Effect of Shiga Toxin on Inhomogeneous Biological Membrane Structure Determined by Small-Angle Scattering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of STxB Purified with a Novel Purification System
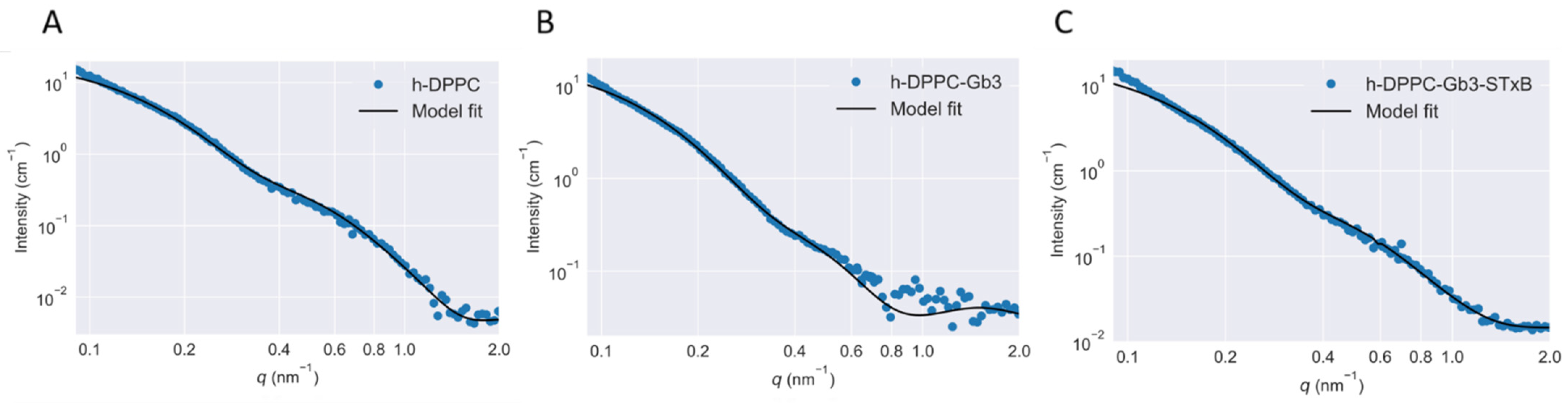
3.2. Contrast Dependence of the Scattering Curves
3.3. Model Membrane Thinning upon STxB Binding
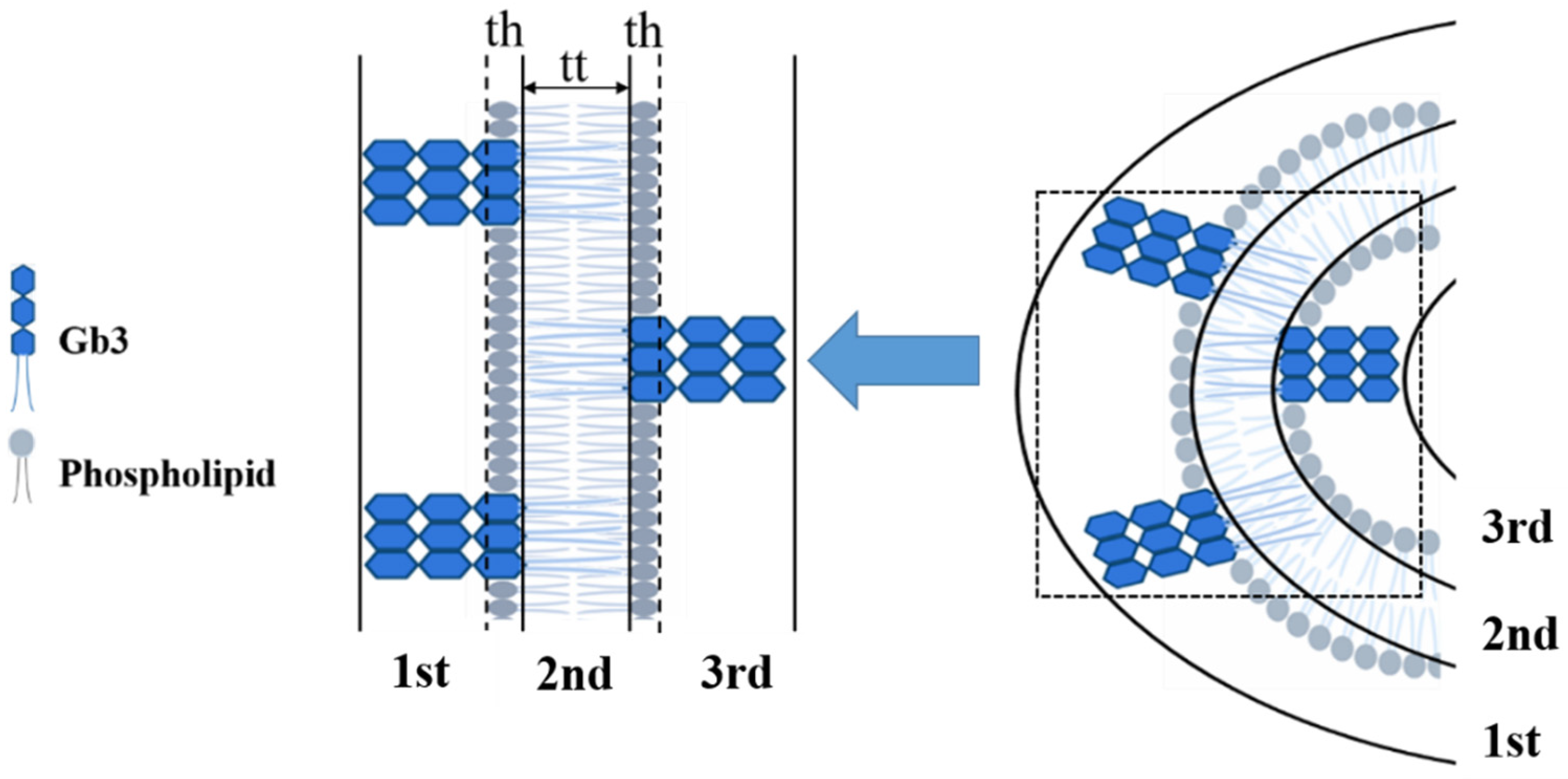
3.4. Glycolipids Form Nanometer-Sized Clusters and Larger Scale Clusters Can Be Induced at Cell Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Hurley, J.H.; Boura, E.; Carlson, L.A.; Rózycki, B. Membrane budding. Cell 2010, 143, 875–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodgers, W.; Farris, D.; Mishra, S. Merging complexes: Properties of membrane raft assembly during lymphocyte signaling. Trends Immunol. 2005, 26, 97–103. [Google Scholar] [CrossRef]
- Van Meer, G.; Sprong, H. Membrane lipids and vesicular traffic. Curr. Opin. Cell Biol. 2004, 16, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Voeltz, G.K.; Prinz, W.A. Sheets, ribbons and tubules—How organelles get their shape. Nat. Rev. Mol. Cell Biol. 2007, 8, 258–264. [Google Scholar] [CrossRef]
- Furne, C.; Corset, V.; Herincs, Z.; Cahuzac, N.; Hueber, A.O.; Mehlen, P. The dependence receptor DCC requires lipid raft localization for cell death signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 4128–4133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, A.; Russell, J.Q.; Rodgers, W.A.; Budd, R.C. Spatial differences in active caspase-8 defines its role in T-cell activation versus cell death. Cell Death Differ. 2008, 15, 1701–1711. [Google Scholar] [CrossRef]
- Baruthio, F.; Quadroni, M.; Ruegg, C.; Mariotti, A. Proteomic analysis of membrane rafts of melanoma cells identifies protein patterns characteristic of the tumor progression stage. Proteomics 2008, 8, 4733–4747. [Google Scholar] [CrossRef]
- Jacobson, K.; Mouritsen, O.G.; Anderson, R.G. Lipid rafts: At a crossroad between cell biology and physics. Nat. Cell Biol. 2007, 9, 7–14. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Howes, M.T.; Mayor, S.; Parton, R.G. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr. Opin. Cell Biol. 2010, 22, 519–527. [Google Scholar] [CrossRef]
