Deterioration of Sandstone in the Historical and Contemporary Sea Walls upon the Impact of the Natural and Man-Made Hazards
Abstract
:1. Introduction
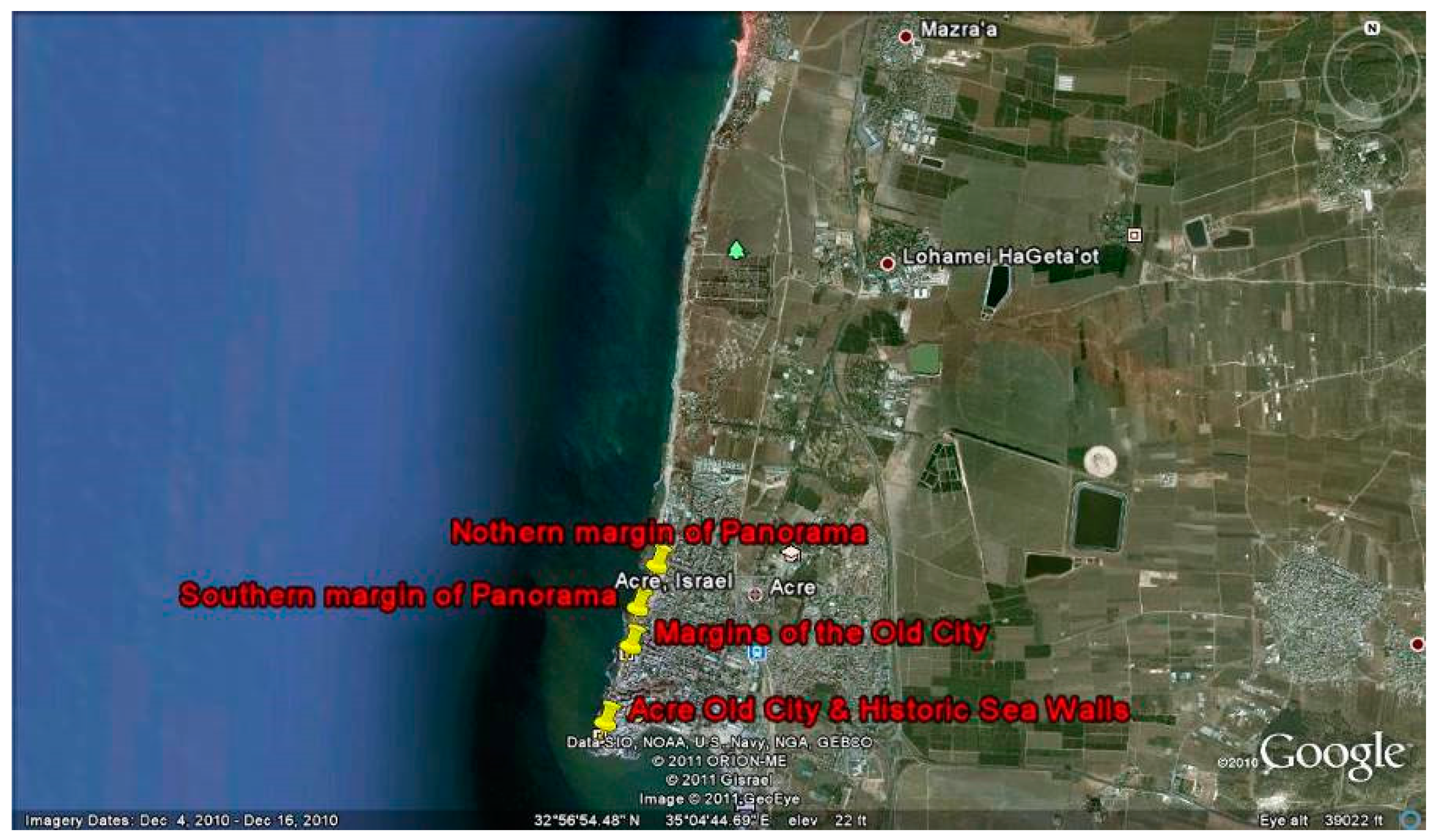
1.1. Historical Background of the Place
1.2. Materials and Construction Methods Used in the Panorama Sea Walls
2. Defects Appeared in the Panorama Sea Walls
- -
- Group 1—Loss of stone material:
- -
- Group 2—Coloration/Deposits
3. Experimental and Results
- Was it realistic to predict kurkar stone durability trends before its implementation in the Panorama Sea Wall?
- What factors have led to the quick deterioration of kurkar stone siding in the contemporary Panorama Sea Wall, although the kurkar stones in Acre’s historic sea wall were less deteriorated?
