Determination of VOCs in Surgical Resected Tissues from Colorectal Cancer Patients by Solid Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and SPME Device
2.2. SPME VOCs Extraction
2.3. GC–MS Apparatus and Analysis Experimental Conditions
3. Results and Discussion
3.1. Optimization of SPME Parameters
3.2. Validation of HS-SPME and DI-SPME Optimized Methods Coupled to GC/MS
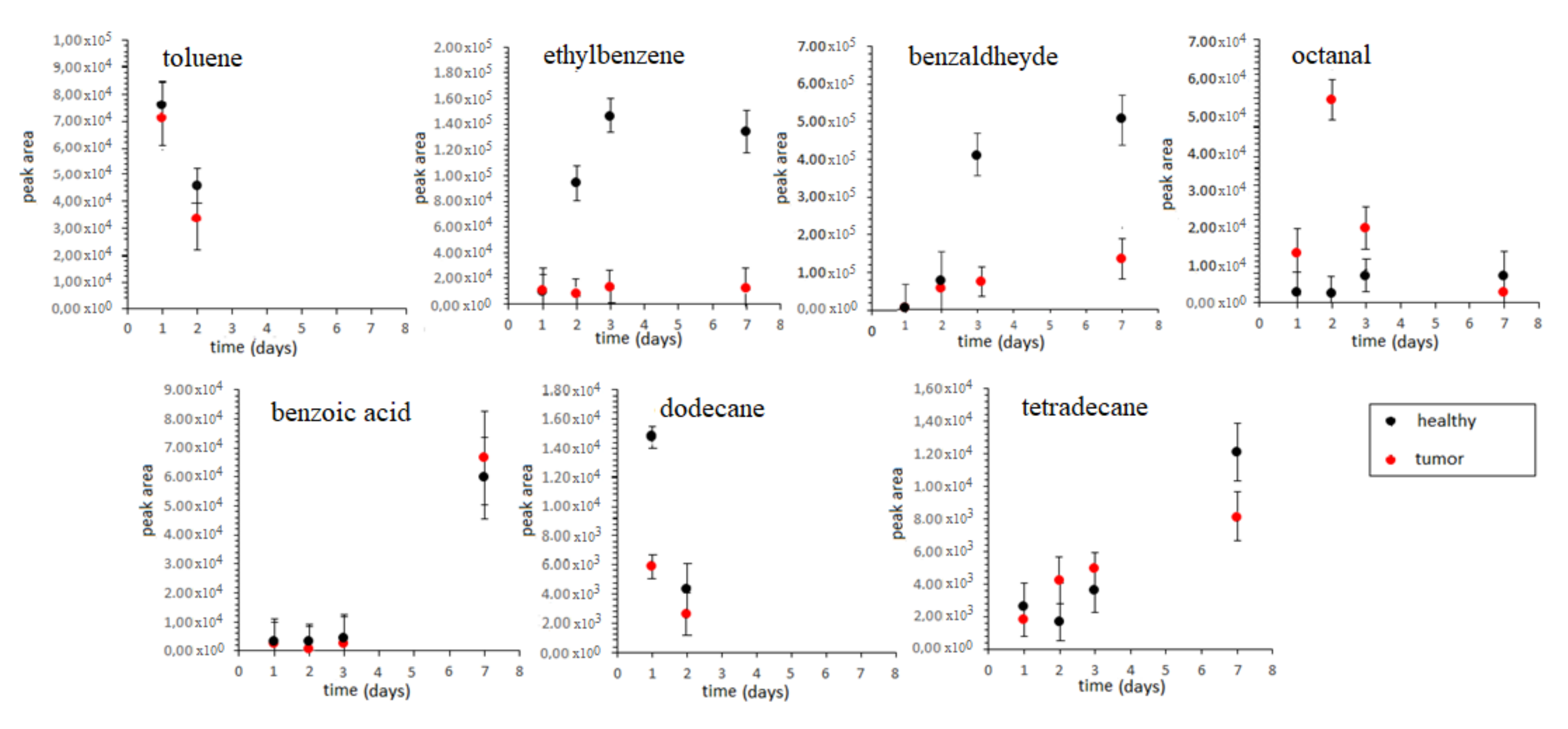
3.3. Analyses of Ex Vivo Colon Tissues from CRC Patients
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Parkin, D.M.; Steliarova-Foucher, E. Estimates of cancer incidence and mortality in Europe in 2008. Eur. J. Cancer 2010, 46, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.; Bording-Jorgensen, M.; Wine, E. Review:The Multifaceted Roles of Diet, Microbes, and Metabolites in Cancer. Cancers 2021, 13, 767. [Google Scholar] [CrossRef]
- Siegel, R.; De Santis, C.; Virgo, K.; Mariotto, A.; Smith, T.; Cooper, D.; Ganslet, T.; Lerro, C.; Fedewa, S.; Lin, C.; et al. Cancer treatment and survivorship statistics. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Favoriti, P.; Cardone, G.; Greco, M.; Pirozzi, F.; Pirozzi, R.E.M.; Corcione, F. Worldwide burden of colorectal cancer: A review. Updates Surg. 2016, 68, 7–11. [Google Scholar] [CrossRef]
