The Effect of Oxygen Admixture with Argon Discharges on the Impact Parameters of Atmospheric Pressure Plasma Jet Characteristics
Abstract
1. Introduction
2. Experimental Set-Up and Procedures
- a.
- Control in the jet dimensions such as width, length, and covered area;
- b.
- Control in the lifetime of the discharge process mode, whether laminar flow mode or turbulent flow mode;
- c.
- Control in the heat impact emerging from the nozzle;
- d.
- Control in the antibacterial effect factors of culture media down the nozzle such as exposure time, optical emission spectra, and irradiance.
3. Results and Discussion
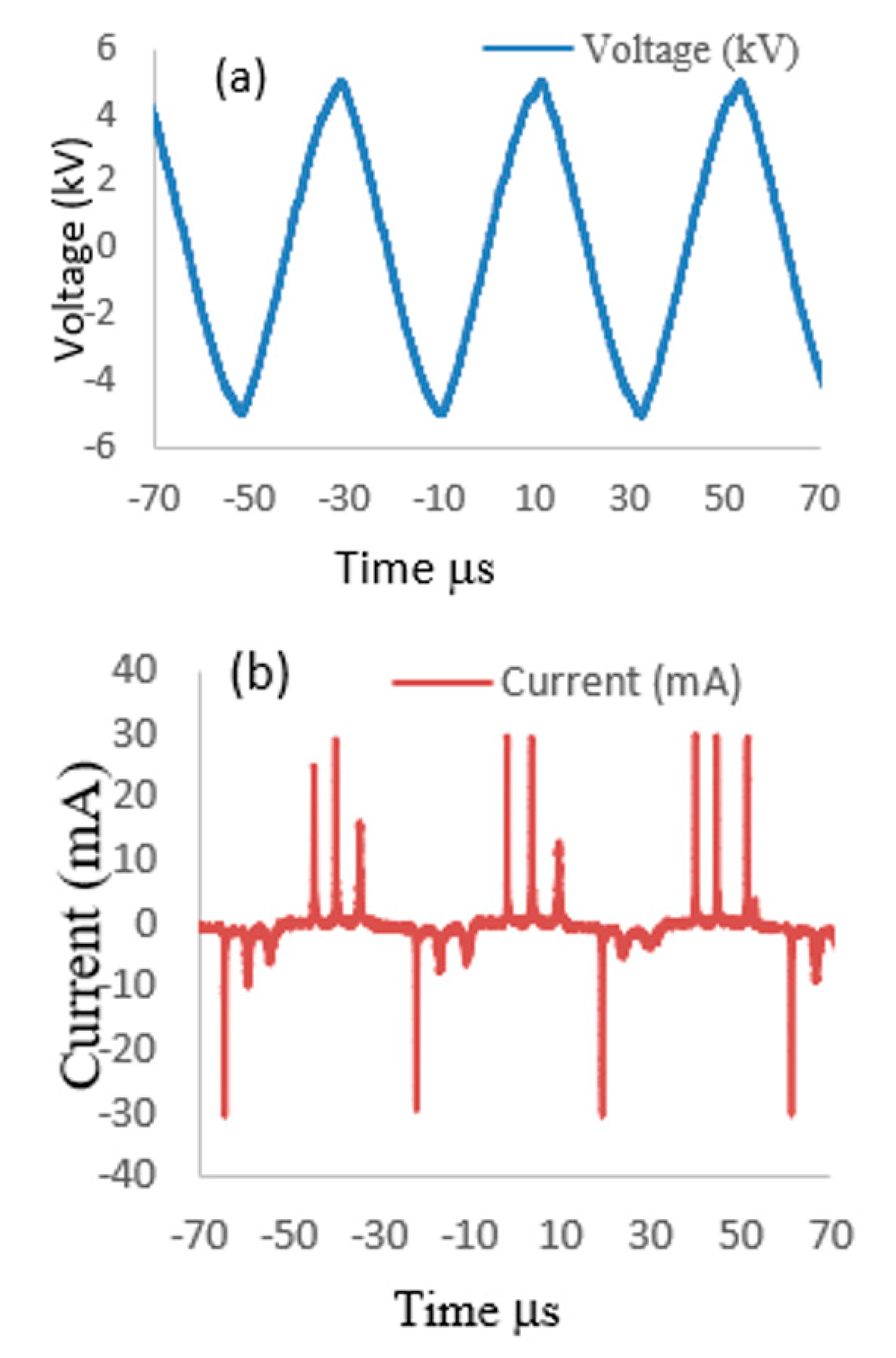
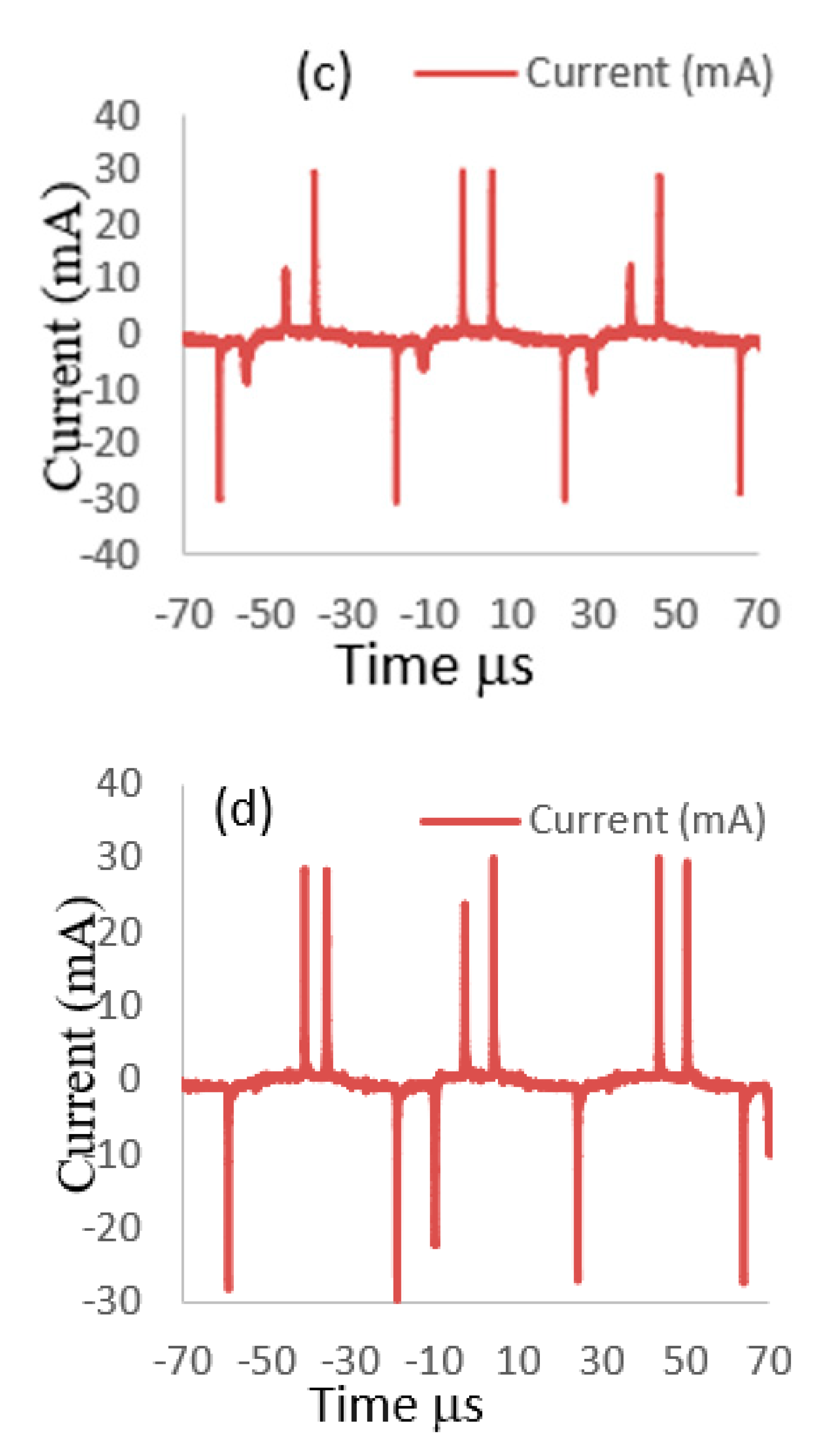
3.1. Voltage–Current Waveform Signals of APPJ Discharge
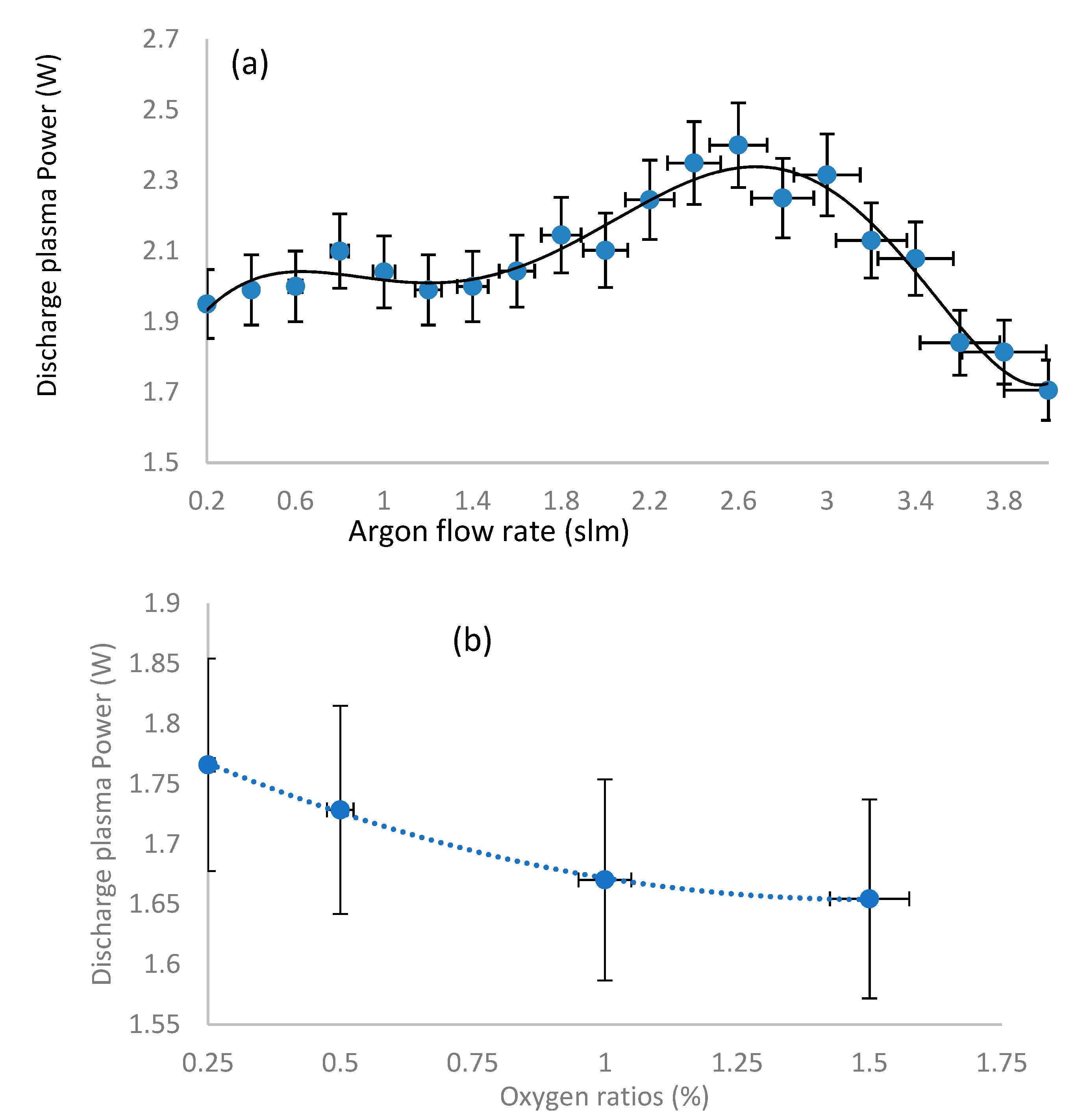
3.2. Gas Flow Rate
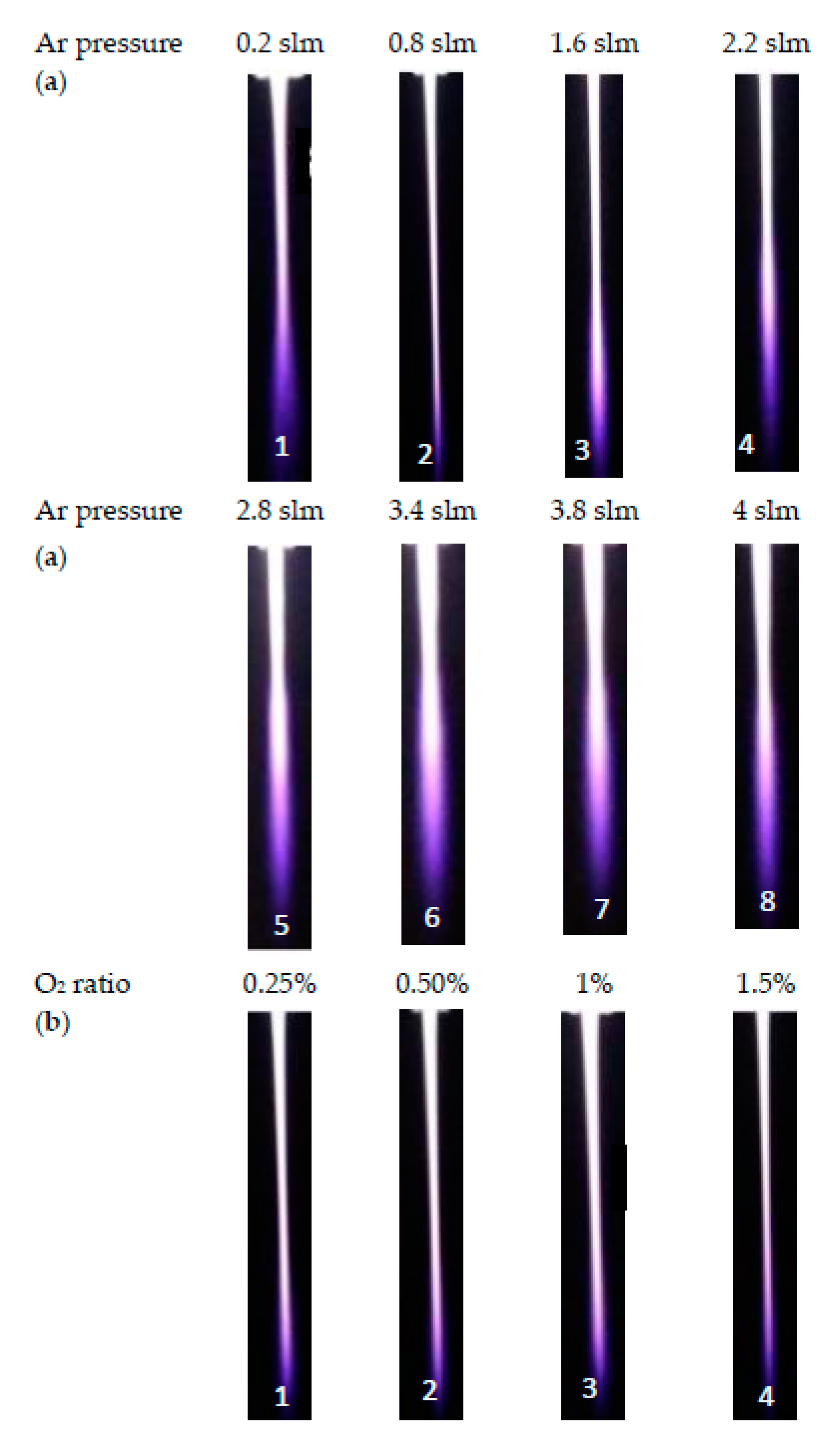
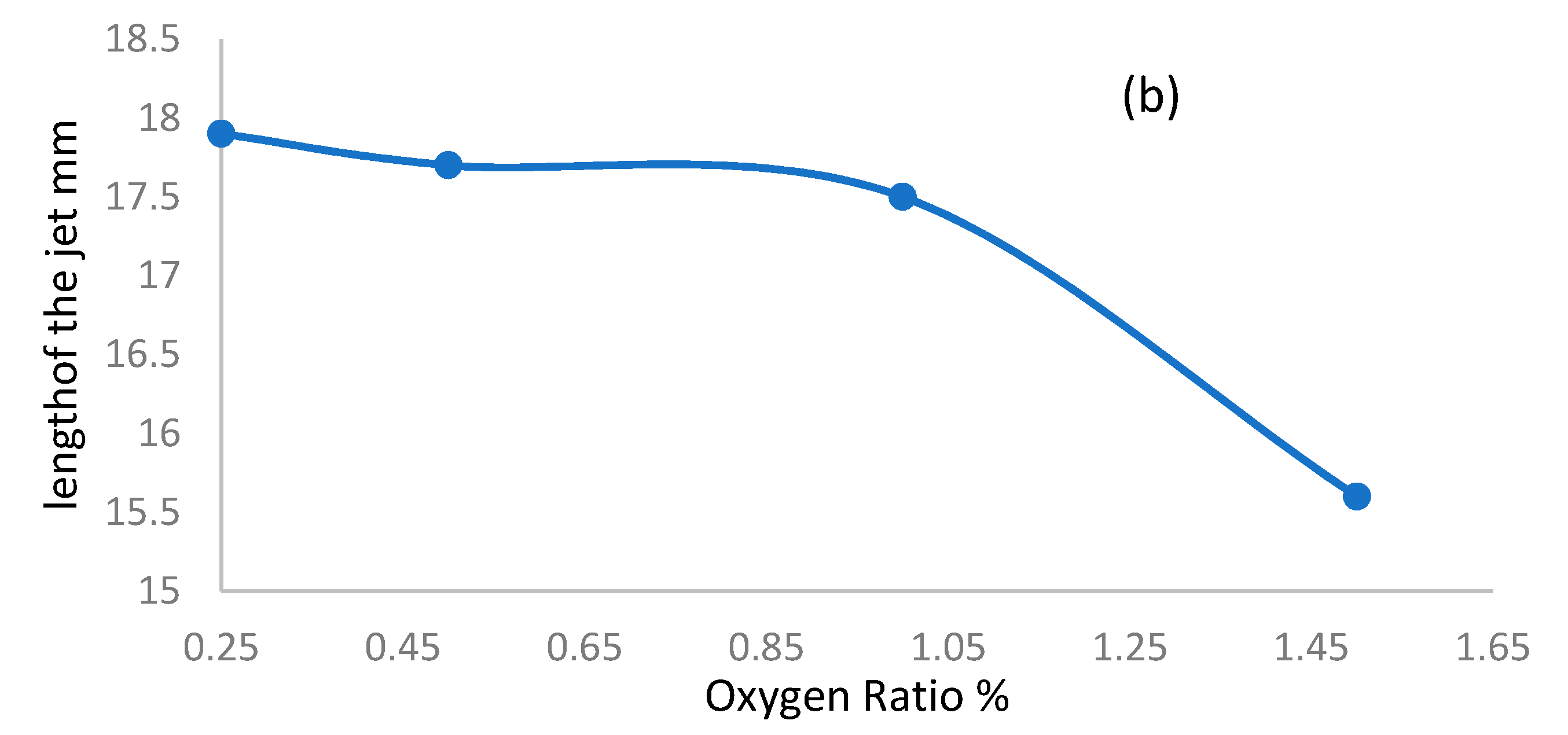
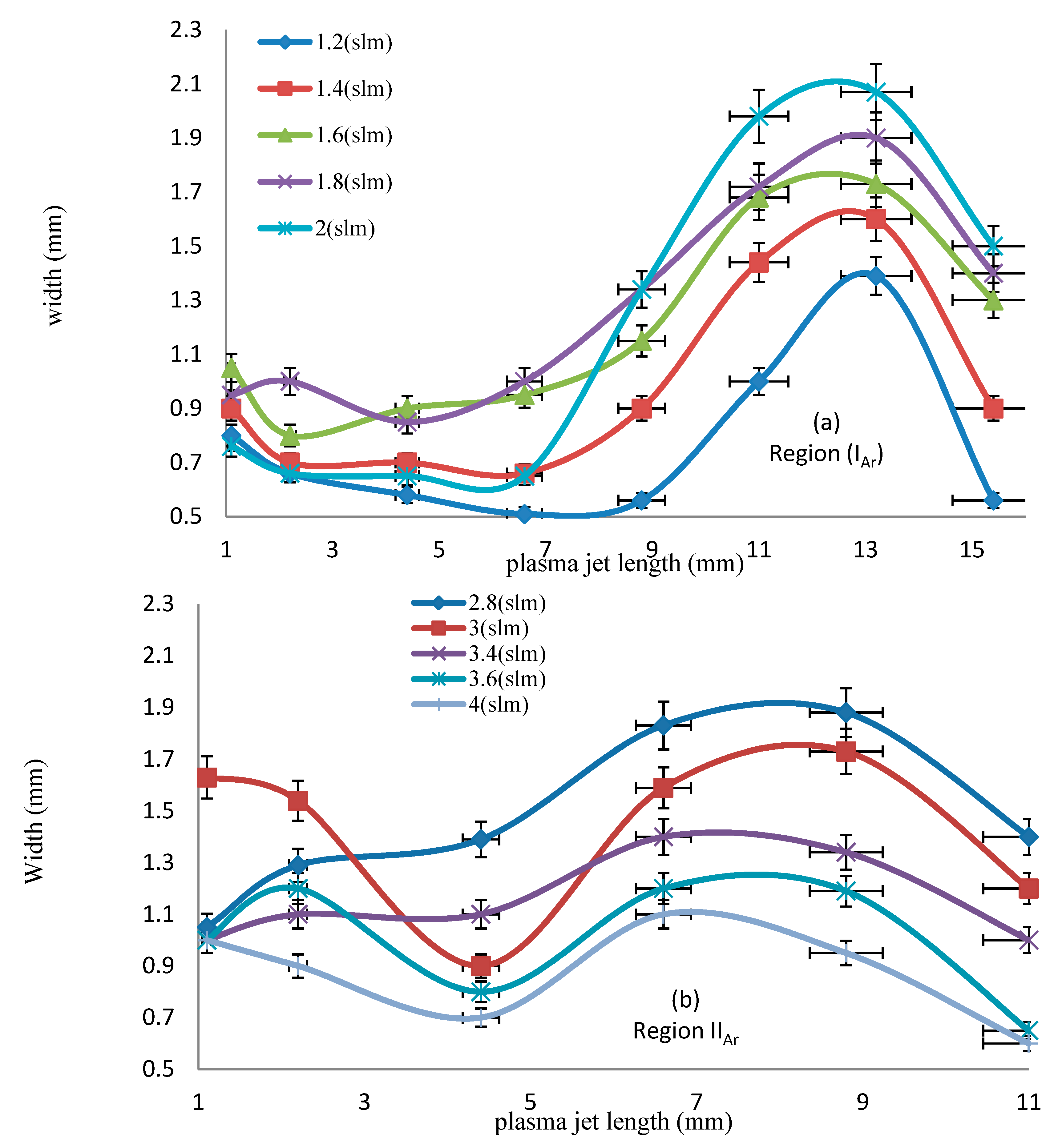
3.3. Length and Width of the Jet
- (a)
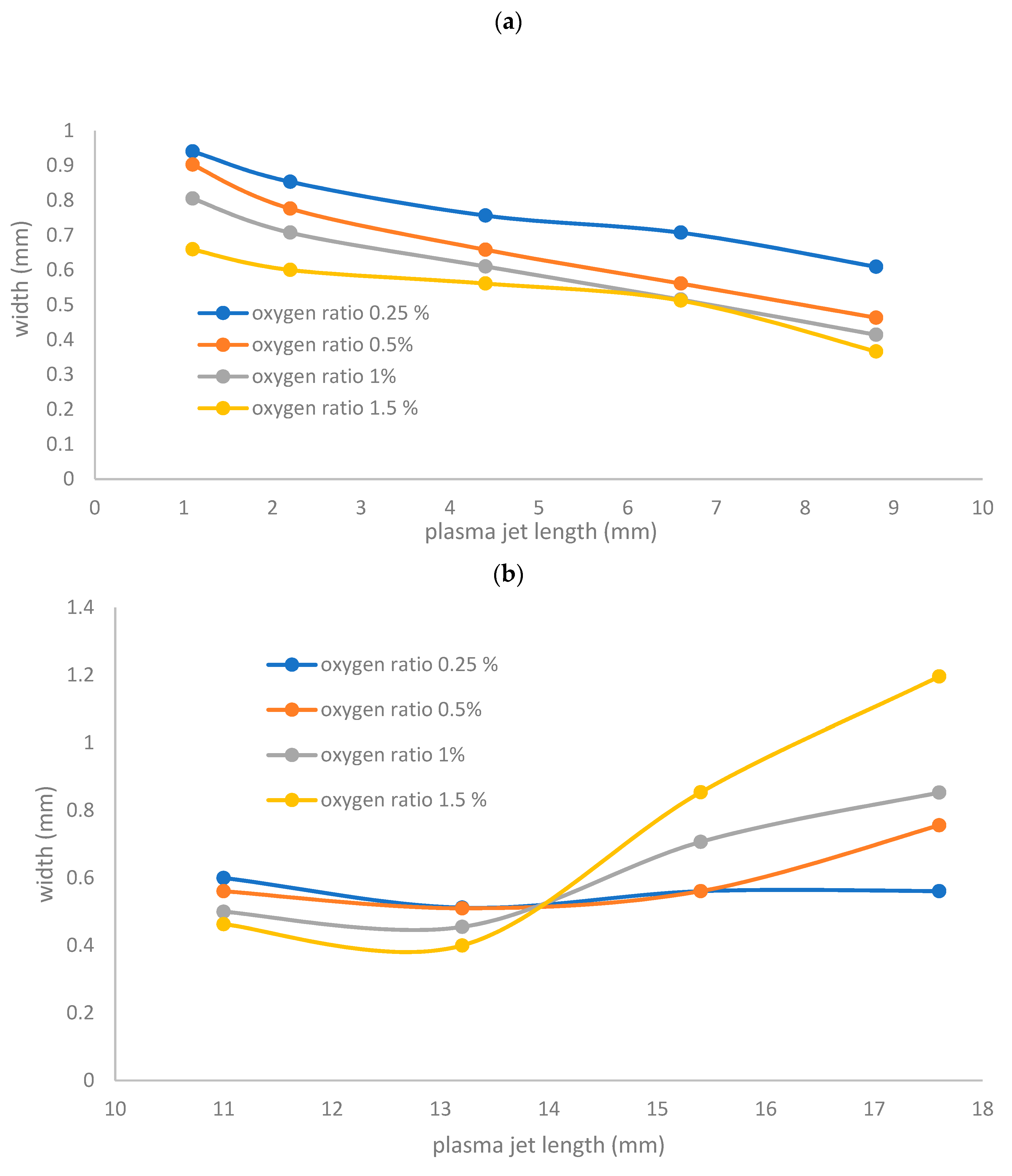
- Figure 7a shows the jet width for wet argon as a function of plasma jet length less than 10 mm, at different applied flow rate ratios of oxygen, ranging from 0.25% to 1.5%. As the flow rate ratio of oxygen increases, the jet width recognizably decreases axially to a minimum of 0.609 to 0.366 mm, for a plasma jet length of 8.8 mm.
- (b)
- Figure 7a shows the jet width for wet argon as a function of plasma jet length, ranging from 11 to 18 mm, at different applied flow rate ratios of oxygen, ranging from 0.25% to 1.5%. As the flow rate ratio of oxygen increases, the jet width begins to increase, reaching a wider scale from 0.561 to 1.196 mm, for a plasma jet length of 17.6 mm. This means that, as in Figure 7b, as the admixture values of oxygen flow rates increase, elongating the axial length dimension of the jet, and increase the width dimensions of the plasma jet. Table 3 and Table 4 give the largest width dimension values at the equivalent length dimensions of jet for an argon and oxygen/argon admixture discharge, respectively. The optical emission spectra and irradiances of the atmospheric pressure plasma jets can be obtained using these dimensions, as briefly presented below in Section 3.5 and Section 3.6.