3.1. Mineral Composition
3.2. Physical Properties and Strength
- S > 0.85—susceptible.
- 0.75 < S < 0.80—moderate resistant.
- S < 0.75—frost resistant.
- -
- The decreased compressive strength and modulus of rupture, i.e., a drop of 16 to 23% and 18 to 35%, respectively.
- -
- The capillary absorption rise: 4.9 to 7.6 times as much as of the original unweathered stone.
- -
- The total absorption has dramatically risen due to weathering, and it was 1.5 times more than in the original unweathered stone.
- -
- A sharp decline of the initial evaporation capacity was observed: about twice as less as in the unweathered stone.
- -
- An increase in the saturation (Hirschwald’s) coefficient of weathered stones is more than twice as high as observed in the unweathered samples.
3.3. Soundness
- -
- Perpendicular to rift plane—from 3291 to 4956 g per m2 per h½ or 2.9 to 4.4 % (w. %).
- -
- Parallel to rift plane—from 3989 to 4227 g per m2 per h½ or 3.5 to 3.8% (w. %).
4. Discussion
- (a)
- Sulphuric and nitric acids, which are supplied by acid precipitation because of air pollution, take over from carbonic acid in weathering reactions of albite, which induced a decrease in the atmospheric/soil CO2 consumption by weathering [32]. The high air pollution in the urban environment causes dissolving CO2 in water and carbonic acid formation afterward. This process promotes the mineral weathering of albite feldspar in kurkar sandstone and results in albite kaolinization [32,33,34]:
- (b)
- Kurkar sandstones in the Panorama Sea Walls have always been exposed to the non-stop dry–wet cycles in acidic and alkaline environments with high levels of air pollution (acidic pH ca. 4.0 (estimated) and seawater breeze (alkaline pH ca. 8.5–9.0), respectively. The dissolution reactions of albite and calcite minerals that form cement in sandstone are too spontaneous in an acidic environment. In an alkaline solution, the dissolution reaction of quartz in sandstone is spontaneous [25,28]. The dissolution of the large amounts of sandstone cement explains kurkar crumbling (see Figure 5) and a sharp decrease in the mechanical properties of naturally weathered kurkar (see Table 2). A sharp rise in capillary absorption could be partially attributed to quartz (aggregate) dissolution leading to the high sandstone porosity, see Figure 7. A dissolution of silicon and a release of iron (II) ions from biotite subjected to the solutions with the low and near neutral (alkali) pH is found to occur within the first ten months of exposure [32,34]. The oxidation of iron (II) to iron (III) in Panorama Sea Walls’ sandstone might be a rapid process given the seawater chlorides absorbed by kurkar. Furthermore, the rusty coloration of kurkar, see Figure 10, has resulted from these redox reactions of iron phases released by biotite because of its degradation.
- (c)
- The walls were cast in-situ against sandstone veneer siding. Thus, one can conclude with confidence that kurkar stone has been, for at least 28 days, in direct contact with fresh and hardening cement-based matrix, which means that kurkar absorbed high pH alkali solutions from concrete. It is common knowledge that the pH of fresh and hardening concrete is ca.12 to 13, and alkalis in it are Ca2+ (major alkali) and Na+& K+ (minor alkalis).). Furthermore, salt-containing seawater breeze and acid air pollution in Acre Bay have promoted the formation of significant efflorescence at kurkar face surface, see Figure 7 and Figure 9 (The chemical composition of such efflorescence might be variable and consists of the following salts: halite, NaCl, thenardite, Na2SO4, mirabilite, Na2SO4·10H2O, thermonatrite, Na2CO3·H2O, gypsum anhydrite, CaSO4 and gypsum, CaSO4·2H2O, depending on (a) seawater moisture content in kurkar sandstone, (b) concentration of alkalis absorbed by sandstone from fresh and hardening mortar/concrete and (c) levels of the local air pollutions [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
- Water absorption and Hirshwald’s coefficient have been substantially increased;
- Water evaporation and mechanical strength have been considerably decreased.
Impact of Construction Technology on Durability of Kurkar Sandstones in the Historic Sea Walls Versus the Panorama Sea Walls in Acre
- The ashlar kurkar blocks, ca. 0.3–0.6 m long, thick, and wide by size, were used in the Historic Walls’ stonework in Acre. In these Walls, the bed joints were made of air lime mortar, [41], see Figure 15. Air lime mortar covered ca. 15% of each kurkar block’s total surface, and the rest of the body has been left breathable and useful for the evaporation of moisture from the historic sea walls.
