Gong, Y.-J.; Xu, J.; et al. ZSM-5 extrudates modified with phosphorus as a super effective MTP catalyst: Impact of the acidity on binder. Fuel Process. Technol. 2017, 168, 105–115. [Google Scholar] [CrossRef]
- Manrique, C.; Guzmán, A.; Solano, R.; Echavarría, A. Phosphorous-Modified Beta Zeolite and Its Performance in Vacuum Gas Oil Hydrocracking Activity. Energy Fuels 2019, 33, 3483–3491. [Google Scholar] [CrossRef]
- Van Der Bij, H.E.; Aramburo, L.R.; Arstad, B.; Dynes, J.J.; Wang, J.; Weckhuysen, B.M. Phosphatation of Zeolite H-ZSM-5: A Combined Microscopy and Spectroscopy Study. ChemPhysChem 2014, 15, 283–292. [Google Scholar] [CrossRef] [PubMed]
- van der Bij, H.E.; Weckhuysen, B.M. Phosphorus promotion and poisoning in zeolite-based materials: Synthesis, characterisation and catalysis. Chem. Soc. Rev. 2015, 44, 7406–7428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danisi, R.M.; Schmidt, J.E.; Paioni, A.L.; Houben, K.; Poplawsky, J.D.; Baldus, M.; Weckhuysen, B.M.; Vogt, E.T.C. Revealing long- and short-range structural modifications within phosphorus-treated HZSM-5 zeolites by atom probe tomography, nuclear magnetic resonance and powder X-ray diffraction. Phys. Chem. Chem. Phys. 2018, 20, 27766–27777. [Google Scholar] [CrossRef]
- de Menezes, S.C.; Lam, Y.; Damodaran, K.; Pruski, M. Modification of H-ZSM-5 zeolites with phosphorus. 1. Identification of aluminum species by 27Al solid-state NMR and characterization of their catalytic properties. Microporous Mesoporous Mater. 2006, 95, 286–295. [Google Scholar] [CrossRef]
- Damodaran, K.; Wiench, J.; de Menezes, S.C.; Lam, Y.; Trébosc, J.; Amoureux, J.P.; Pruski, M. Modification of H-ZSM-5 zeolites with phosphorus. 2. Interaction between phosphorus and aluminum studied by solid-state NMR spectroscopy. Microporous Mesoporous Mater. 2006, 95, 296–305. [Google Scholar] [CrossRef]
