Arabidopsis Disulfide Reductase, Trx-h2, Functions as an RNA Chaperone under Cold Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
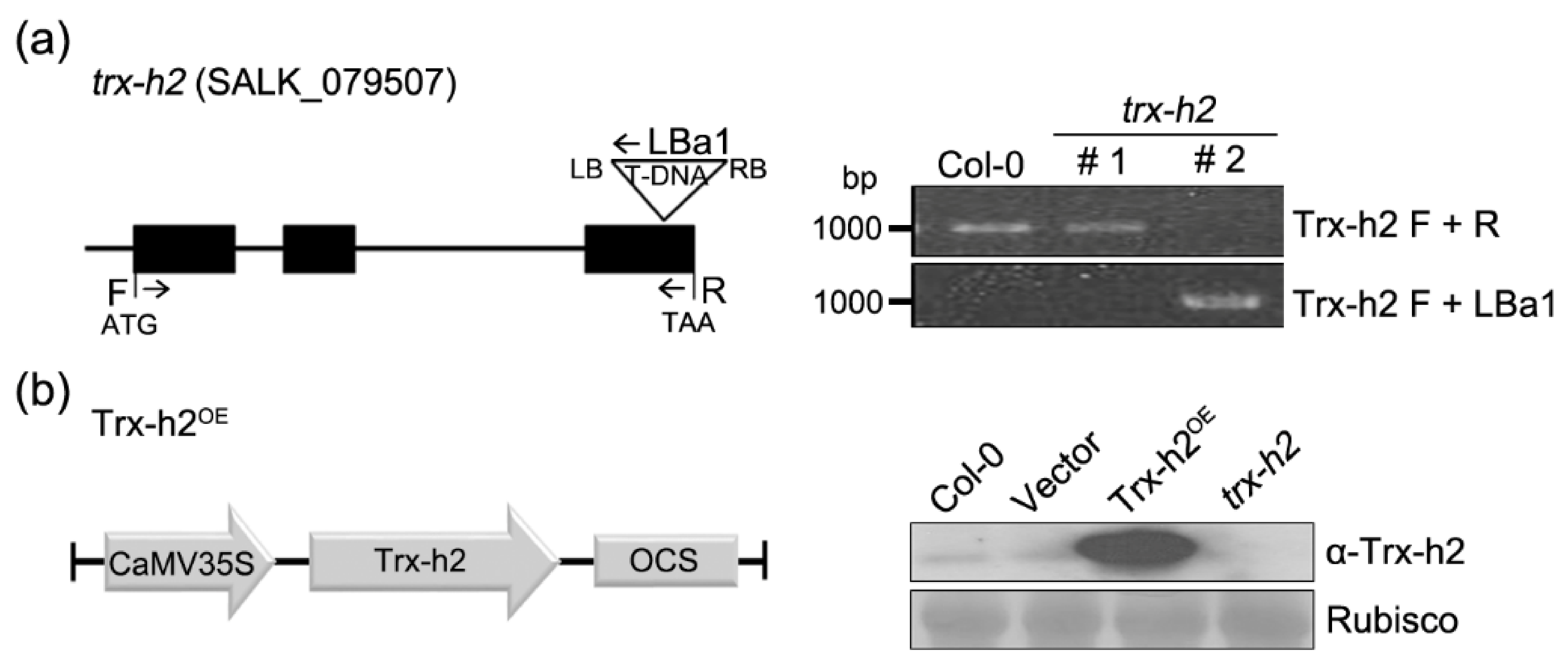
2.2. Identification of Trx-h2 Mutants and Generation of Trx-h2OE Lines
2.3. Western Blot Analysis
2.4. Cold-Shock Treatment
2.5. Expression and Purification of Trx-h2 and Trx-h3 Recombinant Proteins
2.6. Electrophoretic Mobility Shift Assay (EMSA)
2.7. Melting Assay of Nucleic Acids
2.8. Transcription Anti-Termination Assay
2.9. In Situ Hybridization Assay
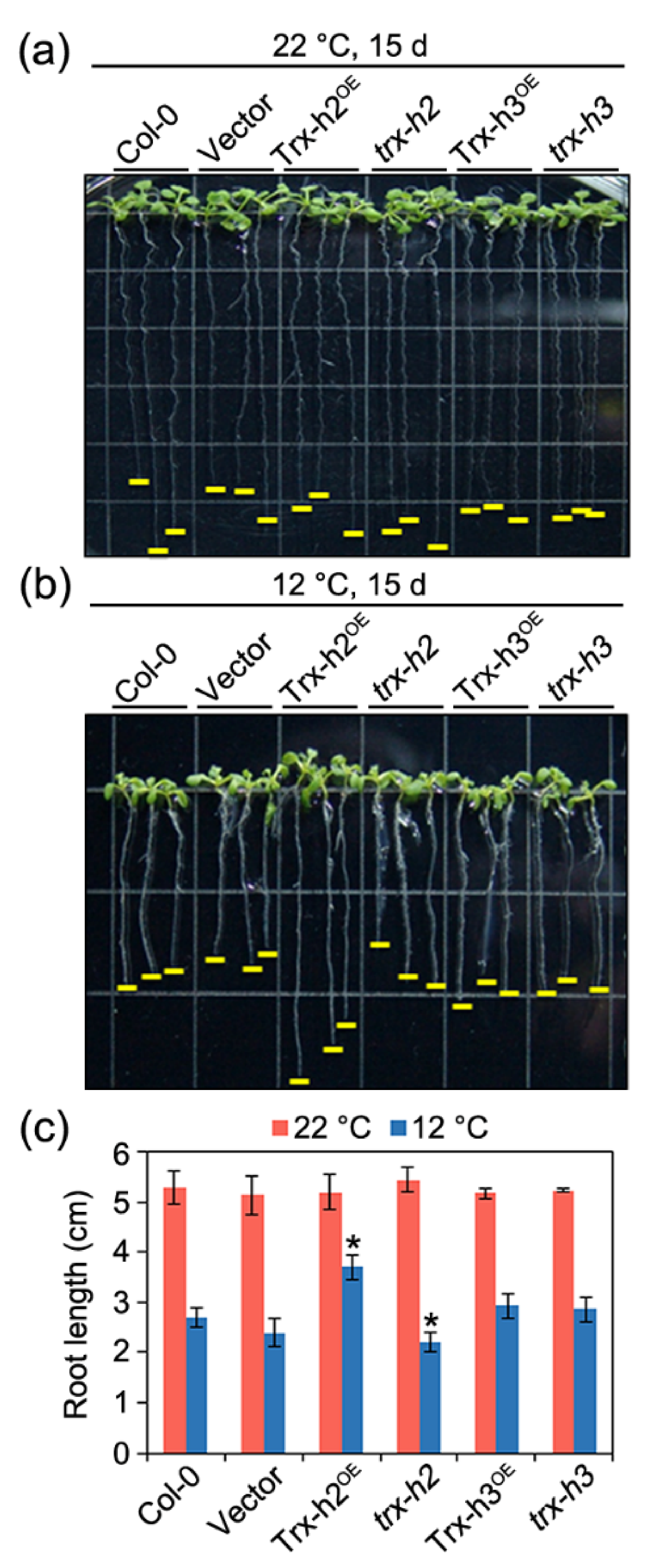
2.10. Seedling Growth under Cold Stress
2.11. Statistical Analysis
3. Results
3.1. Role of Trx-h2 in Seedling Growth under Cold Stress
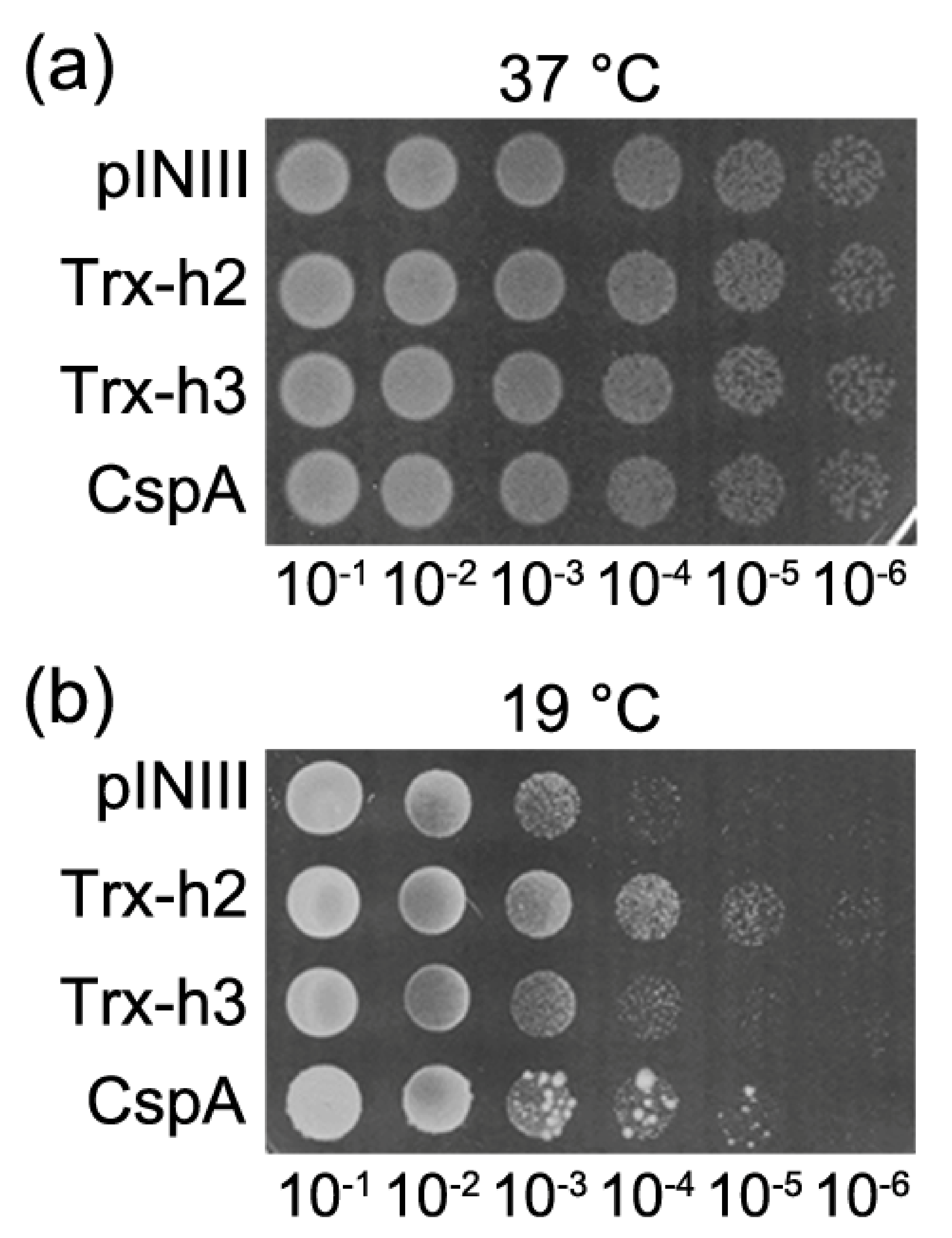
3.2. Trx-h2 Overexpression Complements the Cold-Sensitive Phenotype of the E. coli BX04 Mutant
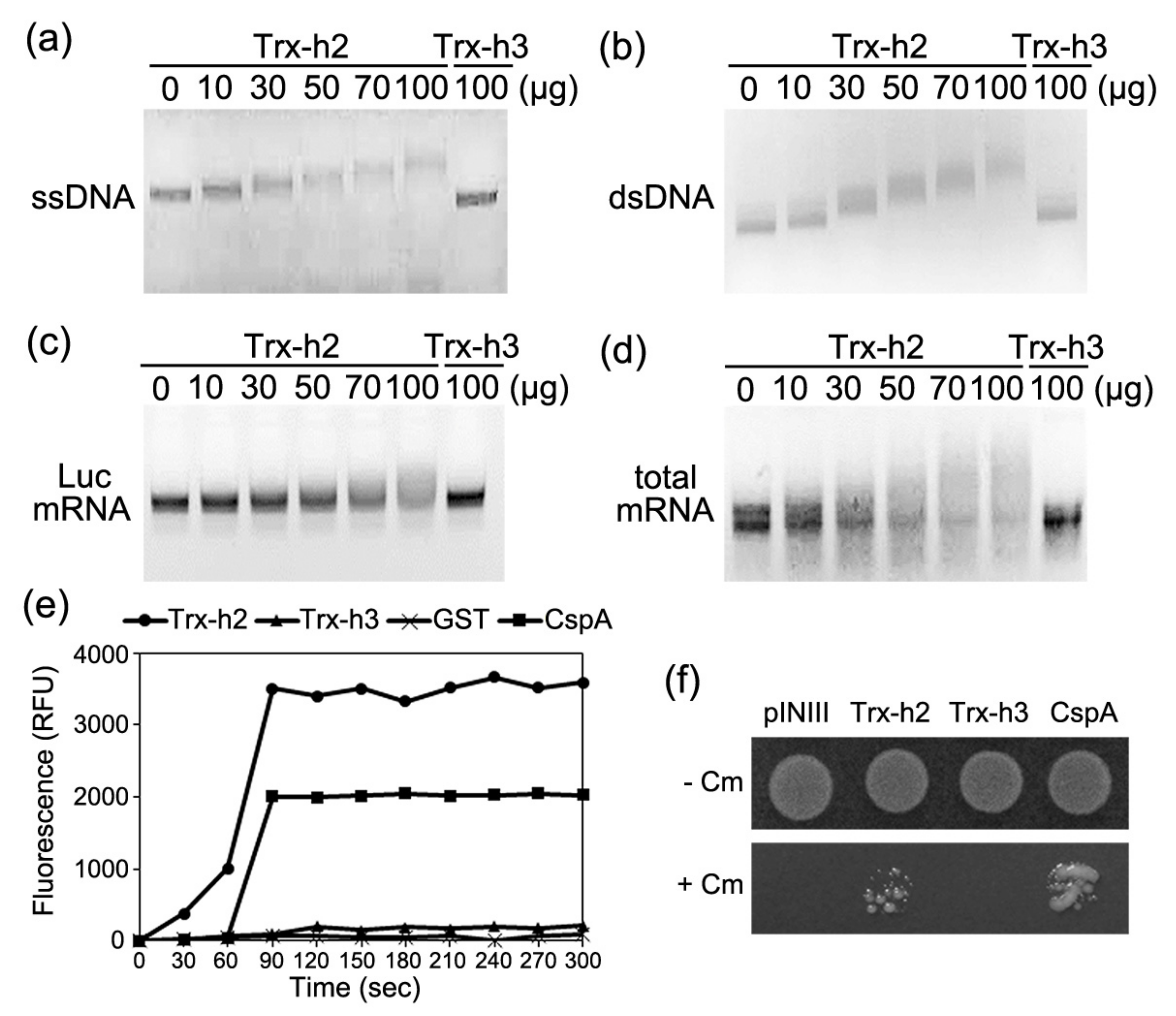
3.3. Trx-h2 Binds to Nucleic Acids
3.4. Trx-h2 Melts to Nucleic Acids
3.5. Trx-h2 Exports Nuclear mRNA to the Cytoplasm under Cold Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Bialasek, M.; Suzuki, N.; Gorecka, M.; Devireddy, A.R.; Karpinski, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelos, E.; Ruberti, C.; Kim, S.J.; Brandizzi, F. Maintaining the factory: The roles of the unfolded protein response in cellular homeostasis in plants. Plant J. 2017, 90, 671–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, C.H.; Nawkar, G.M.; Lee, E.S.; Paeng, S.K.; Chae, H.B.; Chi, Y.H.; Kim, W.Y.; Yun, D.J.; Lee, S.Y. EMR, a cytosolic-abundant ring finger E3 ligase, mediates ER-associated protein degradation in Arabidopsis. New Phytol. 2018, 220, 163–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.H.; Lee, K.O.; Chi, Y.H.; Jung, B.G.; Park, S.K.; Park, J.H.; Lee, J.R.; Lee, S.S.; Moon, J.C.; Yun, J.W.; et al. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 2004, 117, 625–635. [Google Scholar] [CrossRef]