- Johannes, L.; Mayor, S. Induced domain formation in endocytic invagination, lipid sorting, and scission. Cell 2010, 142, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Varma, R.; Sarasij, R.C.; Gousset, K.; Krishnamoorthy, G.; Rao, M.; Mayor, S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 2004, 166, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Antonny, B. Membrane deformation by protein coats. Curr. Opin. Cell Biol. 2006, 18, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Reynwar, B.J.; Illya, G.; Harmandaris, V.A.; Müller, M.M.; Kremer, K.; Deserno, M. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 2007, 447, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Römer, W.; Berland, L.; Chambon, V.; Gaus, K.; Windscheigl, B.; Tenza, D.; Aly, M.R.E.; Fraisier, V.; Florent, J.C.; Perrais, D.; et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 2007, 450, 670–675. [Google Scholar] [CrossRef]
- Pezeshkian, W.; Gao, H.; Arumugam, S.; Bechen, U.; Bassereau, P.; Florent, J.C.; Ipsen, J.H.; Hohannes, L.; Shillcock, J.C. Mechanism of Shiga Toxin Clustering on Membranes. ACS Nano 2017, 11, 314–324. [Google Scholar] [CrossRef] [Green Version]
- Bassereau, P.; Jin, R.; Baumgart, T.; Deserno, M.; Dimova, R.; Frolov, V.A.; Bashkirov, P.V.; Grubmuller, H.; Jahn, R.; Risselada, H.J.; et al. The 2018 biomembrane curvature and remodeling roadmap. J. Phys. D Appl. Phys. 2018, 51, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Casimir, H.B.G.; Polder, D. The Influence of Retardation on the London-van der Waals Forces. Phys. Rev. 1948, 73, 360–372. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, X.; Guan, D.; Xu, J.; Wu, H.; Tong, P.; Zhang, M. Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell 2018, 174, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hirai, M.; lwase, J.; Hayakawa, T.; Koizumi, M.; Takahashi, H. Determination of asymmetric structure of ganglioside-DPPC mixed vesicle using SANS, SAXS, and DLS. Biophys. J. 2003, 85, 1600–1610. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Li, Y.; Wu, H.; Wu, X.; Xu, X.; Wang, W.; Zhang, R.; Li, N. Upgraded SSRF BL19U2 beamline for small-angle X-ray scattering of biological macromolecules in solution. J. Appl. Cryst. 2018, 51, 1633–1640. [Google Scholar] [CrossRef]
- Vogtt, K.; Siebenburger, M.; Clemens, D.; Rabe, C.; Lindner, P.; Russina, M.; Fromme, M.; Mezei, F.; Ballauff, M. A new time-of-flight small-angle scattering instrument at the Helmholtz-Zentrum Berlin: V16/VSANS. J. Appl. Cryst. 2014, 47, 237–244. [Google Scholar] [CrossRef]