- Kakiuchi, Y.; Yamasaki, Y.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. One-pot Synthesis of Phosphorus-modified AEI Zeolites Derived by the Dual-template Method as a Durable Catalyst with Enhanced Thermal/Hydrothermal Stability for Selective Catalytic Reduction of NOx by NH3. Chem. Lett. 2016, 45, 122–124. [Google Scholar] [CrossRef]
- Tsunoji, N.; Tsuchiya, K.; Nakazawa, N.; Inagaki, S.; Kubota, Y.; Nishitoba, T.; Yokoi, T.; Ohnishi, T.; Ogura, M.; Sadakane, M.; et al. Multiple templating strategy for the control of aluminum and phosphorus distributions in AFX zeolite. Microporous Mesoporous Mater. 2021, 321, 111124. [Google Scholar] [CrossRef]
- Simancas, R.; Chokkalingam, A.; Elangovan, S.P.; Liu, Z.; Sano, T.; Iyoki, K.; Wakihara, T.; Okubo, T. Recent progress in the improvement of hydrothermal stability of zeolites. Chem. Sci. 2021, 12, 7677–7695. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Synthesis of phosphorus-modified small-pore zeolites utilizing tetraalkyl phosphonium cations as both structure-directing and phosphorous modification agents. Microporous Mesoporous Mater. 2016, 223, 129–139. [Google Scholar] [CrossRef]
- Mitani, E.; Yamasaki, Y.; Tsunoji, N.; Sadakane, M.; Sano, T. Synthesis of phosphorus-modified AFX zeolite using a dual-template method with tetraethylphosphonium hydroxide as phosphorus modification agent. Microporous Mesoporous Mater. 2018, 267, 192–197. [Google Scholar] [CrossRef]