- Hanschmann, E.M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Sign. 2013, 19, 1539–1605. [Google Scholar] [CrossRef]
- Lee, E.S.; Kang, C.H.; Park, J.H.; Lee, S.Y. Physiological Significance of Plant Peroxiredoxins and the Structure-Related and Multifunctional Biochemistry of Peroxiredoxin 1. Antioxid. Redox Sign. 2018, 28, 625–639. [Google Scholar] [CrossRef]
- Meyer, Y.; Buchanan, B.B.; Vignols, F.; Reichheld, J.P. Thioredoxins and glutaredoxins: Unifying elements in redox biology. Annu. Rev. Genet. 2009, 43, 335–367. [Google Scholar] [CrossRef]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Bio. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Chae, H.B.; Kim, M.G.; Kang, C.H.; Park, J.H.; Lee, E.S.; Lee, S.U.; Chi, Y.H.; Paeng, S.K.; Bae, S.B.; Wi, S.D.; et al. Redox sensor QSOX1 regulates plant immunity by targeting GSNOR to modulate ROS generation. Mol. Plant 2021. [Google Scholar] [CrossRef]
- Lee, J.R.; Lee, S.S.; Jang, H.H.; Lee, Y.M.; Park, J.H.; Park, S.C.; Moon, J.C.; Park, S.K.; Kim, S.Y.; Lee, S.Y.; et al. Heat-shock dependent oligomeric status alters the function of a plant-specific thioredoxin-like protein, AtTDX. Proc. Nati. Acad. Sci. USA 2009, 106, 5978–5983. [Google Scholar] [CrossRef] [Green Version]
- Aslund, F.; Beckwith, J. Bridge over troubled waters: Sensing stress by disulfide bond formation. Cell 1999, 96, 751–753. [Google Scholar] [CrossRef] [Green Version]