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenstein, J. Ultimate strip loading on anisotropic calcareous sandstone. Rock Mech. Felsmech. Mec. Roches 1975, 7, 73–81. [Google Scholar] [CrossRef]
- Fort, R.; Varas, M.J.; de Buergo, M.A.; Martin-Freire, D. Determination of anisotropy to enhance the durability of natural stone. J. Geophys. Eng. 2011, 8, S132–S144. [Google Scholar] [CrossRef]
- Kovacs, T. Durability of Crystalline Monumental Stones in Terms of Their Petrophysical Characteristics; Alma Mater Studiorum—Università di Bologna: Bologna BO, Italy, 2009. [Google Scholar]
- Petersen, A. A Gazetteer of Buildings in Muslim Palestine (Part 1); Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- 14th century BCE COJS—Center for Online Judaic The Amarna Letters. The Amarna Letters, 14th Century BCE. Available online: http://cojs.org/the_amarna_letters-_14th_century_bce (accessed on 1 December 2020).
- Aharoni, Y. The Land of the Bible: A Historical Geography; Westminster Press: Philadelphia, PA, USA, 1979. [Google Scholar]
- Acco, Ptolemais, Acre. Available online: https://www.bibleplaces.com/acco/ (accessed on 1 December 2020).
- New Testament, Acts 21:7. Available online: https://biblehub.com/acts/21-7.htm (accessed on 1 December 2020).
- Michaud, F.; Robson, W. The History of the Crusades, Volume 3. Available online: http://books.google.co.uk/books?id=mAcMAAAAYAAJ&client=firefox-a&pg=PA70#v=onepage&q&f=false (accessed on 1 December 2020).
- Sivan, D.; Porat, N. Evidence from luminescence for Late Pleistocene formation of calcareous aeolianite (kurkar) and paleosol (hamra) in the Carmel Coast, Israel. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 211, 95–106. [Google Scholar] [CrossRef]
- SI 2378 Part 2. Natural Stone Cladded Wall: Walls Cladded Using the Wet Fixing Method; Israeli Standard Institute: Tel Aviv-Yafo, Israel, 2005; p. 44. [Google Scholar]
- ASTM C97/C97M-15. Standard Test Methods for Absorption and Bulk Specific Gravity of Dimension Stone; ASTM Compass: West Conshohocken, PA, USA, 2015. [Google Scholar]
- EN 1925. Natural Stone Test Methods—Determination of Water Absorption Coefficient by Capillarity; BSI—British Standards Institution: London, UK, 1999; p. 10. [Google Scholar]
- C99/C99M-15. Standard Test Method for Modulus of Rupture of Dimension Stone; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Fitzner, B.; Heinrics, K. Photo Atlas of Weathering Forms on Stone Monuments. 2004. Available online: http://www.stone.rwth-aachen.de/atlas.htm (accessed on 1 December 2020).
- Bullock, P.; Fedoroff, N.; Iongerius, A.; Stoops, G.; Tursina, T. Handbook for Soil Thin Section Description; Waine Research Publication: Shropshire, UK, 1985. [Google Scholar]
- AAdams, E.; MacKenzie, W.S. A Color Atlas of Carbonate Sediments and Rocks under the Microscope, 1st ed.; Manson Publishing Ltd.: Copenhagen, Denmark, 1998. [Google Scholar]
- Hirschwald, J. Systematic research of the stone materials of ancient buildings. 2. The weathering of the Otto Heinrich building in the Heidelberg castle. In Bautechnische Gesteinuntersuchungen; Available online: https://www.worldcat.org/title/bautechnische-gesteinsuntersuchungen-mitteilungen-aus-dem-mineralog-geolog-institut-der-kgl-technischen-hochschule-berlin/oclc/891572775/editions?referer=di&editionsView=true (accessed on 1 December 2020).