- Van Koningsveld, H.; Van Bekkum, H.; Jansen, J.C. On the location and disorder of the tetrapropylammonium (TPA) ion in zeolite ZSM-5 with improved framework accuracy. Acta Crystallogr. Sect. B Struct. Sci. 1987, 43, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Olson, D.H.; Kokotailo, G.T.; Lawton, S.L.; Meier, W.M. Crystal structure and structure-related properties of ZSM-5. J. Phys. Chem. 1981, 85, 2238–2243. [Google Scholar] [CrossRef]
- Koller, H.; Wölker, A.; Villaescusa, L.; Díaz-Cabañas, M.J.; Valencia, S.; Camblor, M. Five-Coordinate Silicon in High-Silica Zeolites. J. Am. Chem. Soc. 1999, 121, 3368–3376. [Google Scholar] [CrossRef]
- Fyfe, C.A.; Brouwer, D.H.; Lewis, A.A.R.; Chézeau, J.-M. Location of the Fluoride Ion in Tetrapropylammonium Fluoride Silicalite-1 Determined by 1H/19F/29Si Triple Resonance CP, REDOR, and TEDOR NMR Experiments. J. Am. Chem. Soc. 2001, 123, 6882–6891. [Google Scholar] [CrossRef]
- Martinez-Ortigosa, J.; Simancas, J.; Vidal-Moya, J.A.; Gaveau, P.; Rey, F.; Alonso, B.; Blasco, T.; Vidal-Moya, A. Host–Guest and Guest–Guest Interactions of P- and N-Containing Structure Directing Agents Entrapped inside MFI-Type Zeolite by Multinuclear NMR Spectroscopy. J. Phys. Chem. C 2019, 123, 22324–22334. [Google Scholar] [CrossRef]
- Dib, E.; Gimenez, A.; Mineva, T.; Alonso, B. Preferential orientations of structure directing agents in zeolites. Dalton Trans. 2015, 44, 16680–16683. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, Q. Solid State NMR Spectroscopy Studies of the Nature of Structure Direction of OSDAs in Pure-Silica Zeolites ZSM-5 and Beta. J. Phys. Chem. C 2017, 121, 13211–13217. [Google Scholar] [CrossRef]
- Koller, H.; Lobo, R.F.; Burkett, S.L.; Davis, M.E. SiO-···HOSi Hydrogen Bonds in As-Synthesized High-Silica Zeolites. J. Phys. Chem. 1995, 99, 12588–12596. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Rojas, A.; Gómez-Hortigüela, L.; Camblor, M.A. Zeolite Structure Direction by Simple Bis(methylimidazolium) Cations: The Effect of the Spacer Length on Structure Direction and of the Imidazolium Ring Orientation on the 19F NMR Resonances. J. Am. Chem. Soc. 2012, 134, 3845–3856. [Google Scholar] [CrossRef]