- Amann, A.; de Lacy Costello, B.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef]
- Van Berkel, J.J.B.N.; Dallinga, J.W.; Möller, G.M.; Godschalk, R.W.L.; Moonen, E.J.; Wouters, E.F.M.; Van Schooten, F.J. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respir. Med. 2010, 104, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Schmekel, B.; Winquis, F.; Vikström, A. Analysis of breath samples for lung cancer survival. Anal. Chim. Acta 2014, 840, 82–86. [Google Scholar] [CrossRef]
- Antoniou, S.X.; Gaude, E.; Ruparel, M.; van der Schee, M.P.; Janes, S.M.; Rintoul, R.C.; LuCID Research Group. The potential of breath analysis to improve outcome for patients with lung cancer. J. Breath Res. 2019, 13, 034002. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, C.; Turner, C. Breath analysis in disease diagnosis: Methodological considerations and applications. Metabolites 2014, 4, 465–498. [Google Scholar] [CrossRef] [PubMed]
- Anh, D.T.V.; Olthuis, W.; Bergveld, P. A Hydrogen peroxide sensor for exaheled breath measurement. Sens. Actuators B Chem. 2005, 111–112, 494–499. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Peng, Y.; Liu, Y.; Li, W.; Jin, Y.; Tang, Z.; Duan, Y. Investigation of potential breath biomerkers for early diagnosis of breast cancer using gas chromatography-mass spectrometry. Clin. Chim. Acta 2014, 436, 59–67. [Google Scholar] [CrossRef]