- Breβler, L.; Kohlbrecher, J.; Thünemann, A.F. SASfit: A tool for small-angle scattering data analysis using a library of analytical expressions. J. Appl. Crystallogr. 2015, 20, 1587–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armen, R.S.; Olivia, D.U.; Scott, E.F. Phospholipid component volumes: Determination and application to bilayer structure calculations. Biophys. J. 1998, 75, 734–744. [Google Scholar] [CrossRef] [Green Version]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Bophys. Acta 2000, 1512, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Hirai, M.; Takizawa, T. Intensive extrusion and occlusion of water in ganglioside micelles with thermal reversibility. Biophys. J. 1998, 74, 3010–3014. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Hirai, M. Hydration and thermal reversibility of glycolipids depending on sugar chains. Eur. Biophys. J. 2002, 31, 62–72. [Google Scholar] [CrossRef]
- Vrljic, M.; Nishimura, S.Y.; Brasselet, S.; Moerner, W.E.; McConnell, H.M. Translational diffusion of individual class II MHC membrane proteins in cells. Biophys. J. 2002, 83, 2681–2692. [Google Scholar] [CrossRef] [Green Version]
- Goswami, D.; Gowrishankar, K.; Bilgrai, S.; Ghosh, S.; Raghupath, R.; Chadda, R.; Vishwakarma, R.; Rao, M.; Mayor, S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 2008, 135, 1085–1097. [Google Scholar] [CrossRef] [Green Version]
- Fujita, A.; Cheng, J.; Hirakawa, M.; Furukawa, K.; Kusunoki, S.; Fujimoto, T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol. Biol. Cell 2007, 18, 2112–2122. [Google Scholar] [CrossRef] [Green Version]
- Plowman, S.J.; Muncke, C.; Parton, R.G.; Hancock, J.F. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 2005, 102, 15500–15505. [Google Scholar] [CrossRef] [Green Version]
- Doktorova, M.; Cheng, J.; Heberle, F.A.; Marquardt, D.; Rusinova, R.; Sanford, R.L.; Peyear, T.A.; Katsaras, J.; Feigenson, G.W.; Weinstein, H.; et al. Gramicidin Increases Lipid Flip-Flop in Symmetric and Asymmetric Lipid Vesicles. Biophy. J. 2019, 116, 860–873. [Google Scholar] [CrossRef] [Green Version]
- Vogtt, K.; Jeworrek, C.; Garamus, V.M.; Winter, R. Microdomains in lipid vesicles: Structure and distribution assessed by small-angle neutron scattering. J. Phys. Chem. B 2010, 114, 5643–5648. [Google Scholar] [CrossRef]