- Bourgès, A. Holistic Correlation of Physical and Mechanical Properties of Selected Natural Stones for Assessing Durability and Weathering in the Natural Environment; Ludwigs-Maximilians-Universität München: Munich, Germany, 2006. [Google Scholar]
- Robert, R. Analytical Characterization of Porous Geomaterials Reference Assessment in Some Sedimentary Rocks; Humboldt-Universität: Berlin, Germany, 2004. [Google Scholar]
- Rousset, B.; Gentile, S.; James, J. Injection Grouts for Molasse Sandstones: Preliminary Assesment; Expert-Center for Conservation of Monuments and Sites of Lausanne: Lausanne, Switzerland; Delft, The Netherlands, 2005; p. 11. [Google Scholar]
- Ghobadi, M.H.; Babazadeh, R. Experimental Studies on the Effects of Cyclic Freezing–Thawing, Salt Crystallization, and Thermal Shock on the Physical and Mechanical Characteristics of Selected Sandstones. Rock Mech. Rock Eng. 2015, 48, 1001–1016. [Google Scholar] [CrossRef]
- Ruedrich, J.; Siegesmund, S. Salt and ice crystallisation in porous sandstones. Environ. Geol. 2007, 52, 225–249. [Google Scholar] [CrossRef]
- EN 12370. Natural Stone Test Methods. Determination of Resistance to Salt Crystallization; BSI—British Standards Institution: London, UK, 1999. [Google Scholar]
- Winkler, E.M. Stone in Architecture; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Suchet, P.A.; Probst, A.; Probst, J.L. Influence of acid rain on CO2 consumption by rock weathering: Local and global scales. Water Air Soil Pollut. 1995, 85, 1563–1568. [Google Scholar] [CrossRef]
- Schiavon, N. Kaolinisation of granite in an urban environment. Environ. Geol. 2007, 52, 399–407. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, X.; Fu, Y. Chemical thermodynamics and chemical kinetics analysis of sandstone dissolution under the action of dry–wet cycles in acid and alkaline environments. Bull. Eng. Geol. Environ. 2019, 78, 793–801. [Google Scholar] [CrossRef]
- Diko, M.L. Physicochemical and mineralogical considerations of Ediki sandstone-hosted kaolin occurrence, South West Cameroon. Int. J. Phys. Sci. 2012, 7, 501–507. [Google Scholar]
- Malmström, M.; Banwart, S. Biotite dissolution at 25 °C: The pH dependence of dissolution rate and stoichiometry. Geochim. Cosmochim. Acta 1997, 61, 2779–2799. [Google Scholar] [CrossRef]
- Savage, D.; Bateman, K.; Hill, P.; Hughes, C.; Milodowski, A.; Pearce, J.; Rae, E.; Rochelle, C. Rate and mechanism of the reaction of silicates with cement pore fluids. Appl. Clay Sci. 1992, 7, 33–45. [Google Scholar] [CrossRef]
- Malmström, M.; Banwart, S.; Lewenhagen, J.; Duro, L.; Bruno, J. The dissolution of biotite and chlorite at 25 °C in the near-neutral pH region. J. Contam. Hydrol. 1996, 21, 201–213. [Google Scholar] [CrossRef]
- SSamson, D.; Nagy, K.L.; Cotton, W.B. Transient and quasi-steady-state dissolution of biotite at 22–25°C in high pH, sodium, nitrate, and aluminate solutions. Geochim. Cosmochim. Acta 2005, 69, 399–413. [Google Scholar] [CrossRef]
- Sharvit, J.; Planer, D. Akko, the Southern Seawall. Preliminary Report. 2014. Available online: http://www.hadashot-esi.org.il/report_detail_eng.aspx?id=10574&mag_id=121 (accessed on 5 January 2021).
- Mitchell, D.; Torney, C. Inform: The Use of Lime and Cement in Traditional Builings; Historic Scotland: Edinburgh, Scotland, 2015. [Google Scholar]
- Vitruvius, P. The Ten Books on Architecture. Book II: Stone. Available online: http://www.perseus.tufts.edu/hopper/text?doc=Vitr.2&lang=original (accessed on 3 December 2020).
- Osborne, F.F. Rift, grain, and hardway in some pre-Cambrian granites, Quebec. Econ. Geol. 1935, 30, 540–551. [Google Scholar] [CrossRef]
- Rivas, T.; Prieto, B.; Silva, B. Influence of rift and bedding plane on the physico-mechanical properties of granitic rocks. Implications for the deterioration of granitic monuments. Build. Environ. 2000, 35, 387–396. [Google Scholar] [CrossRef]
- NNaghoj, M.; Youssef, N.A.R.; Maaitah, O.N. Mechanical Properties of Natural Building Stone: Jordanian Building Limestone as an Example. Jordan J. Earth Environ. Sci. 2010, 3, 37–48. [Google Scholar]
- Israel Ministry of Environmental Protection. Reduction of Pollution from Transportation in Haifa Bay (Hebrew, Updated January 2017); Israel Ministry of Environmental Protection: Jerusalem, Israel, 2017. [Google Scholar]
- Israel Ministry of Environmental Protection. Haifa Bay Air Quality Values (Hebrew, Updated January 2017); Israel Ministry of Environmental Protection: Jerusalem, Israel, 2017. [Google Scholar]
- NUMBEO. Pollution in Acre, Israel. 2020. Available online: https://www.numbeo.com/pollution/in/Acre (accessed on 30 December 2020).
