Positively Charged Lipid as Potential Tool to Influence the Fate of Ethosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Physico-Chemical Characterization of Vesicles
2.4. Cell Cultures
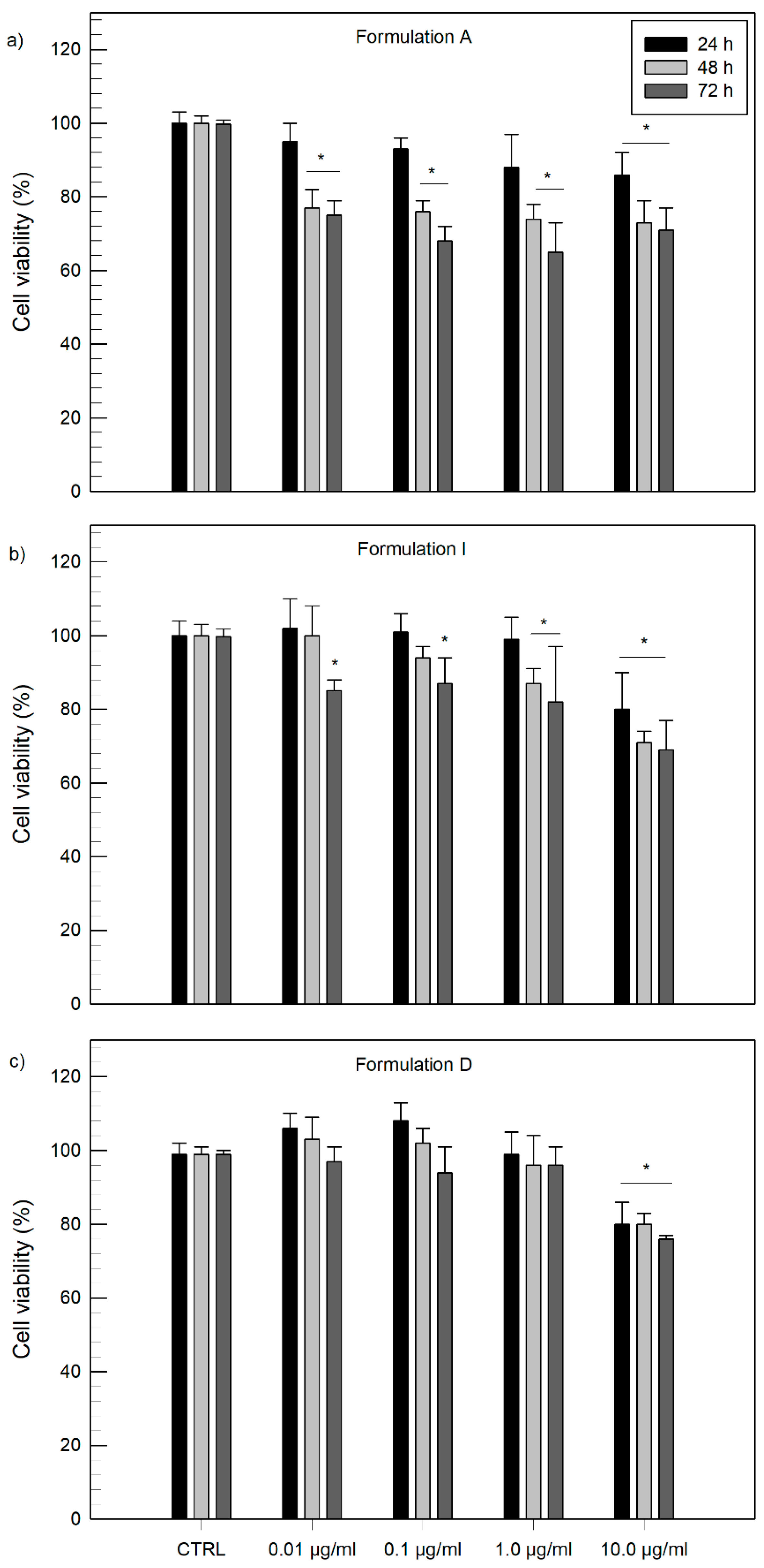
Evaluation of Cytotoxicity on NCTC2544 Cells
2.5. Evaluation of Entrapment Efficiency
HPLC Analysis
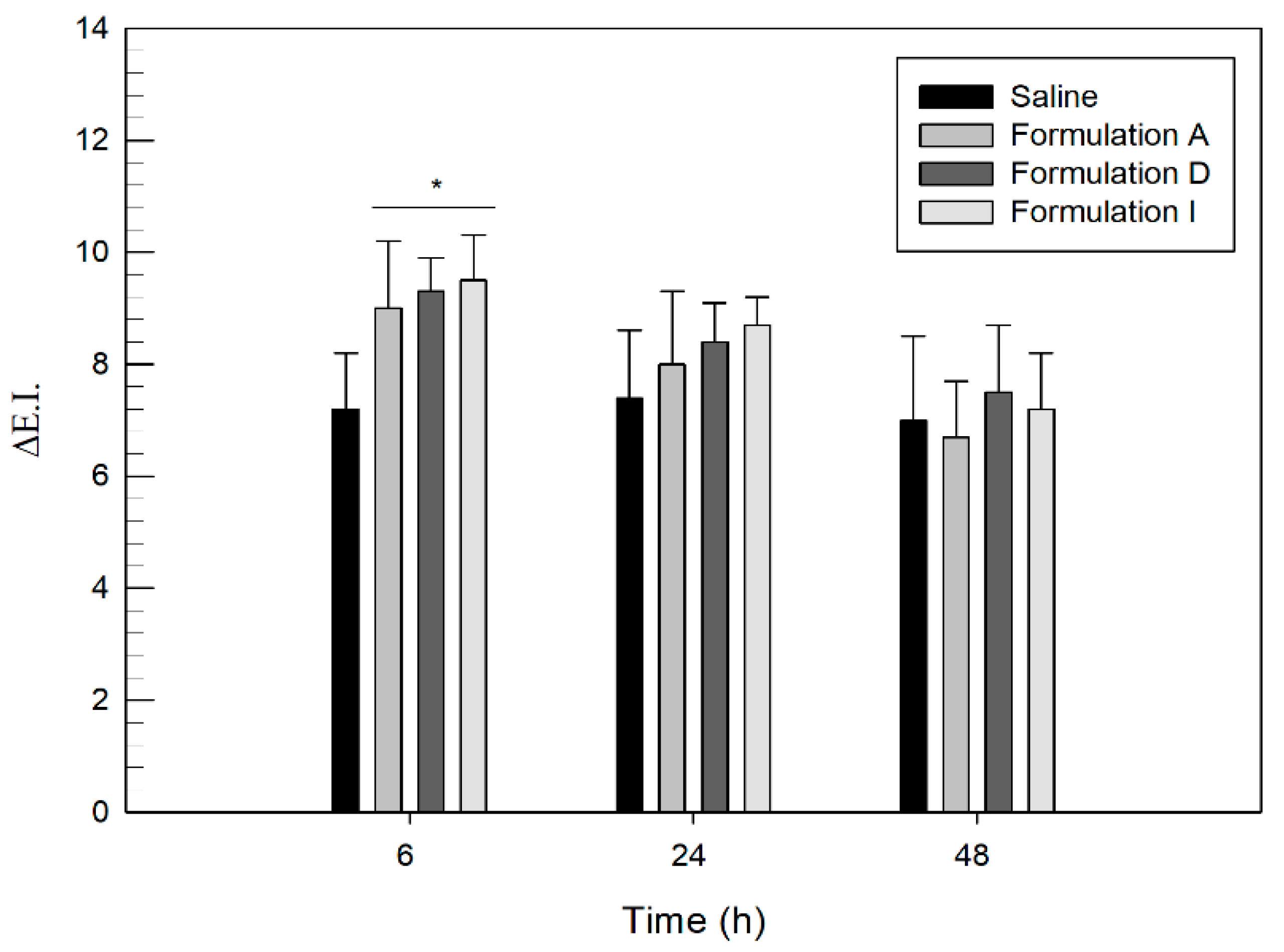
2.6. In Vivo Tolerability Studies
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characterization Studies
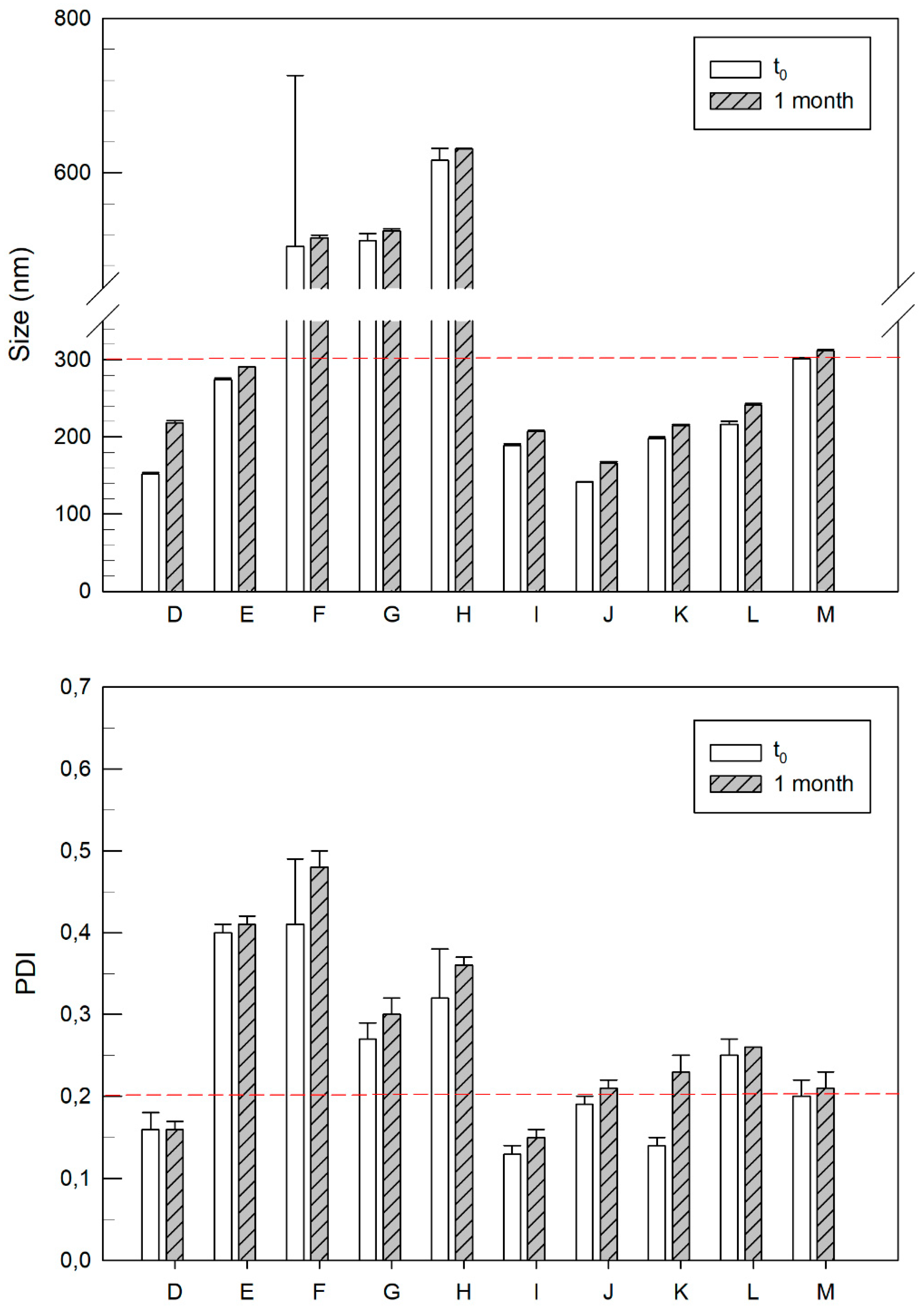
3.2. Characterization of Cationic Vesicles Made of Different Amount of DOTAP
3.3. In Vitro Cytotoxicity Studies
3.4. Evaluation of Encapsulation Efficiency
3.5. In Vivo Human Skin Tolerability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Song, C.; Baik, S.; Kim, D.; Hyeon, T.; Kim, D.H. Device-assisted transdermal drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 35–45. [Google Scholar] [CrossRef]
- Leppert, W.; Malec-Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and topical drug administration in the treatment of pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Peng, K.; Sarode, A.; Prakash, S.; Zhao, Z.; Filippov, S.K.; Todorova, K.; Sell, B.R.; Lujano, O.; Bakre, S.; et al. Hyaluronic acid conjugates for topical treatment of skin cancer lesions. Sci. Adv. 2021, 7, eabe6627. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.-C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin Barrier and Transdermal Drug Delivery STRUCTURE AND ORIGIN OF THE SKIN BARRIER Stratum Corneum Structure and Organization. Med. Ther. 2012, 124, 2065–2073. [Google Scholar]
- Lee, W.R.; Shen, S.C.; Aljuffali, I.A.; Li, Y.C.; Fang, J.Y. Impact of different vehicles for laser-assisted drug permeation via skin: Full-surface versus fractional ablation. Pharm. Res. 2014, 31, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Kirkby, M.; Hutton, A.R.J.; Donnelly, R.F. Microneedle Mediated Transdermal Delivery of Protein, Peptide and Antibody Based Therapeutics: Current Status and Future Considerations. Pharm. Res. 2020, 37, 117. [Google Scholar] [CrossRef]