- Marzorati, D.; Mainardi, L.; Sedda, G.; Gasparri, R.; Spaggiari, L.; Cerveri, P. A review of exhaled breath: A key role in lung cancer diagnosis. J. Breath Res. 2019, 13, 034001. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, D.; Kumar, P.; Mohanty, S.K.; Smith, Y.R.; Misra, M. Detection of four distinct volatile indicators of colorectal cancer using functionalized titania nanotubular arrays. Sensors 2017, 17, 1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.Q.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br. J. Cancer 2013, 108, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Altomare, D.F.; Picciariello, A.; Rotelli, M.T.; De Fazio, M.; Aresta, A.M.; Zambonin, C.G.; Vincenti, L.; Trerotoli, P.; De Vietro, N. Chemical signature of colorectal cancer: Case–control study for profiling the breath print. BJS 2020, 4, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- De Vietro, N.; Aresta, A.; Rotelli, M.T.; Zambonin, C.; Lippolis, C.; Picciariello, A.; Altomare, D.F. Relationship between cancer tissue derived and exhaled volatile organic compound from colorectal cancer patients. Preliminary results. J. Pharm. Biomed. Anal. 2020, 180, 113055–113065. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Solid Phase Microextraction. In A Century of Separation Science; Marcel Dekker Inc.: New York, NY, USA, 2002; pp. 399–419. [Google Scholar]
- Aresta, A.; De Vietro, N.; Zambonin, C. Ultra-Trace Determination of Sudan I, II, III, and IV in Wastewater by Solid-Phase Microextraction (SPME) and on-line Solid-Phase Extraction (SPE) with High-Performance Liquid Chromatography (HPLC). Anal. Lett. 2020, 53, 2559–2570. [Google Scholar] [CrossRef]
- Moein, M.M.; Said, R.; Bassyouni, F.; Abdel-Rehim, M. Solid Phase Microextraction and Related Techniques for Drugs in Biological Samples. J. Anal. Methods Chem. 2014, 10, 921350. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, B.; Zhang, M.; Shi, J.; Xu, Y. Headspace solid-phase microextraction-gas chromatography–mass spectrometry analysis of the volatile components of longan (Dimocarpus longan Lour.). Eur. Food Res. Technol. 2009, 229, 457–465. [Google Scholar] [CrossRef]
- Ma, Q.L.; Hamid, N.; Bekhit, A.E.D.; Robertson, J.; Law, T.F. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC–MS) analysis of aroma compounds in cooked beef using response surface methodology. Microchem. J. 2013, 111, 16–24. [Google Scholar] [CrossRef]