- Cabrillac, D.; Cock, J.M.; Dumas, C.; Gaude, T. The S-locus receptor kinase is inhibited by thioredoxins and activated by pollen coat proteins. Nature 2001, 410, 220–223. [Google Scholar] [CrossRef]
- Huber, H.E.; Tabor, S.; Richardson, C.C. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J. Biol. Chem. 1987, 262, 16224–16232. [Google Scholar] [CrossRef]
- Gelhaye, E.; Rouhier, N.; Jacquot, J.P. The thioredoxin h system of higher plants. Plant Physiol. Bioch. 2004, 42, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Menges, M.; Hennig, L.; Gruissem, W.; Murray, J.A. Cell cycle-regulated gene expression in Arabidopsis. J. Biol. Chem. 2002, 277, 41987–42002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehelin, C.; Mouaheb, N.; Verdoucq, L.; Lancelin, J.M.; Meyer, Y. Characterization of determinants for the specificity of Arabidopsis thioredoxins h in yeast complementation. J. Biol. Chem. 2000, 275, 31641–31647. [Google Scholar] [CrossRef] [Green Version]
- Park, S.K.; Jung, Y.J.; Lee, J.R.; Lee, Y.M.; Jang, H.H.; Lee, S.S.; Park, J.H.; Kim, S.Y.; Moon, J.C.; Lee, S.Y.; et al. Heat-shock and redox-dependent functional switching of an h-type Arabidopsis thioredoxin from a disulfide reductase to a molecular chaperone. Plant Physiol. 2009, 150, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweat, T.A.; Wolpert, T.J. Thioredoxin h5 is required for victorin sensitivity mediated by a CC-NBS-LRR gene in Arabidopsis. Plant Cell 2007, 19, 673–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tada, Y.; Spoel, S.H.; Pajerowska-Mukhtar, K.; Mou, Z.; Song, J.; Wang, C.; Zuo, J.; Dong, X. Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 2008, 321, 952–956. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.J.; Chi, Y.H.; Chae, H.B.; Shin, M.R.; Lee, E.S.; Cha, J.Y.; Paeng, S.K.; Lee, Y.; Park, J.H.; Kim, W.Y.; et al. Analysis of Arabidopsis thioredoxin-h isotypes identifies discrete domains that confer specific structural and functional properties. Biochem. J. 2013, 456, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Melencion, S.M.B.; Chi, Y.H.; Pham, T.T.; Paeng, S.K.; Wi, S.D.; Lee, C.; Ryu, S.W.; Koo, S.S.; Lee, S.Y. RNA Chaperone Function of a Universal Stress Protein in Arabidopsis Confers Enhanced Cold Stress Tolerance in Plants. Int. J. Mol. Sci. 2017, 18, 2546. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Dong, C.H.; Lee, H.; Zhu, J.; Xiong, L.; Gong, D.; Stevenson, B.; Zhu, J.K. A DEAD box RNA helicase is essential for mRNA export and important for development and stress responses in Arabidopsis. Plant Cell 2005, 17, 256–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Nati. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Ke, H.; Inouye, M. Acquirement of cold sensitivity by quadruple deletion of the cspA family and its suppression by PNPase S1 domain in Escherichia coli. Mol. Microbiol. 2001, 40, 179–188. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, W.Y.; Kwak, K.J.; Oh, S.H.; Han, Y.S.; Kang, H. Glycine-rich RNA-binding proteins are functionally conserved in Arabidopsis thaliana and Oryza sativa during cold adaptation process. J. Exp. Bot. 2010, 61, 2317–2325. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kim, W.Y.; Kwak, K.J.; Oh, S.H.; Han, Y.S.; Kang, H. Zinc finger-containing glycine-rich RNA-binding protein in Oryza sativa has an RNA chaperone activity under cold stress conditions. Plant Cell Environ. 2010, 33, 759–768. [Google Scholar] [CrossRef]