- Singh Malik, D.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef]
- Liakopoulou, A.; Mourelatou, E.; Hatziantoniou, S. Exploitation of traditional healing properties, using the nanotechnology’s advantages: The case of curcumin. Toxicol. Rep. 2021, 8, 1143–1155. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Schmid, M.H.; Korting, H.C. Therapeutic progress with topical liposome drugs for skin disease. Adv. Drug Deliv. Rev. 1996, 18, 335–342. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int. J. Pharm. 2006, 322, 60–66. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes–Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 322, 60–66. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Iannone, M.; Fresta, M.; Fiorito, S.; Celia, C.; Paolino, D. In vitro and in vivo trans-epidermal water loss evaluation following topical drug delivery systems application for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2020, 186, 113295. [Google Scholar] [CrossRef]
- Ainbinder, D.; Touitou, E. Testosterone ethosomes for enhanced transdermal delivery. Drug Deliv. J. Deliv. Target. Ther. Agents 2005, 12, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Ibaraki, H.; Kanazawa, T.; Oogi, C.; Takashima, Y.; Seta, Y. Effects of surface charge and flexibility of liposomes on dermal drug delivery. J. Drug Deliv. Sci. Technol. 2019, 57, 101754. [Google Scholar] [CrossRef]
- Barone, A.; Cristiano, M.C.; Cilurzo, F.; Locatelli, M.; Iannotta, D.; Di Marzio, L.; Celia, C.; Paolino, D. Ammonium glycyrrhizate skin delivery from ultradeformable liposomes: A novel use as an anti-inflammatory agent in topical drug delivery. Colloids Surf. B 2020, 193, 111152. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Cristiano, M.C.; Fresta, M.; Paolino, D. The challenge of nanovesicles for selective topical delivery for acne treatment: Enhancing absorption whilst avoiding toxicity. Int. J. Nanomed. 2020, 15, 9197–9210. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kasamaki, M. Enhanced Delivery of Retinoic Acid to Skin by Cationic Liposomes. Chem. Pharm. Bull. 2006, 54, 242–244. [Google Scholar] [CrossRef] [Green Version]
- Fresta, M.; Mancuso, A.; Cristiano, M.C.; Urbanek, K.; Cilurzo, F.; Cosco, D.; Iannone, M.; Paolino, D. Targeting of the pilosebaceous follicle by liquid crystal nanocarriers: In vitro and in vivo effects of the entrapped minoxidil. Pharmaceutics 2020, 12, 1127. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Fan, Y.; Fan, C.; Li, X.; Wang, X.; Li, M.; Liu, Y. Tacrolimus-loaded ethosomes: Physicochemical characterization and in vivo evaluation. Eur. J. Pharm. Biopharm. 2012, 82, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Date, M.; Arai, S.; Kawano, K.; Yonemochi, E.; Maitani, Y. Transdermal Delivery of Small Interfering RNA with Elastic Cationic Liposomes in Mice. J. Pharm. 2013, 2013, 149695. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.W.; Xie, Q.C.; Huang, X.; Ban, J.F.; Wang, B.; Wei, X.; Chen, Y.Z.; Lu, Z.F. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int. J. Nanomed. 2018, 2018, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Otberg, N.; Thiede, G.; Richter, H.; Sterry, W.; Panzner, S.; Lademann, J. Innovative liposomes as a transfollicular drug delivery system: Penetration into porcine hair follicles. J. Investig. Dermatol. 2006, 126, 1728–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-laded ufasomes improve the nutraceutical efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Mota, A.H.; Rijo, P.; Molpeceres, J.; Reis, C.P. Broad overview of engineering of functional nanosystems for skin delivery. Int. J. Pharm. 2017, 532, 710–728. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, T.; Boonme, P.; Khongkow, P.; Amnuaikit, T. Ethosomes of Phenylethyl Resorcinol as Vesicular Delivery System for Skin Lightening Applications. Biomed Res. Int. 2017, 2017, 8310979. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, S.; Tilak, A.; Sharma, J.; Dave, V.; Sharma, S.; Yadav, R.; Patel, S.; Verma, K.; Tak, K. Flurbiprofen loaded ethosomes–Transdermal delivery of anti-inflammatory effect in rat model. Lipids Health Dis. 2019, 18, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilch, E.; Musiał, W. Liposomes with an ethanol fraction as an application for drug delivery. Int. J. Mol. Sci. 2018, 19, 3806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-Loaded Ultradeformable Vesicles as a Potential Natural Nanomedicine for the Treatment of Skin Cancer Diseases. Pharmaceutics 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, J.D.; Meinardi, M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, Q.; Hu, H.; He, Z.; Wu, T.; Guo, T.; Feng, N. Sodium dodecyl sulfate improved stability and transdermal delivery of salidroside-encapsulated niosomes via effects on zeta potential. Int. J. Pharm. 2020, 580, 119183. [Google Scholar] [CrossRef]
- Andleeb, M.; Shoaib Khan, H.M.; Daniyal, M. Development, Characterization and Stability Evaluation of Topical Gel Loaded with Ethosomes Containing Achillea millefolium L. Extract. Front. Pharmacol. 2021, 12, 603227. [Google Scholar] [CrossRef]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan lab expert analysis of the stability of ethosomes and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef]
- Wang, C.; Hong, H.H.; Chen, M.; Ding, Z.; Rui, Y.; Qi, J.; Li, Z.-C.; Liu, Z. Cationic Micelle as An In Vivo Catalyst for Tumor-Localized Cleavage Chemistry. Angew. Chem. Int. Ed. 2021. online ahead of print. [Google Scholar] [CrossRef]