- Risticevic, S.; Lord, H.; Górecki, T.; Arthur, C.L.; Pawliszyn, J. Protocol for solid-phase microextraction method development. Nat. Protoc. 2010, 5, 122–139. [Google Scholar] [CrossRef]
- Razmi, H.; Pasandideh, Y. Introduction of commercial heating elements of resistance metal alloys as the novel solid-phase microextraction fibers for chromatographic monitoring of organic pollutants. J. Iran. Chem. Soc. 2020, 17, 1111–1121. [Google Scholar] [CrossRef]
- Pereira, A.; Silva, E.; Cerejeira, M.J. Applicability of the New 60 µm Polyethylene Glycol Solid-Phase Microextraction Fiber Assembly for the Simultaneous Analysis of Six Pesticides in Water. J. Chromatogr. Sci. 2014, 52, 423–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
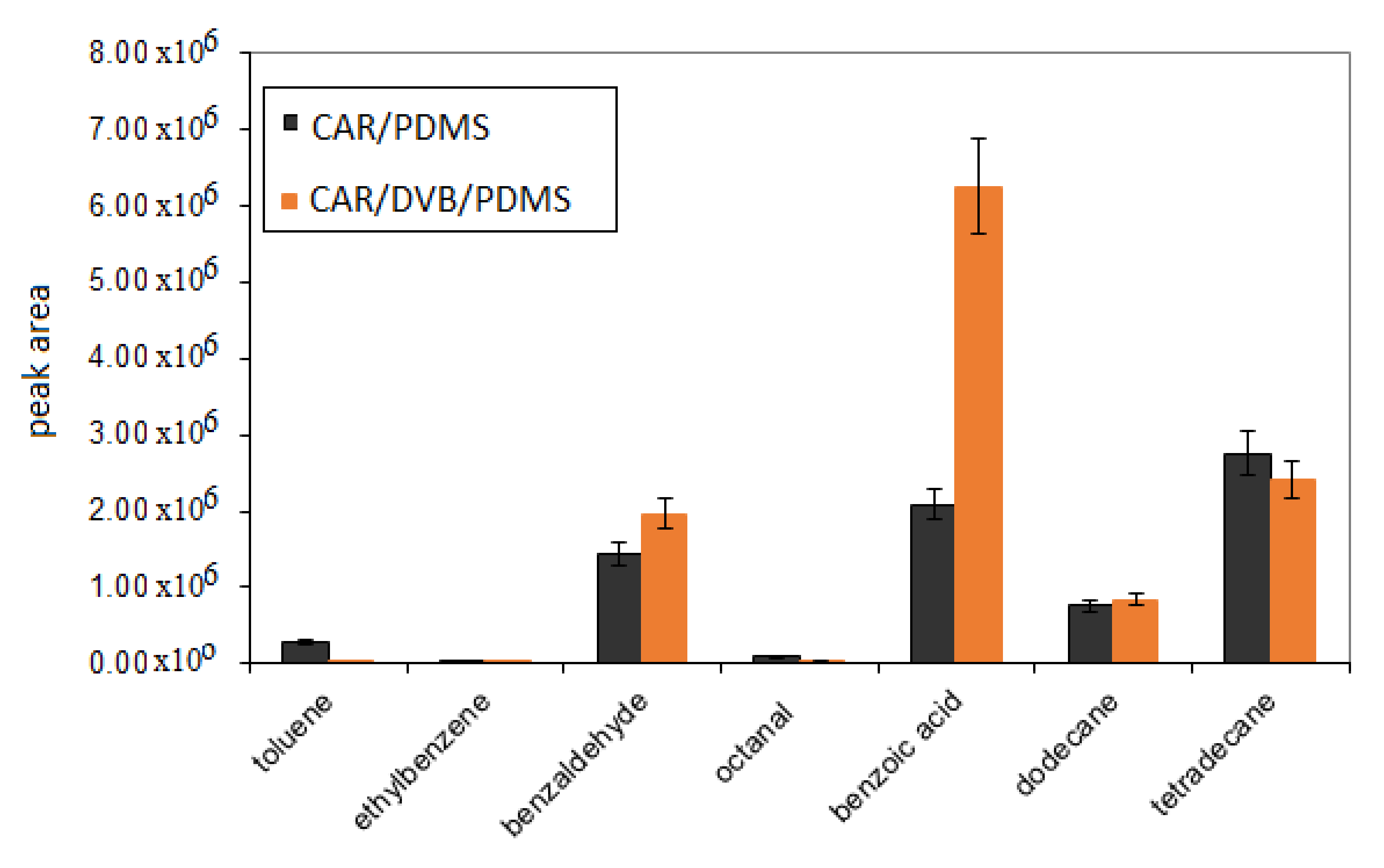
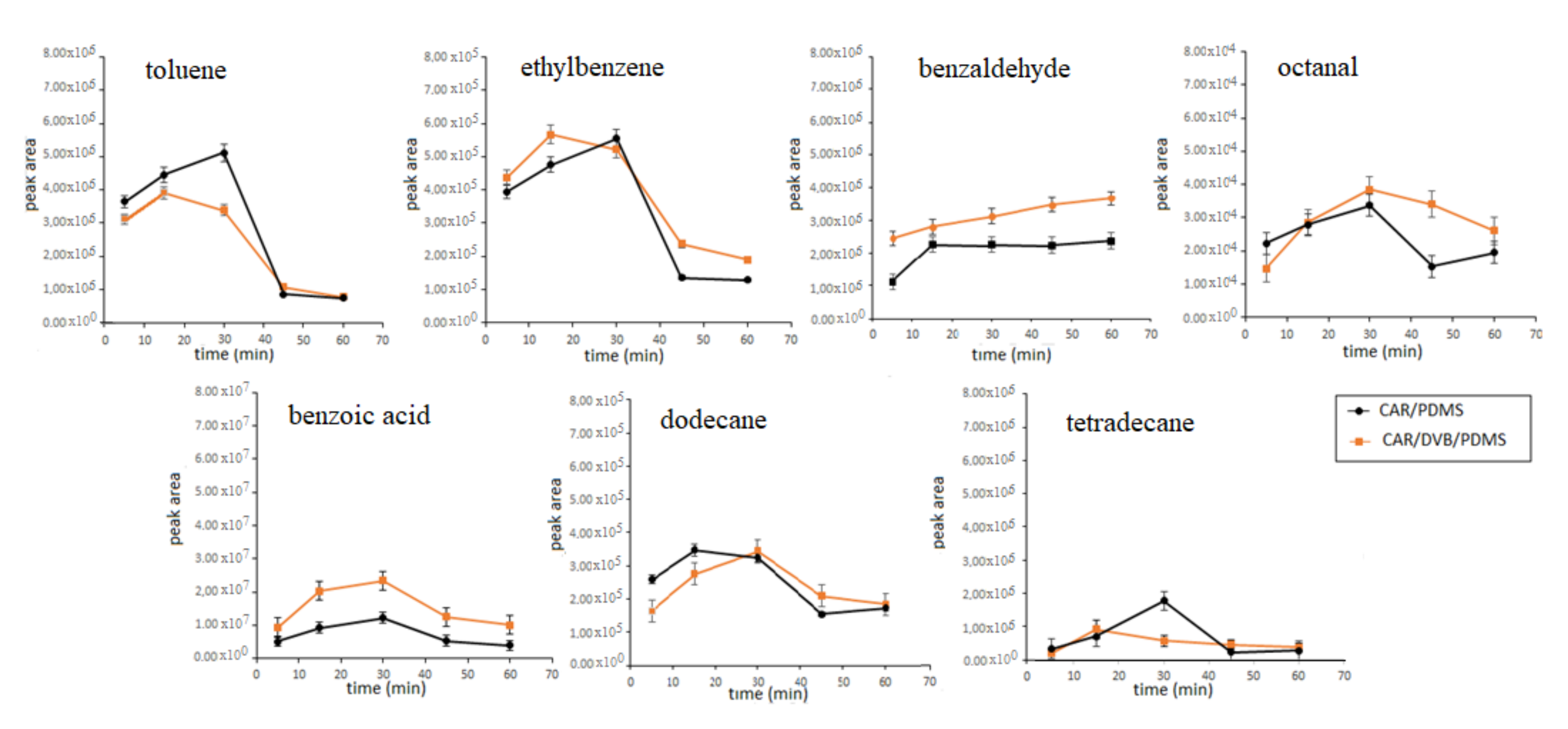
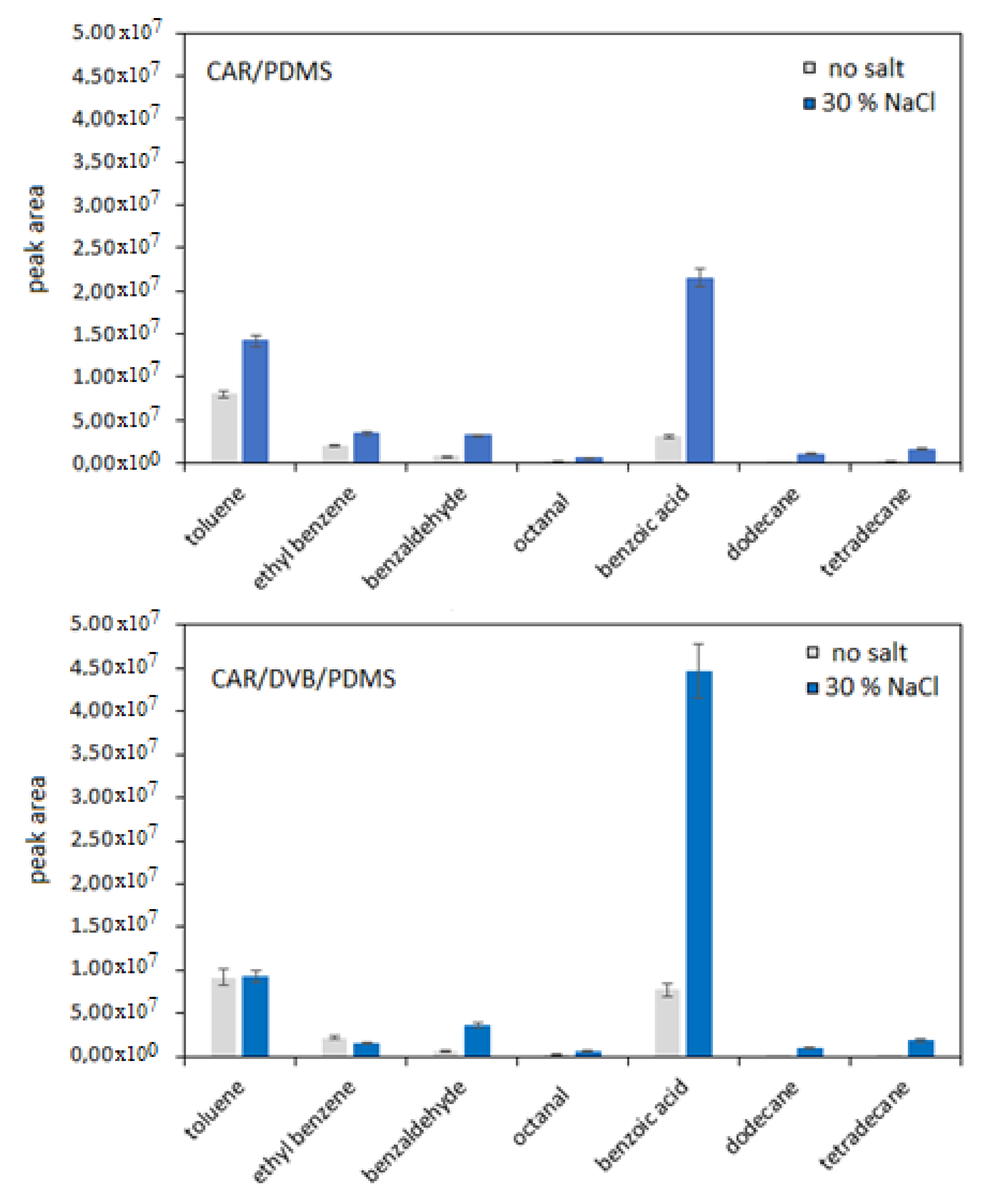
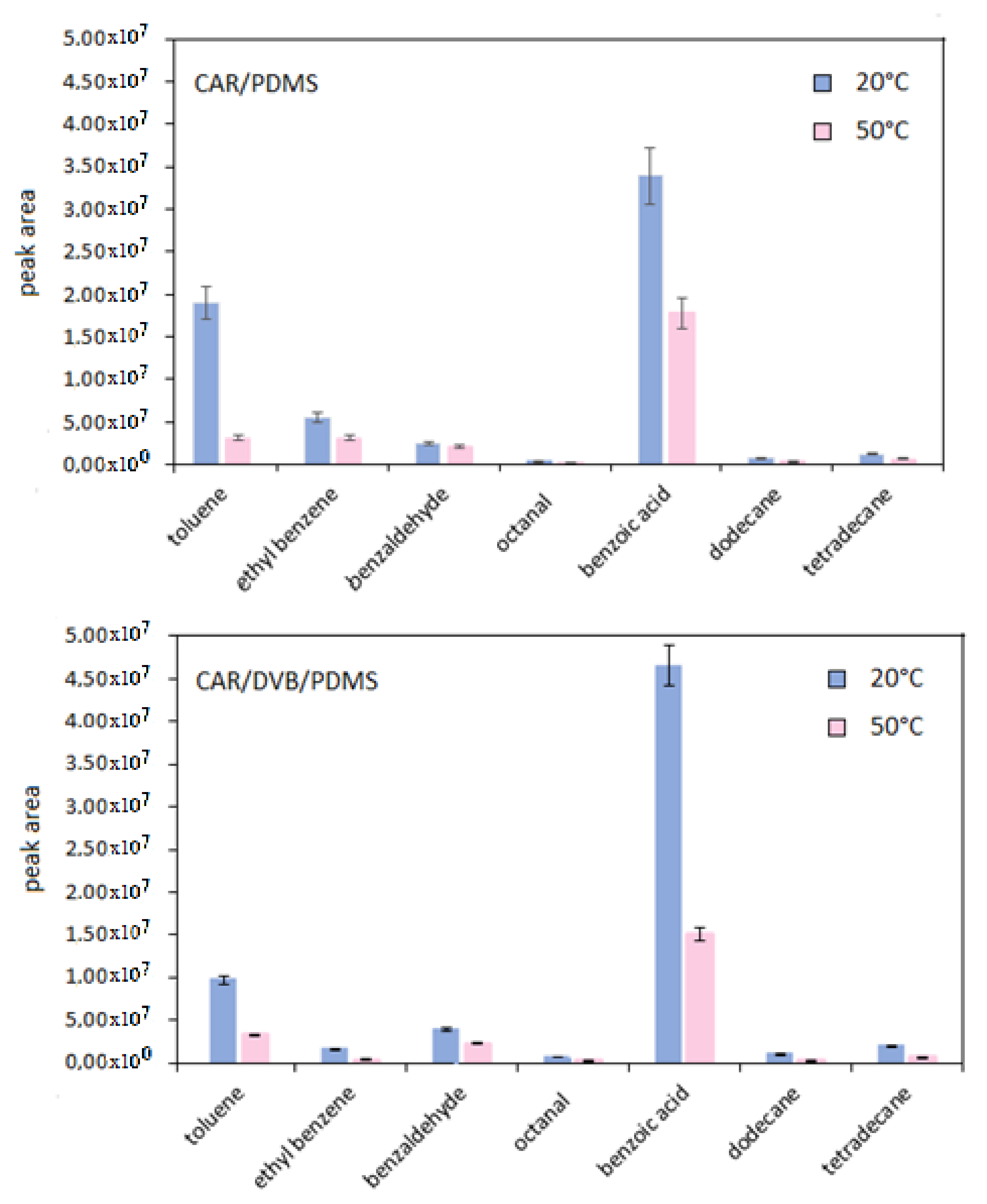
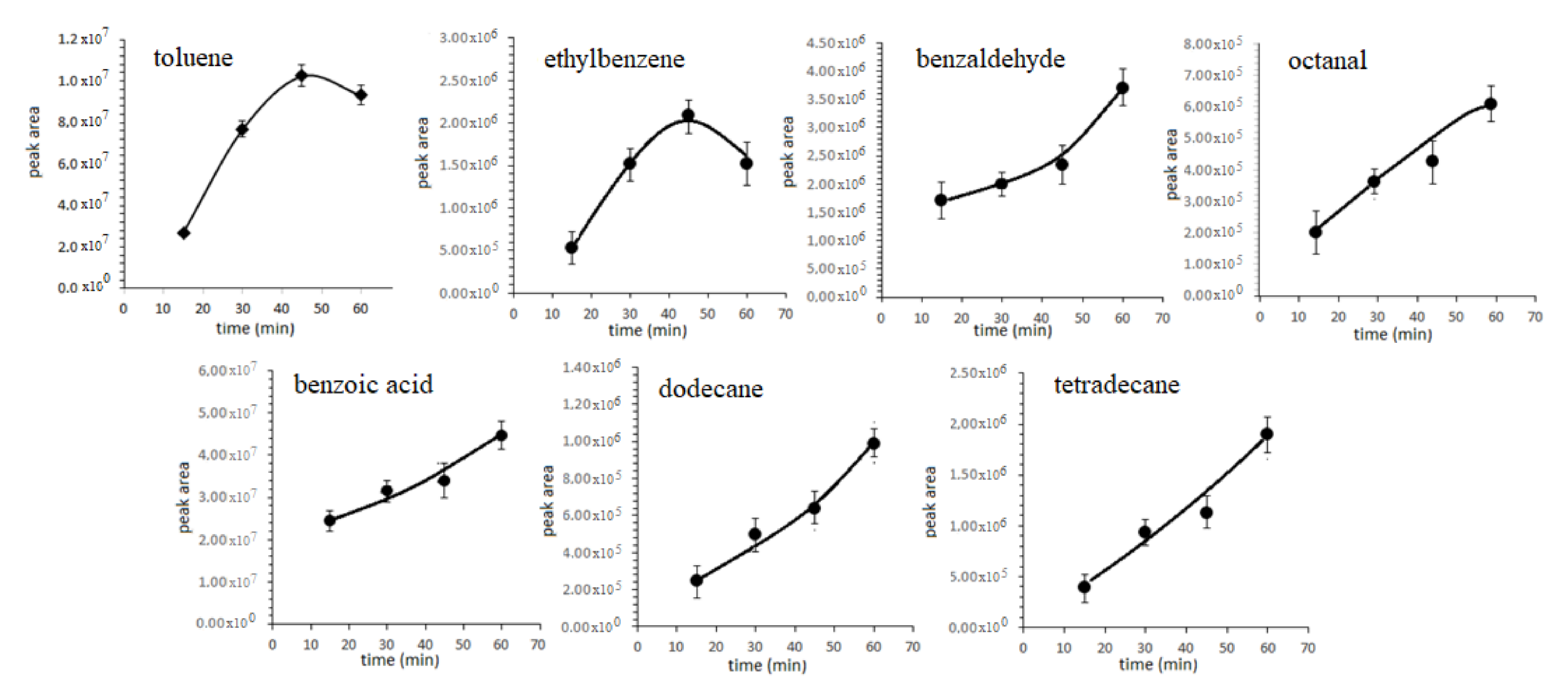
| RT * | Compound | Segment Start–End (min) | Characteristic Ions ** |
|---|---|---|---|
| 5.79 ± 0.03 | toluene | 4.00–7.50 | 65, 91 |
| 8.44 ± 0.02 | ethylbenzene | 7.50–10.50 | 91, 106 |
| 11.01 ± 0.02 | benzaldheyde | 10.50–11.50 | 77, 105 |
| 11.66 ± 0.01 | octanal | 10.50–13.50 | 43, 56 |