- Chi, Y.H.; Moon, J.C.; Park, J.H.; Kim, H.S.; Zulfugarov, I.S.; Fanata, W.I.; Jang, H.H.; Lee, J.R.; Lee, Y.M.; Kim, S.T.; et al. Abnormal chloroplast development and growth inhibition in rice thioredoxin m knock-down plants. Plant Physiol. 2008, 148, 808–817. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.S.; Park, J.H.; Wi, S.D.; Kang, C.H.; Chi, Y.H.; Chae, H.B.; Paeng, S.K.; Ji, M.G.; Kim, W.-Y.; Kim, M.G.; et al. Redox-dependent structural switch and CBF activation confer freezing tolerance in plants. Nat. Plants 2021, 7, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Laloi, C.; Mestres-Ortega, D.; Marco, Y.; Meyer, Y.; Reichheld, J.P. The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol. 2004, 134, 1006–1016. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Wong, J.H.; Feldman, L.J.; Lemaux, P.G.; Buchanan, B.B. A membrane-associated thioredoxin required for plant growth moves from cell to cell, suggestive of a role in intercellular communication. Proc. Nati. Acad. Sci. USA 2010, 107, 3900–3905. [Google Scholar] [CrossRef] [Green Version]
- Traverso, J.A.; Micalella, C.; Martinez, A.; Brown, S.C.; Satiat-Jeunemaitre, B.; Meinnel, T.; Giglione, C. Roles of N-terminal fatty acid acylations in membrane compartment partitioning: Arabidopsis h-type thioredoxins as a case study. Plant Cell 2013, 25, 1056–1077. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Lee, E.S.; Chae, H.B.; Paeng, S.K.; Wi, S.D.; Bae, S.B.; Thi Phan, K.A.; Lee, S.Y. Disulfide reductase activity of thioredoxin-h2 imparts cold tolerance in Arabidopsis. Biochem. Bioph. Res. Commun. 