- de Lima, M.; Neves, S.; Filipe, A.; Duzgunes, N.; Simoes, S. Cationic Liposomes for Gene Delivery: From Biophysics to Biological Applications. Curr. Med. Chem. 2003, 10, 1221–1231. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, E.J.; Jang, H.S.; Park, J.S. New cationic liposomes for gene transfer into mammalian cells with high efficiency and low toxicity. Bioconjug. Chem. 2001, 12, 108–113. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Rodríguez, A.; Delgado, D.; Solinís, M.A.; Gascón, A.R.; Pedraz, J.L. Solid lipid nanoparticles: Formulation factors affecting cell transfection capacity. Int. J. Pharm. 2007, 339, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Mohd Izham, M.N.; Hussin, Y.; Aziz, M.N.M.; Yeap, S.K.; Rahman, H.S.; Masarudin, M.J.; Mohamad, N.E.; Abdullah, R.; Alitheen, N.B. Preparation and Characterization of Self Nano-Emulsifying Drug Delivery System Loaded with Citraland Its Antiproliferative Effect on Colorectal Cells In Vitro. Nanomaterials 2019, 9, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Formulation | Lipid Composition | |
|---|---|---|
| Lipidic Mixture | Molar Ratio | |
| A | PL90G 1 | - |
| B | PL90G:DSPE 2 | 10:1 |
| C | PL90G:DOPE 3 | 10:1 |
| D | PL90G:DOTAP 4 | 10:1 |
| E | PL90G:DOTAP | 10:2 |
| F | PL90G:DOTAP | 10:3 |
| G | PL90G:DOTAP | 10:4 |
| H | PL90G:DOTAP | 10:5 |
| I | PL90G:DOTAP | 10:0.5 |
| J | PL90G:DOTAP | 10:0.4 |
| K | PL90G:DOTAP | 10:0.3 |
| L | PL90G:DOTAP | 10:0.2 |
| M | PL90G:DOTAP | 10:0.1 |
| Formulation | Mean Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| A | 224 ± 3 | 0.16 ± 0.01 | −25 ± 0 |
| B | 208 ± 1 | 0.20 ± 0.02 | −24 ± 1 |
| C | 241 ± 5 | 0.23 ± 0.02 | −27 ± 1 |
| D | 152 ± 2 | 0.16 ± 0.02 | +56 ± 1 |
| Formulation | Lipid Composition | Size (nm) | Polydispersity Index | Zeta Potential (mV) | |
|---|---|---|---|---|---|
| Lipidic Mixture | Molar Ratio | ||||
| E | PL90G:DOTAP | 10:2 | 274 ± 2 | 0.40 ± 0.01 | +55 ± 1 |
| F | PL90G:DOTAP | 10:3 | 505 ± 22 | 0.41 ± 0.08 | +58 ± 3 |
| G | PL90G:DOTAP | 10:4 | 512 ± 9 | 0.27 ± 0.02 | +64 ± 1 |