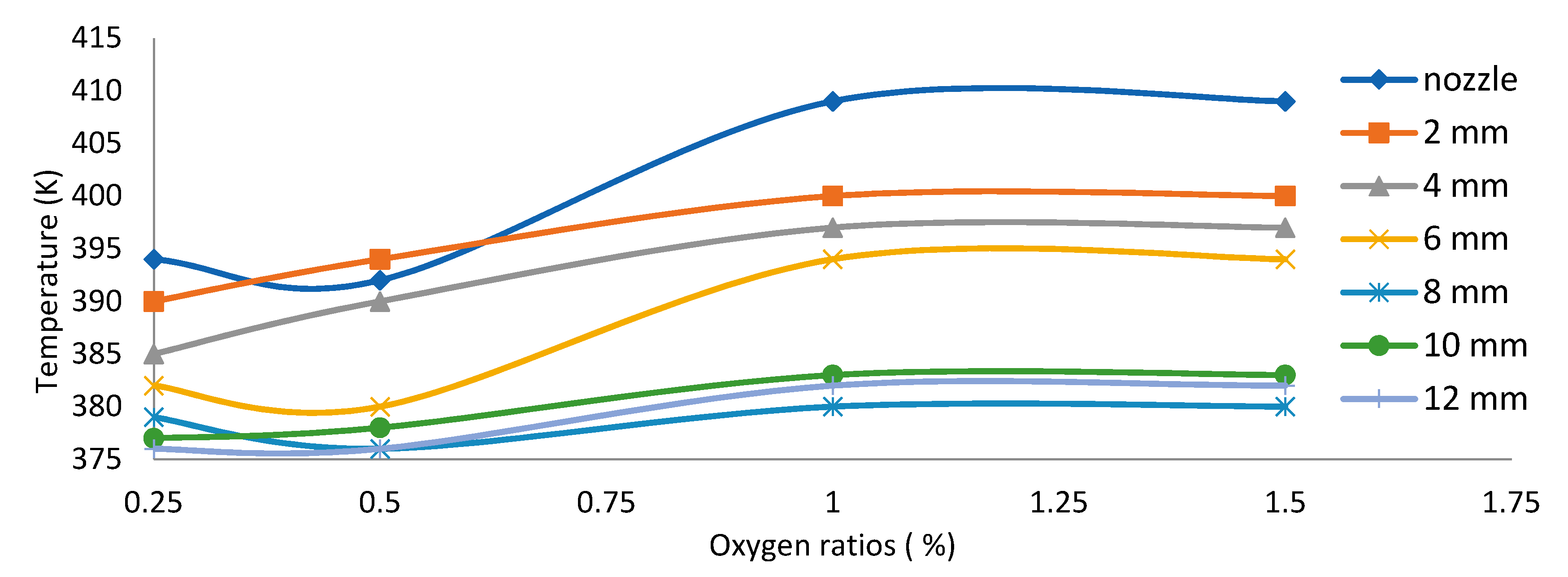
3.4. Axial Temperature Distribution
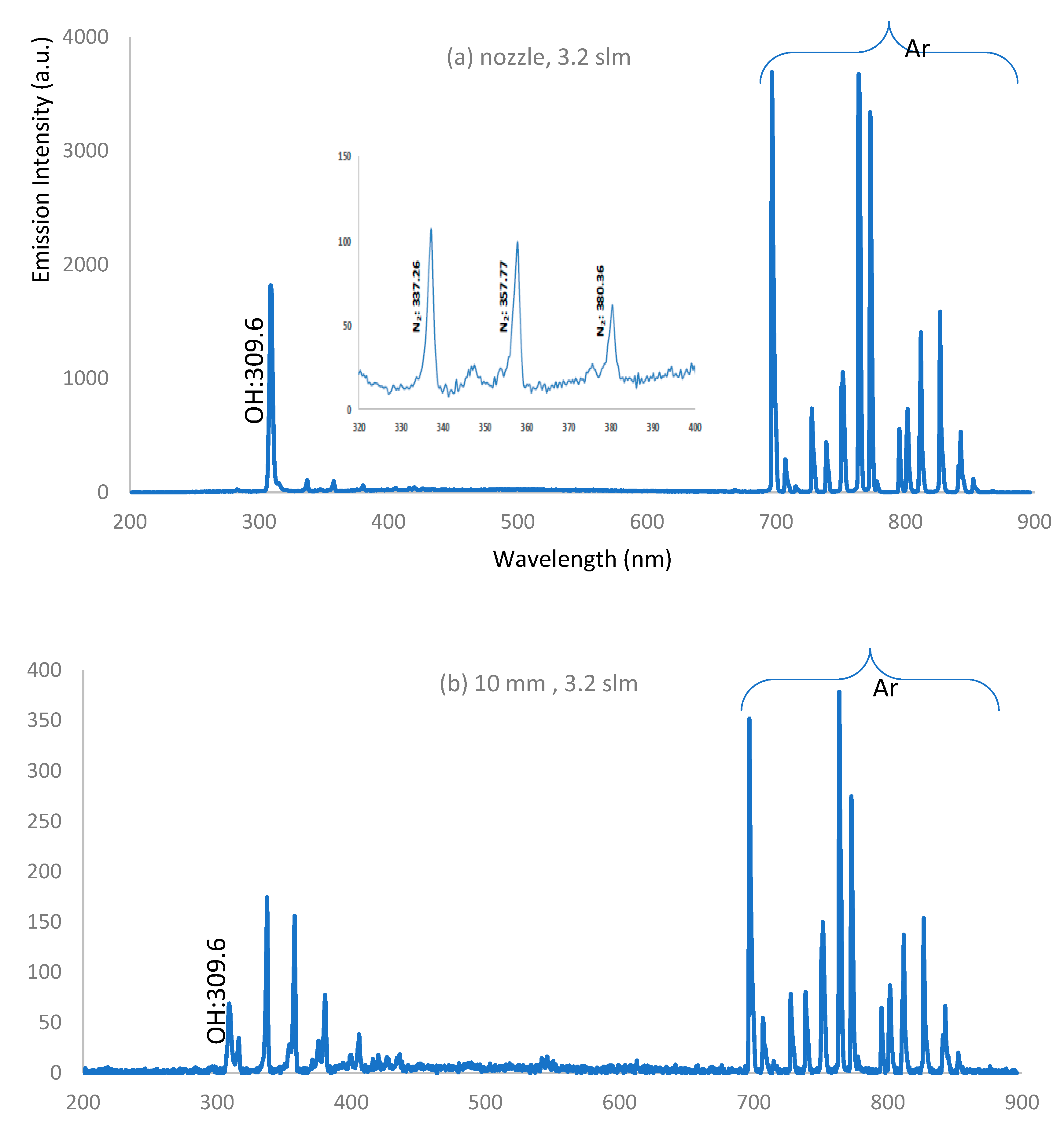
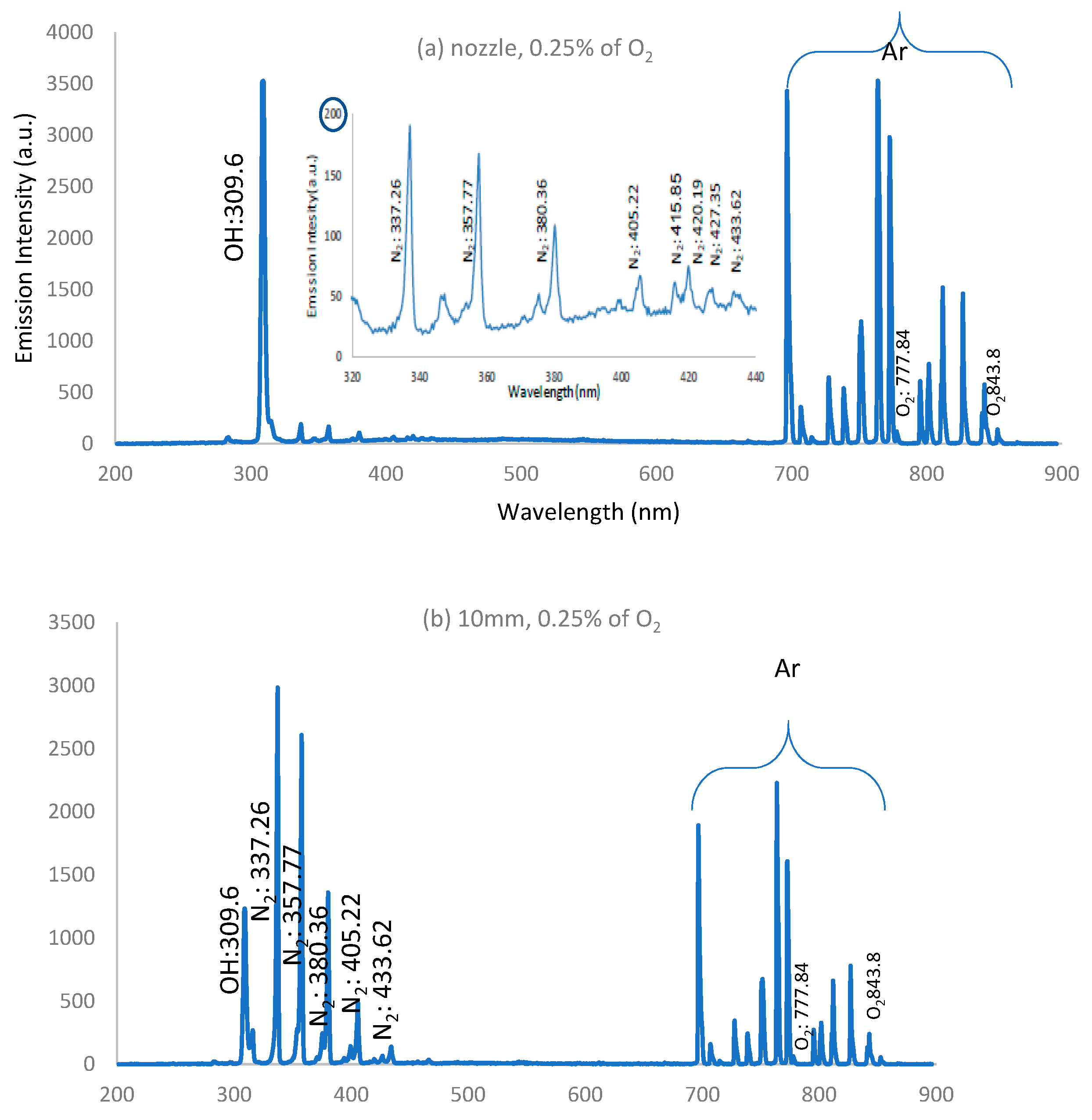
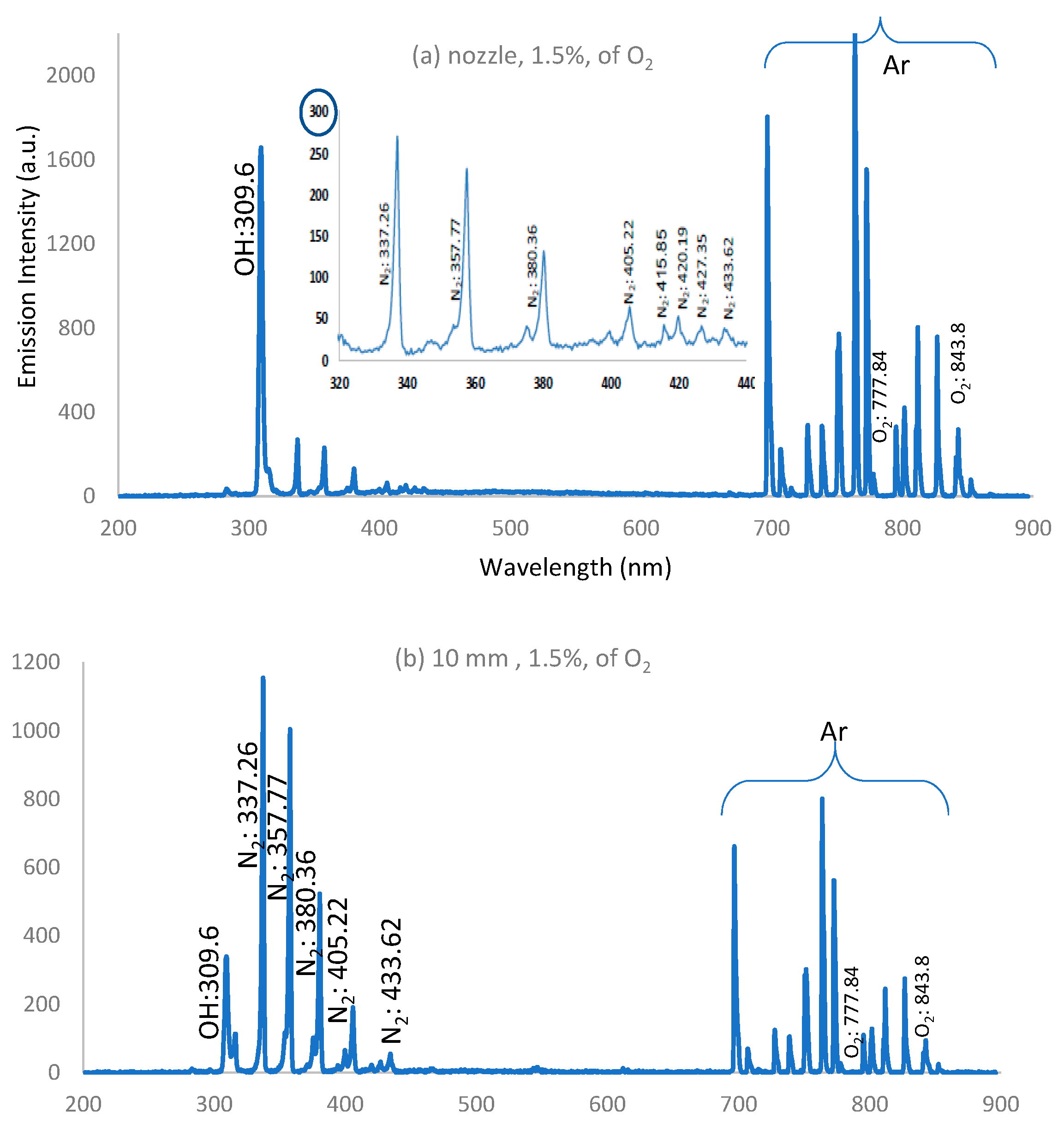
3.5. Optical Emission Spectra of APPJ
- a-
- IES decreases along the jet length and decreases apart from the nozzle due to the presence of the strong electric field near the nozzle and because of the high rate of charge carrier generation by electron impacts near the nozzle.
- b-
- As the admixture of oxygen increases, IES decreases due to the decrease in electron density and temperature resulting from significant dissociation of the oxygen molecules in the discharge processes [34].
- c-
- As the admixture of oxygen increases, IES for the concentration of O, OH, and NO radicals increases far away from the nozzle, compared with the dry argon case.