| 14.30 ± 0.03 | benzoic acid | 13.50–15.50 | 91, 121 |
| 16.59 ± 0.03 | dodecane | 15.50–18.50 | 57, 71, 85 |
| 23.05 ± 0.04 | tetradecane | 18.50–24.50 | 57, 71, 85 |
| Compound | Equation | R2 | Linear Range (mg/mL) | LOD * (mg/mL) | LOQ * (mg/mL) |
|---|---|---|---|---|---|
| HS-SPME-GC/MS-SIM | |||||
| toluene | y = 5110x + 225 | 0.8681 | 0.05–100 | 0.02 | 0.05 |
| ethylbenzene | y = 2436x + 262 | 0.9941 | 0.14–100 | 0.04 | 0.14 |
| benzaldheyde | y = 18762x + 327 | 0.9531 | 0.09–100 | 0.03 | 0.09 |
| octanal | y = 11427x + 1283 | 0.8974 | 0.08–100 | 0.02 | 0.08 |
| benzoic acid | y = 194091x + 5000 | 0.9891 | 0.01–100 | 3 × 10−3 | 9 × 10−3 |
| dodecane | y = 1732x + 306 | 0.8717 | 1.40–100 | 0.40 | 1.40 |
| tetradecane | y = 17293x−5202 | 0.9883 | 0.10–100 | 0.03 | 0.10 |
| DI-SPME-GC/MS-SIM | |||||
| toluene | y = 18135x + 1617 | 0.9987 | 0.02–1000 | 6 × 10−3 | 0.02 |
| ethylbenzene | y = 2270x + 3031 | 0.9977 | 0.04–1000 | 0.04 | 0.14 |
| benzaldheyde | y = 1704x + 384 | 0.9995 | 0.90–1000 | 0.27 | 0.90 |
| octanal | y = 1990x + 648 | 0.9997 | 0.40–1000 | 0.12 | 0.40 |
| benzoic acid | y = 242162x + 4204 | 0.9974 | 7 × 10−3–100 | 2 × 10−3 | 7 × 10−3 |
| dodecane | y = 1245x − 930 | 0.9996 | 2.00–1000 | 0.60 | 2.00 |
| tetradecane | y = 13800x + 1127 | 0.9969 | 0.17–1000 | 0.05 | 0.17 |