| H | PL90G:DOTAP | 10:5 | 616 ± 16 | 0.32 ± 0.06 | +69 ± 1 |
| I | PL90G:DOTAP | 10:0.5 | 189 ± 2 | 0.13 ± 0.01 | +52 ± 1 |
| J | PL90G:DOTAP | 10:0.4 | 141 ± 1 | 0.19 ± 0.01 | +50 ± 0 |
| K | PL90G:DOTAP | 10:0.3 | 198 ± 2 | 0.14 ± 0.01 | +47 ± 3 |
| L | PL90G:DOTAP | 10:0.2 | 216 ± 4 | 0.25 ± 0.02 | +47 ± 2 |
| M | PL90G:DOTAP | 10:0.1 | 301 ± 2 | 0.20 ± 0.02 | +42 ± 1 |
| Dye | Formulation | Mean Size (nm) | Polydispersity Index | Zeta Potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| Oil red O | A | 100 ± 1 | 0.19 ± 0.02 | −25 ± 1 | 43 ± 3 |
| D | 112 ± 3 | 0.29 ± 0.01 | +38 ± 2 | 55 ± 2 | |
| I | 105 ± 1 | 0.33 ± 0.01 | +29 ± 3 | 50 ± 2 | |
| Rhodamine B | A | 132 ± 2 | 0.17 ± 0.02 | −14 ± 1 | 69 ± 4 |
| D | 108 ± 2 | 0.13 ± 0.01 | +33 ± 2 | 52 ± 2 | |
| I | 118 ± 1 | 0.08 ± 0.01 | +31 ± 1 | 69 ± 6 | |
| Bromophenol blue | A | 88 ± 3 | 0.18 ± 0.01 | −16 ± 1 | 27 ± 2 |
| D | 94 ± 2 | 0.16 ± 0.00 | +28 ± 2 | 15 ± 6 | |
| I | 135 ± 2 | 0.08 ± 0.02 | +13 ± 2 | 36 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, A.; Cristiano, M.C.; Fresta, M.; Torella, D.; Paolino, D. Positively Charged Lipid as Potential Tool to Influence the Fate of Ethosomes. Appl. Sci. 2021, 11, 7060. https://doi.org/10.3390/app11157060
Mancuso A, Cristiano MC, Fresta M, Torella D, Paolino D. Positively Charged Lipid as Potential Tool to Influence the Fate of Ethosomes. Applied Sciences. 2021; 11(15):7060. https://doi.org/10.3390/app11157060
Chicago/Turabian StyleMancuso, Antonia, Maria Chiara Cristiano, Massimo Fresta, Daniele Torella, and Donatella Paolino. 2021. "Positively Charged Lipid as Potential Tool to Influence the Fate of Ethosomes" Applied Sciences 11, no. 15: 7060. https://doi.org/10.3390/app11157060
APA StyleMancuso, A., Cristiano, M. C., Fresta, M., Torella, D., & Paolino, D. (2021). Positively Charged Lipid as Potential Tool to Influence the Fate of Ethosomes. Applied Sciences, 11(15), 7060. https://doi.org/10.3390/app11157060