- Lu, P.; Gómez-Hortigüela, L.; Camblor, M.A. Synthesis of pure silica MFI zeolites using imidazolium-based long dications. A comparative study of structure-directing effects derived from a further spacer length increase. Dalton Trans. 2018, 47, 7498–7504. [Google Scholar] [CrossRef] [Green Version]
- Losch, P.; Pinar, A.B.; Willinger, M.G.; Soukup, K.; Chavan, S.; Vincent, B.; Pale, P.; Louis, B. H-ZSM-5 zeolite model crystals: Structure-diffusion-activity relationship in methanol-to-olefins catalysis. J. Catal. 2017, 345, 11–23. [Google Scholar] [CrossRef]
- Brunklaus, G.; Koller, H.; Zones, S.I. Defect Models of As-Made High-Silica Zeolites: Clusters of Hydrogen-Bonds and Their Interaction with the Organic Structure-Directing Agents Determined from 1H Double and Triple Quantum NMR Spectroscopy. Angew. Chem. Int. Ed. 2016, 55, 14459–14463. [Google Scholar] [CrossRef]
- Dib, E.; Grand, J.; Mintova, S.; Fernandez, C. Structure-Directing Agent Governs the Location of Silanol Defects in Zeolites. Chem. Mater. 2015, 27, 7577–7579. [Google Scholar] [CrossRef]
- Shantz, D.F.; der Günne, J.S.A.; Koller, H.; Lobo, R.F. Multiple-Quantum 1H MAS NMR Studies of Defect Sites in As-Made All-Silica ZSM-12 Zeolite. J. Am. Chem. Soc. 2000, 122, 6659–6663. [Google Scholar] [CrossRef]
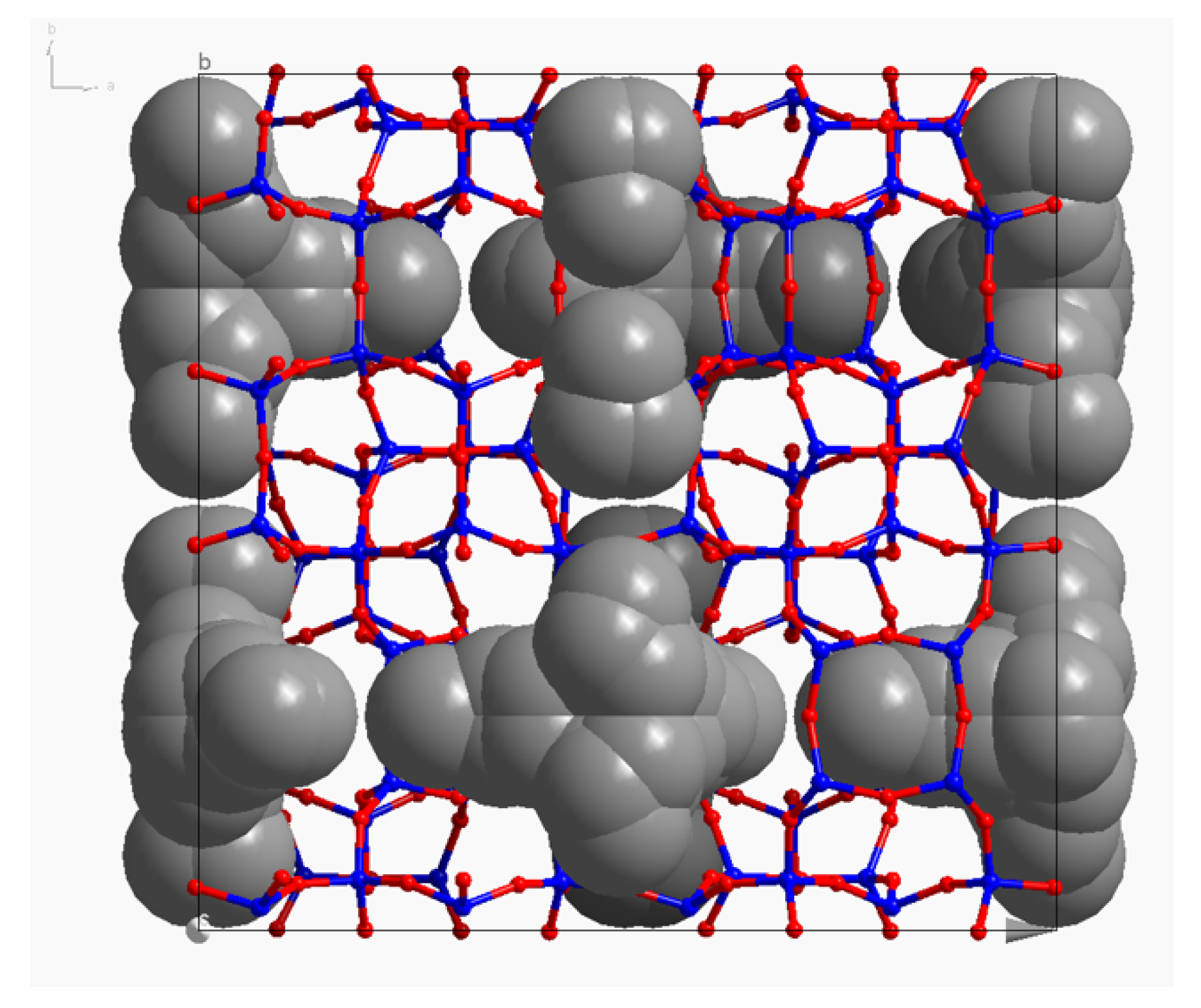
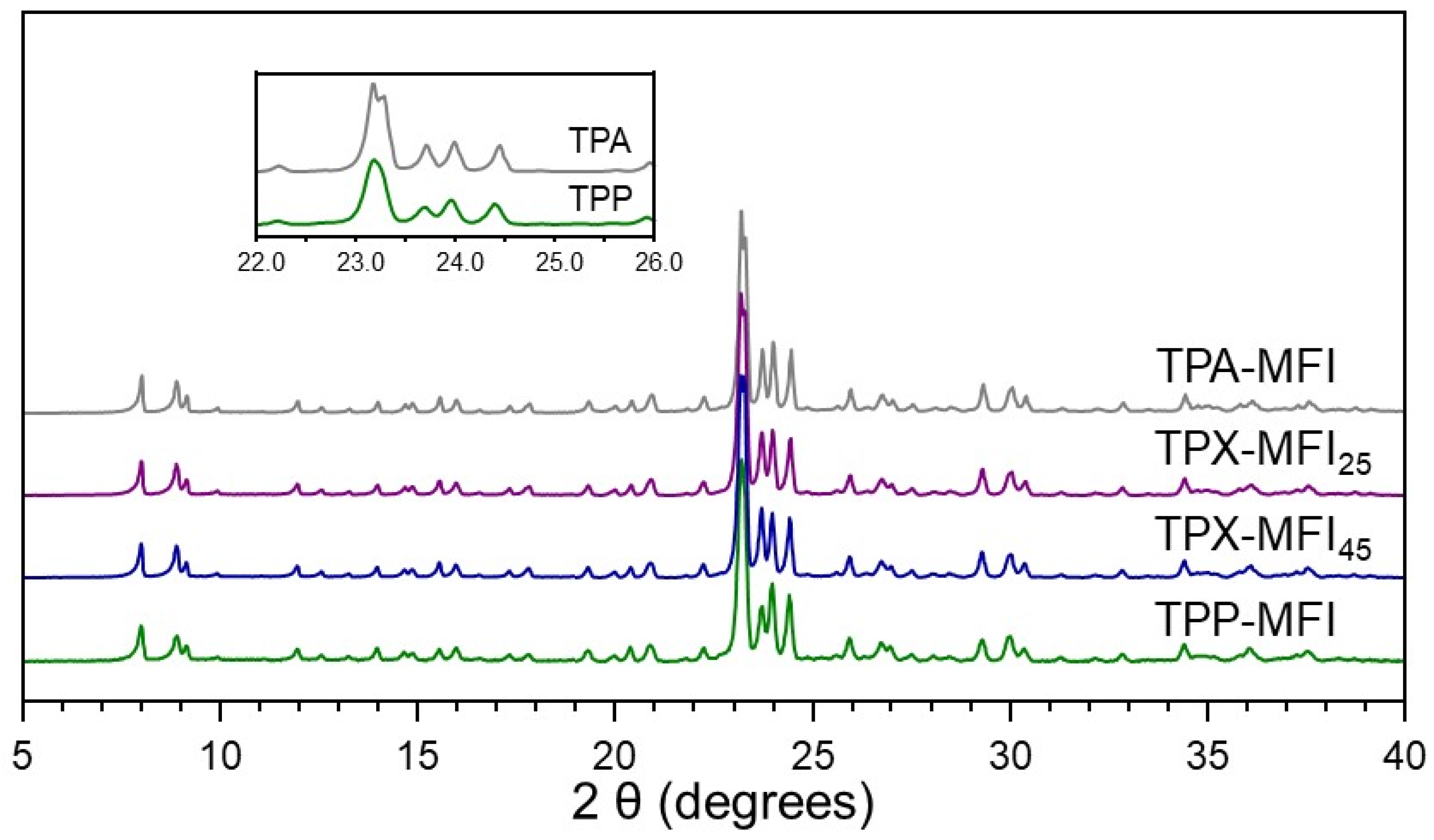


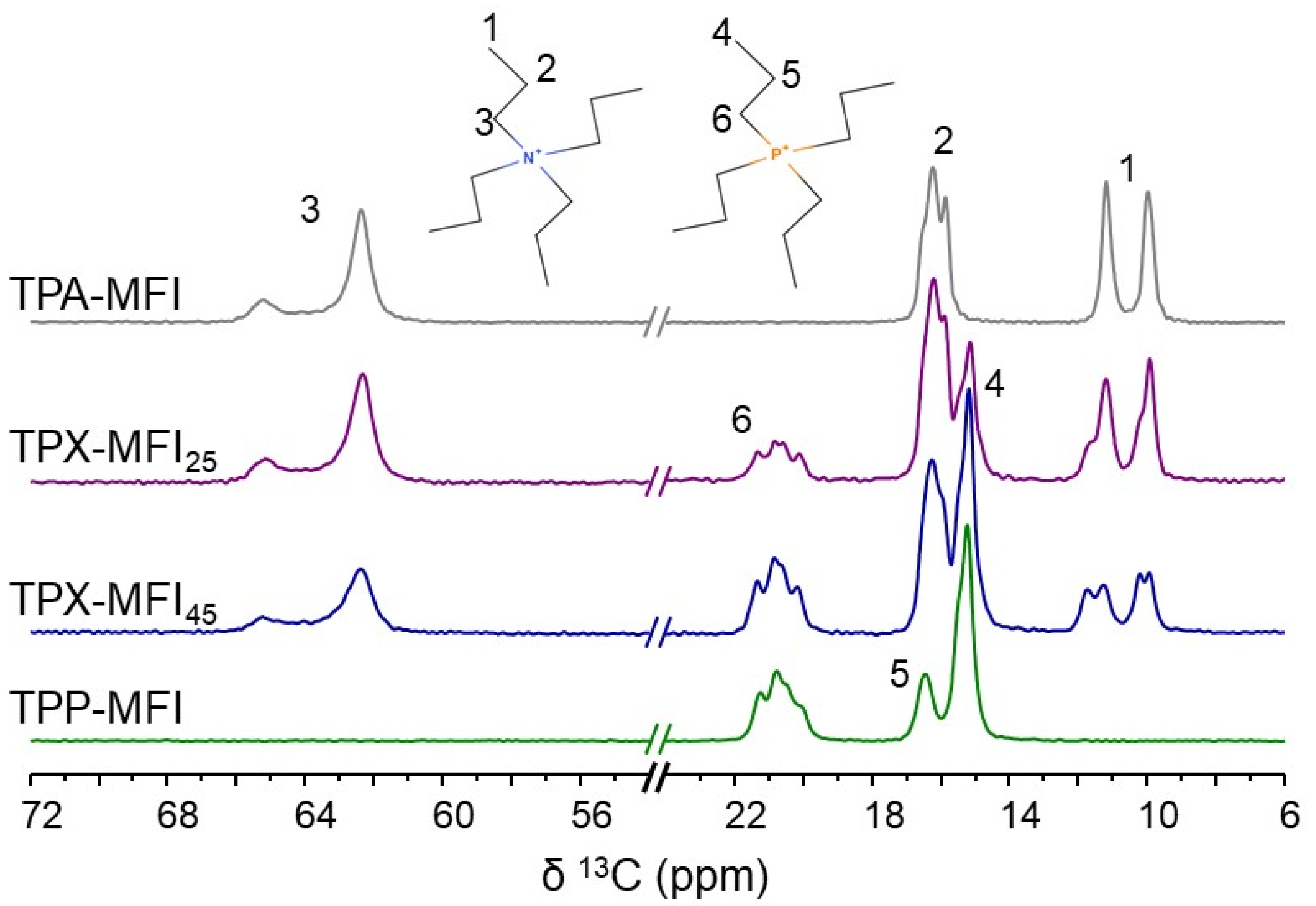
| Sample | OSDA 1 | Crystallization Time 2 (Days) |
|---|---|---|
| TPA-MFI | 1.00 TPA/0.00 TPP | 12 |