- Rodgers, W.; Smith, K. Properties of glycolipid-enriched membrane rafts in antigen presentation. Crit. Rev. Immunol. 2005, 25, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Meer, G.V.; Vaz, W.L.C. Membrane curvature sorts lipids. Stabilized lipid rafts in membrane transport. EMBO Rep. 2005, 6, 418–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touyz, R.M. Lipid rafts take center stage in endothelial cell redox signaling by death receptors. Hypertension 2006, 47, 16–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julicher, F.; Lipowsky, R. Domain-induced budding of vesicles. Phys. Rev. Lett. 1993, 70, 2964–2967. [Google Scholar] [CrossRef] [PubMed]
- Ewers, H.; Romer, W.; Smith, A.E.; Bacia, K.; Dmitrieff, S.; Chai, W.; Mancini, R.; Kartenbeck, J.; Chambon, V.; Berland, L.; et al. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 2010, 12, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Windschiegl, B.; Orth, A.; Romer, W.; Berland, L.; Stechmann, B.; Bassereau, P.; Johannes, L.; Steinem, C. Lipid reorganization induced by Shiga toxin clustering on planar membranes. PLoS ONE 2009, 4, e6238. [Google Scholar] [CrossRef] [PubMed]
- Bacia, K.; Schwille, P.; Kurzchalia, T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc. Natl. Acad. Sci. USA 2005, 102, 3272–3277. [Google Scholar] [CrossRef] [Green Version]




| Sample (Molar Ratio) | Inner Part [h-DPPC/d-DPPC] = 1/0 | Headgroup [h-DPPC/d-DPPC] = 1/0 | Inner Part [h-DPPC/d-DPPC] = 0/1 | Headgroup [h-DPPC/d-DPPC] = 0/1 | Gb3 | D2O | |
|---|---|---|---|---|---|---|---|
| SLD (10−11 cm−2) | |||||||
| Without Gb3 | 2.3 | 11.0 | 66.2 | 11.0 | 4.8 | 63.7 | |
| With Gb3 | 2.5 | 11.2 | 58.2 | 11.2 | |||
| Thickness of Tail (nm) | Thickness of Headgroup (nm) | |
|---|---|---|
| h-DPPC | 3.33 (0.05) | 0.80 (0.08) |
| h-DPPC-Gb3 | 3.10 (0.13) | 0.85 (0.07) |
| h-DPPC-Gb3-STxB | 2.50 (0.15) | 1.13 (0.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, S.; Zhang, H.; Li, Y.; Zhang, Y.; Tian, Q.; Almásy, L.; Xu, X.; Zhang, R.; Zou, A.; Li, N. Effect of Shiga Toxin on Inhomogeneous Biological Membrane Structure Determined by Small-Angle Scattering. Appl. Sci. 2021, 11, 6965. https://doi.org/10.3390/app11156965
Tu S, Zhang H, Li Y, Zhang Y, Tian Q, Almásy L, Xu X, Zhang R, Zou A, Li N. Effect of Shiga Toxin on Inhomogeneous Biological Membrane Structure Determined by Small-Angle Scattering. Applied Sciences. 2021; 11(15):6965. https://doi.org/10.3390/app11156965
Chicago/Turabian StyleTu, Shuyang, Haijiao Zhang, Yawen Li, Yongchao Zhang, Qiang Tian, László Almásy, Xianhui Xu, Rongguang Zhang, Aihua Zou, and Na Li. 2021. "Effect of Shiga Toxin on Inhomogeneous Biological Membrane Structure Determined by Small-Angle Scattering" Applied Sciences 11, no. 15: 6965. https://doi.org/10.3390/app11156965