| N # | RT (min) | RI * | Compound |
|---|---|---|---|
| 1 | 3.2 | 671 | 1-butanol |
| 2 | 3.45 | 687 | 2-pentanone |
| 3 | 4.75 | 723 | 1-butanol, 3-methyl |
| 4 | 5.00 | 732 | disulfide, dimethyl |
| 5 | 5.1 | 753 | pyridine |
| 6 | 5.79 | 761 | toluene |
| 7 | 8.44 | 851 | ethylbenzene |
| 8 | 9.02 | 875 | 2-heptanone |
| 9 | 9.14 | 885 | 2,4-dithiapentane |
| 10 | 9.67 | 895 | 2,5-dimethyl pyrazine |
| 11 | 9.91 | 917 | oxime, methoxy-phenyl |
| 12 | 10.69 | 921 | pentanoic acid |
| 13 | 11.01 | 961 | benzaldehyde |
| 14 | 11.14 | 996 | furan, 2-pentyl |
| 15 | 11.66 | 1001 | octanal |
| 16 | 12.14 | 1023 | benzyl Alcohol |
| 17 | 12.9 | 1027 | phenol, 2-methyl |
| 18 | 13.02 | 1094 | 2-nonanone |
| 19 | 13.28 | 1080 | nonanal |
| 20 | 13.63 | 1083 | phenethyl alcohol |
| 22 | 13.88 | 1143 | disulfide, methyl (methylthio)methyl |
| 23 | 14.30 | 1171 | benzoic acid |
| 24 | 13.90 | 1138 | phenol, 2-ethyl |
| 25 | 13.95 | 1143 | phenol, 3,5-dimethyl |
| 26 | 14.89 | 1185 | decanal |
| 27 | 15.40 | 1202 | tetrasulfide, dimethyl |
| 28 | 16.51 | 1288 | indole |
| 29 | 16.59 | 1250 | dodecane |
| 30 | 23.05 | 1399 | tetradecane |
| 31 | 23.62 | 1568 | dodecanoic acid |
| Compound | Healthy Range (µg/mL) | Tumor Range (µg/mL) |