- d-
- There are many reactions for dry argon discharge, as follows [35]:
- i-
- Argon reacts with an energetic electron (e*) to produce metastable argon (Arm):Ar + e*→ Arm + ē
- ii-
- Arm reacts with e* to produce excited argon (Ar*):e * + Arm → Ar* + ē
- iii-
- Ar* reacts with water to generate OH•:H2O + Ar* → H• + OH• + Ar
- iv-
3.6. Irradiance
- a-
- Figure 15a shows the influence of the flow rate on the discharge plasma power of the emerging jet for pure argon discharge; by increasing the flow rate from 1.8 to 3 slm, the irradiance per unit area of the plasma jet discharge decreases from 55 to 13 μJ/mm2 for a plasma jet distance with 8 mm. With increasing plasma jet length to 18.4 mm, the irradiance decreases to between 15 and 6.09 μJ/mm2.
- b-
- Figure 15b shows the influence of different oxygen ratios; whereas the oxygen ratios increase from 0.25% to 1.5%, the irradiance per unit area of plasma jet discharge decreases from 28.57 to 5.68 μJ/mm2 for a plasma jet length of 8 mm. By increasing the plasma jet length to 18.4 mm, the irradiance decreases to between 6.9 and 4.02 μJ/mm2. The dose rate of irradiance per unit area is lower for the oxygen/argon admixture discharges than for the argon discharges. The oxygen percentages increase; inelastic collisions lead to losses in the discharge process and lower irradiance compared with the case for argon discharges.
- c-
- According to the measured data and the Guidelines of the International Commission on Non-Ionizing Radiation Protection (ICNIRP), the limits of irradiance exposure of the skin must not exceed 30 μJ/mm2 [43,44]. From the results, it is concluded that the measured irradiances under the drawn dashed line in Figure 15a for argon discharges and the irradiances for all oxygen/argon admixture discharges in Figure 15b are compatible with ICNIRP irradiance limits.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Oost, G. Plasma for environment. J. Phys. Conf. Ser. 2017, 941, 012014. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacte-rial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Laroussi, M.; Mendis, D.A.; Rosenberg, M. Plasma interaction and microbes. New J. Phys. 2003, 5, 41. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Leys, C.; Gengembre, L.; Schacht, E.; Payen, E. Deposition of HMDSO-based coatings on PET substrates using an atmospheric pressure dielectric barrier discharge. Prog. Org. Coat. 2009, 64, 304. [Google Scholar] [CrossRef]
- Laroussi, M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Lee, H.W.; Nam, S.H.; Mohamed, A.-A.; Kim, G.C.; Lee, J.K. Atmospheric Pressure Plasma Jet Composed of Three Electrodes: Application to Tooth Bleaching. Plasma Process. Polym. 2010, 7, 274–280. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Verschuren, J.; De Clerck, K.; Kiekens, P.; Leys, C. Non-thermal plasma treatment of textiles. Surf. Coat. Technol. 2008, 202, 3427–3449. [Google Scholar] [CrossRef]
- Machala, Z.; Hensel, K.; Akishev, Y. Plasma for Bio-Decontamination, Medicine and Food Security; Springer: Dordrecht, The Netherlands, 2011; pp. 417–430. [Google Scholar]
- Lee, M.-H.; Min, B.K.; Son, J.S.; Kwon, T.-Y. Influence of Different Post-Plasma Treatment Storage Conditions on the Shear Bond Strength of Veneering Porcelain to Zirconia. Materials 2016, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Wei, C.-K.; Ho, C.-C.; Ding, S.-J. Enhanced Hydrophilicity and Biocompatibility of Dental Zirconia Ceramics by Oxygen Plasma Treatment. Materials 2015, 8, 684–699. [Google Scholar] [CrossRef]
- Nam, S.H.; Lee, H.J.; Hong, J.W.; Kim, G.C. Efficacy of Nonthermal Atmospheric Pressure Plasma for Tooth Bleaching. Sci. World J. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Laroussi, M. Low-Temperature Plasma Jet for Biomedical Applications: A Review. IEEE Trans. Plasma Sci. 2015, 43, 703–712. [Google Scholar] [CrossRef]
- Xiong, Q.; Lu, X.P.; Jiang, Z.H.; Tang, Z.Y.; Hu, J.; Xiong, Z.L.; Pan, Y. An Atmospheric Pressure Nonequilibrium Plasma Jet Device. IEEE Trans. Plasma Sci. 2008, 36, 986–987. [Google Scholar] [CrossRef]
- Seo, Y.S.; Mohamed, A.-A.; Woo, K.C.; Lee, H.W.; Lee, J.K.; Kim, K.T. Comparative Studies of Atmospheric Pressure Plasma Characteristics between He and Ar Working Gases for Sterilization. IEEE Trans. Plasma Sci. 2010, 38, 2954–2962. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Wang, S. Study on the Bactericidal Mechanism of Atmospheric-Pressure Low-Temperature Plasma against Escherichia coli and Its Application in Fresh-Cut Cucumbers. Molecules 2018, 23, 975. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.-D.; Brandenburg, R.; von Woedtke, T.; Ehlbeck, J.; Foest, R.; Stieber, M.; Kindel, E. Antimicrobial treatment of heat sensitive products by miniaturized atmospheric pressure plasma jets (APPJs). J. Phys. D Appl. Phys. 2008, 41. [Google Scholar] [CrossRef]
- Mohamed, A.-A.H.; Aljuhani, M.M.; Almarashi, J.Q.M.; Alhazime, A. The effect of a second grounded electrode on the atmospheric pressure argon plasma jet. Plasma Res. Express 2020, 2, 015011. [Google Scholar] [CrossRef]
- Galaly, A.R.; Ahmed, O.B.; Asghar, A.H. Antibacterial effects of combined non-thermal plasma and photocatalytic treatment of culture media in the laminar flow mode. Phys. Fluids 2021, 33, 043604. [Google Scholar] [CrossRef]
- Dünnbier, M.; Becker, M.M.; Iséni, S.; Bansemer, R.; Loffhagen, D.; Reuter, S.; Weltmann, K.-D. Stability and excitation dynamics of an argon micro-scaled atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2015, 24, 65018. [Google Scholar] [CrossRef]
- Sarani, A.; Nikiforov, A.; Leys, C. Atmospheric pressure plasma jet in Ar and Ar/H2O mixtures: Optical emission spectroscopy and temperature measurements. Phys. Plasmas 2010, 17, 063504. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Vansteenkiste, E.; De Bock, J.; Philips, W. Measuring the wicking behavior of textiles by the combination of a horizontal wicking experiment and image processing. Rev. Sci. Instrum. 2006, 77, 093502. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Surface Modification of Non-woven Textiles using a Dielectric Barrier Discharge Operating in Air, Helium and Argon at Medium Pressure. Text. Res. J. 2007, 77, 471–488. [Google Scholar] [CrossRef]