| TPX-MFI25 | 0.25 TPP/0.75 TPA | 7 |
| TPX-MFI45 | 0.50 TPP/0.50 TPA | 10 |
| TPP-MFI | 0.00 TPA/1.00 TPP | 7 |
| Sample | P/(P + N) 1 Gel | P/(P + N) 1 Solid | OSDA/ u.c. 2 | F/u.c. 3 | F/OSDA |
|---|---|---|---|---|---|
| TPA-MFI | 0.00 | 0.00 | 4.5 | 3.3 | 0.74 |
| TPX-MFI25 | 0.25 | 0.26 | 4.1 | 2.6 | 0.64 |
| TPX-MFI45 | 0.50 | 0.45 | 4.1 | 2.5 | 0.60 |
| TPP-MFI | 1.00 | 1.00 | 4.3 | 2.4 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Ortigosa, J.; Simancas, J.; Vidal-Moya, J.A.; Rey, F.; Valencia, S.; Blasco, T. A Multi-Nuclear MAS-NMR Study on the Structural Properties of Silicalite-1 Zeolite Synthesized Using N- and P-Based Organic Structure Directing Agents. Appl. Sci. 2021, 11, 6850. https://doi.org/10.3390/app11156850
Martinez-Ortigosa J, Simancas J, Vidal-Moya JA, Rey F, Valencia S, Blasco T. A Multi-Nuclear MAS-NMR Study on the Structural Properties of Silicalite-1 Zeolite Synthesized Using N- and P-Based Organic Structure Directing Agents. Applied Sciences. 2021; 11(15):6850. https://doi.org/10.3390/app11156850
Chicago/Turabian StyleMartinez-Ortigosa, Joaquin, Jorge Simancas, Jose A. Vidal-Moya, Fernando Rey, Susana Valencia, and Teresa Blasco. 2021. "A Multi-Nuclear MAS-NMR Study on the Structural Properties of Silicalite-1 Zeolite Synthesized Using N- and P-Based Organic Structure Directing Agents" Applied Sciences 11, no. 15: 6850. https://doi.org/10.3390/app11156850
APA StyleMartinez-Ortigosa, J., Simancas, J., Vidal-Moya, J. A., Rey, F., Valencia, S., & Blasco, T. (2021). A Multi-Nuclear MAS-NMR Study on the Structural Properties of Silicalite-1 Zeolite Synthesized Using N- and P-Based Organic Structure Directing Agents. Applied Sciences, 11(15), 6850. https://doi.org/10.3390/app11156850