2021, 568, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Cremers, C.M.; Reichmann, D.; Hausmann, J.; Ilbert, M.; Jakob, U. Unfolding of metastable linker region is at the core of Hsp33 activation as a redox-regulated chaperone. J. Biol. Chem. 2010, 285, 11243–11251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, J.M.; Lee, H.N.; Kim, E.K.; Ha, B.; Ahn, S.M.; Jang, H.H.; Lee, S.Y. RNA-binding properties and RNA chaperone activity of human peroxiredoxin 1. Biochem. Bioph. Res. Commun. 2012, 425, 730–734. [Google Scholar] [CrossRef]
- Kim, Y.O.; Kang, H. The role of a zinc finger-containing glycine-rich RNA-binding protein during the cold adaptation process in Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.O.; Kim, J.S.; Kang, H. Cold-inducible zinc finger-containing glycine-rich RNA-binding protein contributes to the enhancement of freezing tolerance in Arabidopsis thaliana. Plant J. 2005, 42, 890–900. [Google Scholar] [CrossRef]
- Jiang, W.; Hou, Y.; Inouye, M. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 1997, 272, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.S.; Park, J.H.; Wi, S.D.; Chae, H.B.; Paeng, S.K.; Bae, S.B.; Phan, K.A.T.; Lee, S.Y. Arabidopsis Disulfide Reductase, Trx-h2, Functions as an RNA Chaperone under Cold Stress. Appl. Sci. 2021, 11, 6865. https://doi.org/10.3390/app11156865
Lee ES, Park JH, Wi SD, Chae HB, Paeng SK, Bae SB, Phan KAT, Lee SY. Arabidopsis Disulfide Reductase, Trx-h2, Functions as an RNA Chaperone under Cold Stress. Applied Sciences. 2021; 11(15):6865. https://doi.org/10.3390/app11156865
Chicago/Turabian StyleLee, Eun Seon, Joung Hun Park, Seong Dong Wi, Ho Byoung Chae, Seol Ki Paeng, Su Bin Bae, Kieu Anh Thi Phan, and Sang Yeol Lee. 2021. "Arabidopsis Disulfide Reductase, Trx-h2, Functions as an RNA Chaperone under Cold Stress" Applied Sciences 11, no. 15: 6865. https://doi.org/10.3390/app11156865