- Vicoveanu, D.; Ohtsu, Y.; Fujita, H. Pulsed Discharge Effects on Bacteria Inactivation in Low-Pressure Radio-Frequency Oxygen Plasma. Jpn. J. Appl. Phys. 2008, 47, 1130–1135. [Google Scholar] [CrossRef]
- Singh, M.K.; Ogino, A.; Nagatsu, M. Inactivation factors of spore-forming bacteria using low-pressure microwave plasmas in an N2 and O2 gas mixture. New J. Phys. 2009, 11. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Proc. Polym. 2005, 2, 391. [Google Scholar] [CrossRef]
- Deng, X.; Leys, C.; Vujosevic, D.; Vuksanovic, V.; Cvelbar, U.; De Geyter, N.; Morent, R.; Nikiforov, A. Engineering of Composite Organosilicon Thin Films with Embedded Silver Nanoparticles via Atmospheric Pressure Plasma Process for Antibacterial Activity. Plasma Process. Polym. 2014, 11, 921. [Google Scholar] [CrossRef]
- Ploux, L.; Mateescu, M.; Anselme, K.; Vasilev, K. Antibacterial Properties of Silver-Loaded Plasma Polymer Coatings. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Deng, X.L.; Nikiforov, A.; Vanraes, P.; Leys, C. Direct current plasma jet at atmospheric pressure operating in nitrogen and air. J. Appl. Phys. 2013, 113, 023305. [Google Scholar] [CrossRef]
- Asghar, A.H.; Ahmed, O.B.; Galaly, A.R. Inactivation of E. coli Using Atmospheric Pressure Plasma Jet with Dry and Wet Argon Discharges. Membranes 2021, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Soloshenko, I.; Tsiolko, V.; Khomich, V. Sterilization of medical products in low- pressure glow discharges. Plasma Phys. Rep. 2000, 26, 792. [Google Scholar] [CrossRef]
- Lu, X.; Ye, T.; Cao, Y.; Sun, Z.; Xiong, Q.; Tang, Z.; Xiong, Z.; Hu, J.; Jiang, Z.; Pan, Y. The roles of the various plasma agents in the inactivation of bacteria. J. Appl. Phys. 2008, 104, 053309. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, C.; Shidong, F.; Hongbing, X.; Yan, L.; Guohua, N.; Yuedong, M.; Jiarong, L.; Xiangke, W. Sterilization of Bacillus subtilis Spores Using an Atmospheric Plasma Jet with Argon and Oxygen Mixture Gas. Appl. Phys. Express 2012, 5, 3. [Google Scholar] [CrossRef]
- Cheng, C. Atmospheric pressure plasma jet utilizing Ar and Ar/H2O mixtures and its applications to bacteria inactivation. Chin. Phys. B 2014, 23, 7. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Bruggeman, P.; Schram, D.; González, M.A.; Rego, R.; Kong, M.G.; Leys, C. Characterization of a direct dc-excited discharge in water by optical emission spectroscopy. Plasma Sources Sci. Technol. 2009, 18, 5–17. [Google Scholar] [CrossRef]
- Dodet, B.; Odic, E.; Goldman, A.; Goldman, M.; Renard, D. Hydrogen Peroxide Formation by Discharges in Argon/Water Vapor Mixtures at Atmospheric Pressure. J. Adv. Oxid. Technol. 2005, 8, 91–97. [Google Scholar] [CrossRef]
- Kirkpatrick, M.; Dodet, B.; Odic, E. Atmospheric pressure humid argon DBD plasma for the application of sterilization measurement and simulation of hydrogen, oxygen, and hydrogen peroxide formation. Int. J. Plasma Environ. Sci. Technol. 2007, 1, 96–101. [Google Scholar]
- Taghizadeh, L.; Brackman, G.; Nikiforov, A.; van der Mullen, J.; Leys, C.; Coenye, T. Inactivation of biofilms using a low power atmospheric pressure argon plasma jet; the role of en-trained nitrogen. Plasma Process. Polym. 2015, 12, 75–81. [Google Scholar] [CrossRef]
- Galaly, A.R.; Zahran, H.H. Disinfection of Microbes by Magnetized DC Plasma. J. Mod. Phys. 2014, 5, 781–791. [Google Scholar] [CrossRef][Green Version]
- Galaly, A.R.; Zahran, H.H. Inactivation of Bacteria using Combined Effects of Magnetic Field, Low Pressure and Ultra Low Frequency Plasma Discharges (ULFP). J. Phys. Conf. Ser. (IOP) 2013, 431, 012014. [Google Scholar] [CrossRef]
- Lam, Y.L.; Kan, C.W.; Yuen, C.W. Effect of oxygen plasma pre-treatment and titanium dioxide overlay coating on flame retardant finished cotton fabrics. Bioresources 2011, 6, 1454–1474. [Google Scholar]
- Deng, X.; Nikiforov, A.; Coenye, T.; Cools, P.; Aziz, G.; Morent, R.; De Geyter, N.; Leys, C. Antimicrobial nano-silver non-woven polyethylene terephthalate fabric via an atmospheric pressure plasma deposition process. Sci. Rep. 2015, 5, 10138. [Google Scholar] [CrossRef] [PubMed]
- The International Commission on Non-Ionizing Radiation Protection. Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm and 400 nm incoherent optical radiation. Health Phys. 2004, 87, 171–179. [Google Scholar] [CrossRef] [PubMed]
- The International Commission on Non-Ionizing Radiation Protection. General approach to protection against non-ionizing radiation. Health Phys. 2002, 82, 540–545. [Google Scholar] [CrossRef] [PubMed]







| Tube dimensions | Ceramic | Material |
| Cylindrical | Shape | |
| 70 mm | Length | |
| 2 mm | Outer diameter | |
| 1.5 mm | Inner diameter (Nozzle) | |
| Powering characteristics | AC high voltage | Power source |
| 2.5 to 25 kV | Applied voltage | |
| 60 kHz | Frequency |
| O2 Flow Rate | Equivalent O2 Ratio |
|---|---|
| 2.5 mslm | 0.25% |
| 5 mslm | 0.50% |
| 10 mslm | 1% |
| 15 mslm | 1.5% |
| Flow Rate slm | Width mm | Length mm |
|---|---|---|
| 1.8 | 1.9 | 13.2 |
| 2 | 2.07 | 13.2 |
| 2.8 | 1.8 | 8.8 |
| 3 | 1.73 | 8.8 |
| Axial Length of the Jet (mm) | Width (mm) | Oxygen Ratio 0.25% | Oxygen Ratio 0.50% | Oxygen Ratio 1% | Oxygen Ratio 1.5% |
| 13.2 | 0.455 | 0.51 | 0.455 | 0.4 | |
| 15.4 | 0.561 | 0.561 | 0.707 | 0.854 | |
| 17.6 | 0.561 | 0.756 | 0.853 | 1.196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, A.H.; Galaly, A.R. The Effect of Oxygen Admixture with Argon Discharges on the Impact Parameters of Atmospheric Pressure Plasma Jet Characteristics. Appl. Sci. 2021, 11, 6870. https://doi.org/10.3390/app11156870
Asghar AH, Galaly AR. The Effect of Oxygen Admixture with Argon Discharges on the Impact Parameters of Atmospheric Pressure Plasma Jet Characteristics. Applied Sciences. 2021; 11(15):6870. https://doi.org/10.3390/app11156870
Chicago/Turabian StyleAsghar, Atif H., and Ahmed Rida Galaly. 2021. "The Effect of Oxygen Admixture with Argon Discharges on the Impact Parameters of Atmospheric Pressure Plasma Jet Characteristics" Applied Sciences 11, no. 15: 6870. https://doi.org/10.3390/app11156870
APA StyleAsghar, A. H., & Galaly, A. R. (2021). The Effect of Oxygen Admixture with Argon Discharges on the Impact Parameters of Atmospheric Pressure Plasma Jet Characteristics. Applied Sciences, 11(15), 6870. https://doi.org/10.3390/app11156870