|---|---|---|
| benzaldehyde | 1.14–67.62 | 1.31–394.86 |
| benzoic acid | LOD-0.26 | LOD-0.23 |
| dodecane | LOD -5.43 | LOD-12.59 |
| ethylbenzene | 2.81–62.77 | 3.21–4.38 |
| octanal | 0.96–27.06 | 0.97–3.24 |
| tetradecane | 0.05–0.5 | 0.11–0.79 |
| toluene | nd–3.84 | nd–4.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vietro, N.; Aresta, A.M.; Picciariello, A.; Rotelli, M.T.; Zambonin, C. Determination of VOCs in Surgical Resected Tissues from Colorectal Cancer Patients by Solid Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry. Appl. Sci. 2021, 11, 6910. https://doi.org/10.3390/app11156910
De Vietro N, Aresta AM, Picciariello A, Rotelli MT, Zambonin C. Determination of VOCs in Surgical Resected Tissues from Colorectal Cancer Patients by Solid Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry. Applied Sciences. 2021; 11(15):6910. https://doi.org/10.3390/app11156910
Chicago/Turabian StyleDe Vietro, Nicoletta, Antonella Maria Aresta, Arcangelo Picciariello, Maria Teresa Rotelli, and Carlo Zambonin. 2021. "Determination of VOCs in Surgical Resected Tissues from Colorectal Cancer Patients by Solid Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry" Applied Sciences 11, no. 15: 6910. https://doi.org/10.3390/app11156910