- Charola, A.E.; Pühringer, J.; Steiger, M. Gypsum: A review of its role in the deterioration of building materials. Environ. Geol. 2007, 52, 339–352. [Google Scholar] [CrossRef]
- Sánchez, J.S.; Alves, C.A.S.; Romaní, J.R.V.; Mosquera, D.F. Origin of Gypsum-rich Coatings on Historic Buildings. Water. Air. Soil Pollut. 2009, 204, 53–68. [Google Scholar] [CrossRef]
- Sánchez, J.S.; Romaní, J.R.V.; Alves, C. Deposition of particles on gypsum-rich coatings of historic buildings in urban and rural environments. Constr. Build. Mater. 2011, 25, 813–822. [Google Scholar] [CrossRef]
- Schaffer, Y.; Sharvit, J.; Planer, D. Structural and conservation solutions for the treatment of the sea front walls of old Akko (Acre), Israel. In Proceedings of the SAHC2014: 9th International Conference on Structural Analysis of Historical Constructions, Mexico City, Mexico, 14–17 October 2014; pp. 1–23. [Google Scholar]














| Property | Minimal Value | Maximal Value | Average | Standard Deviation | Requirements for Average Value [11] | Test Method |
|---|---|---|---|---|---|---|
| Bulk density, kg/m³ | 1850 | 2120 | 2010 | 106 | ≥2500 | ASTM C97-18 [12] |
| Total water absorption, mass % | 3.4 | 8.64 | 6.1 | 1.6 | <2 | ASTM C97-18 [12] |
| Capillary water absorption, g per m2 per hour | 2400 | 2680 | 2600 | 55 | ≤500 | EN 1925–1999 [13] |
| Modulus of rupture, MPa | 2.7 | 8.6 | 4.9 | 1.7 | > 3 | ASTM C99-2000 [14] |
| Property | Unweathered Stone | Weathered Stone (Cored from the Wall) | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimal Value | Maximal Value | Average | Standard Deviation | Minimal Value | Maximal Value | Average | Standard Deviation | |
| ‘Capillary absorption coefficient, g per m2 per hour½ | 1747 | 25,398 | 13,569 | 8924 | ||||
| 1601 | 1907 | 1777 | 158 | ||||
| 2073 | 3176 | 2765 | 603 | ||||
| Total water absorption, W, mass % | 3.3 | 10.4 | 4.8 | n/a | 3.7 | 13.0 | 7.3 | n/a |
| Saturation (Hirschwald’s) coefficient [22] | 0.76 | 0.80 | 0.78 | n/a | ||||
| 0.3 | 0.5 | 0.35 | n/a | ||||
| 0.4 | 0.6 | 0.52 | n/a | ||||
| Water evaporation capacity, w. %, after | ||||||||
| 68% | 30% | ||||||
| 90% | 83% | ||||||
| n/a | n/a | 100% | n/a | n/a | n/a | 91% | n/a |
| Compressive strength, MPa | ||||||||
| 6.3 | 34.4 | 14.0 | 8.8 | 6.8 | 25.6 | 11.7 | 8.6 |
| 3.7 | 12.5 | 6.6 | 3.0 | 2.1 | 10.1 | 5.1 | 2.2 |
| Modulus of rupture, MPa | ||||||||
| 4.2 | 8.8 | 6.2 | 1.7 | 2.8 | 4.0 | 4.0 | 1.4 |
| 3.3 | 6.0 | 4.9 | 1.1 | n/a | n/a | n/a | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasserman, R. Deterioration of Sandstone in the Historical and Contemporary Sea Walls upon the Impact of the Natural and Man-Made Hazards. Appl. Sci. 2021, 11, 6892. https://doi.org/10.3390/app11156892
Wasserman R. Deterioration of Sandstone in the Historical and Contemporary Sea Walls upon the Impact of the Natural and Man-Made Hazards. Applied Sciences. 2021; 11(15):6892. https://doi.org/10.3390/app11156892
Chicago/Turabian StyleWasserman, Rina (Irena). 2021. "Deterioration of Sandstone in the Historical and Contemporary Sea Walls upon the Impact of the Natural and Man-Made Hazards" Applied Sciences 11, no. 15: 6892. https://doi.org/10.3390/app11156892
APA StyleWasserman, R. (2021). Deterioration of Sandstone in the Historical and Contemporary Sea Walls upon the Impact of the Natural and Man-Made Hazards. Applied Sciences, 11(15), 6892. https://doi.org/10.3390/app11156892





