Insights on the Adaptation of Foeniculum vulgare Mill to Iron Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Fe Scarcity Regime and Mineral Analysis
2.2. Photosynthetic Pigments
2.3. Phenolic Compounds
2.4. Antioxidant Activities
2.4.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity Assay
2.4.2. Chemical Nitric Oxide (NO●) Quenching Assay
2.4.3. Oxygen Radical Capacity (ORAC) Test
2.4.4. Ferric Reducing Power Assay (FRAP)
2.4.5. Lipid Peroxidation Inhibition in the Presence of Thiobarbituric Acid Reactive Substances (TBARS)
2.5. Enzymatic Inhibitory Ability
2.5.1. Lipoxygenase (LOX)
2.5.2. Xanthine Oxidase (XO)
2.5.3. Acetylcholinesterase (AChE)
2.6. Data Analysis
3. Results and Discussion
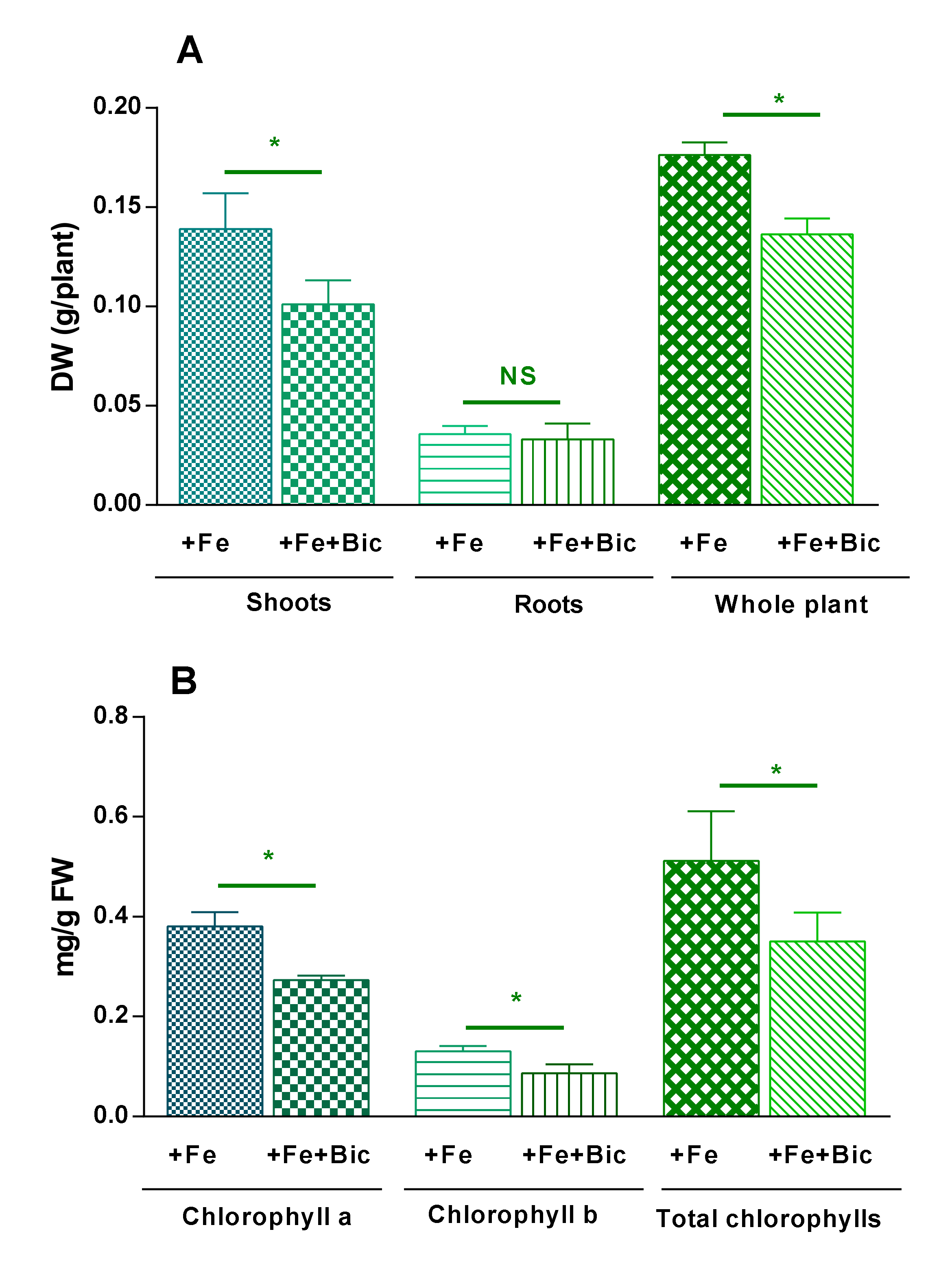
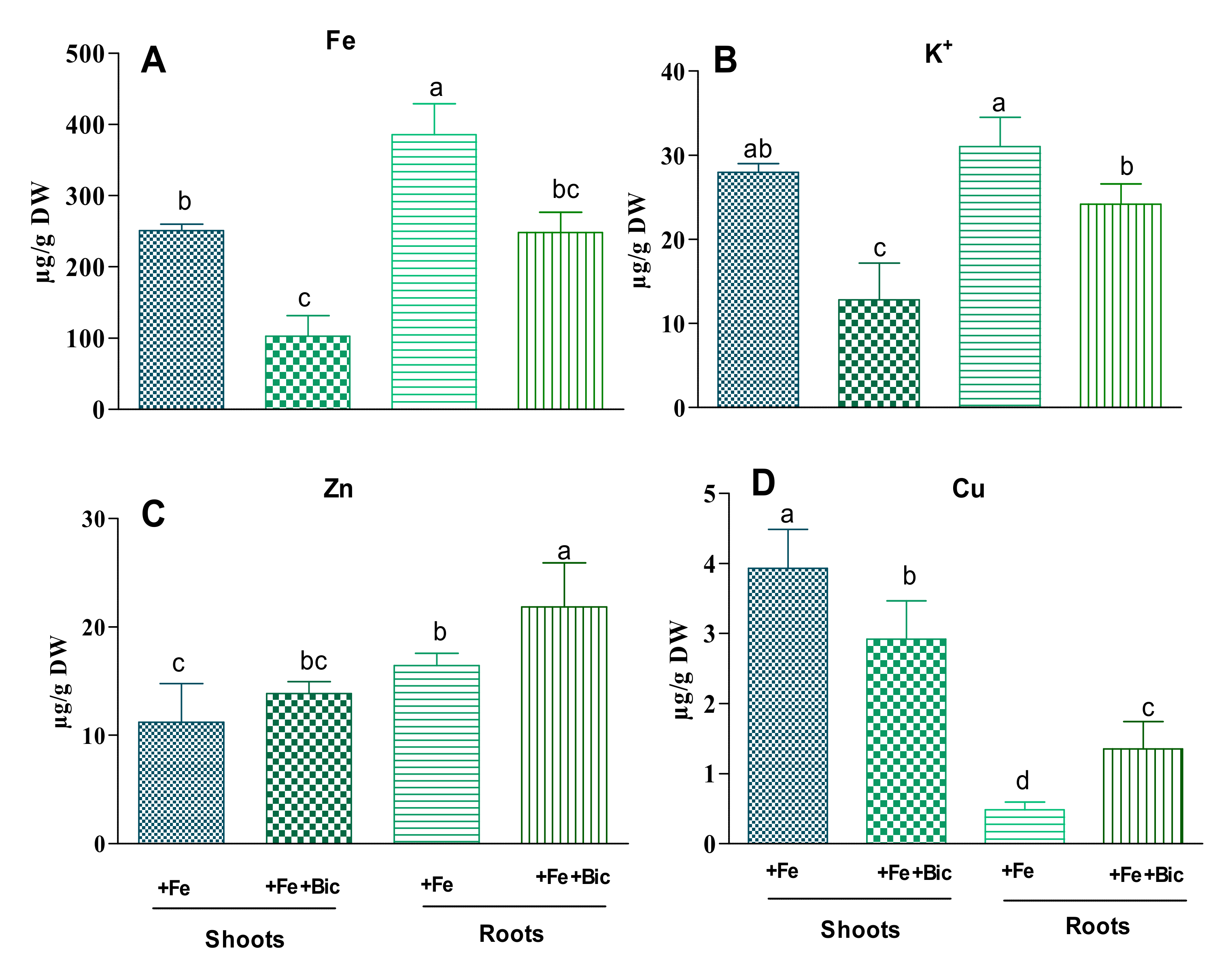
3.1. Effect of HCO3-Induced Fe Deficiency on Plant Growth and Levels of Photosynthetic Pigments and Nutrients
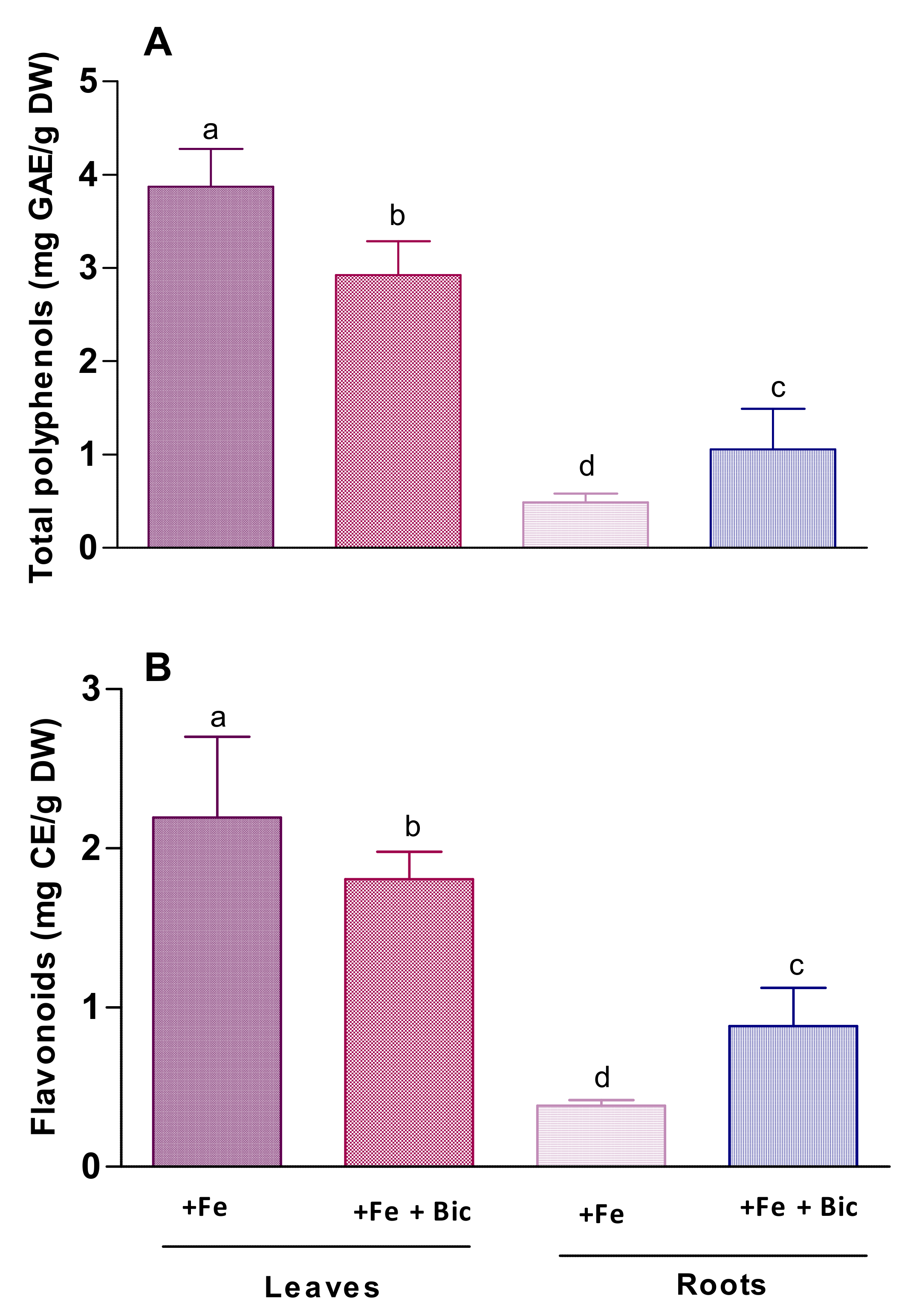
3.2. Effect of HCO3-Induced Fe Deficiency on Phenolic Compounds
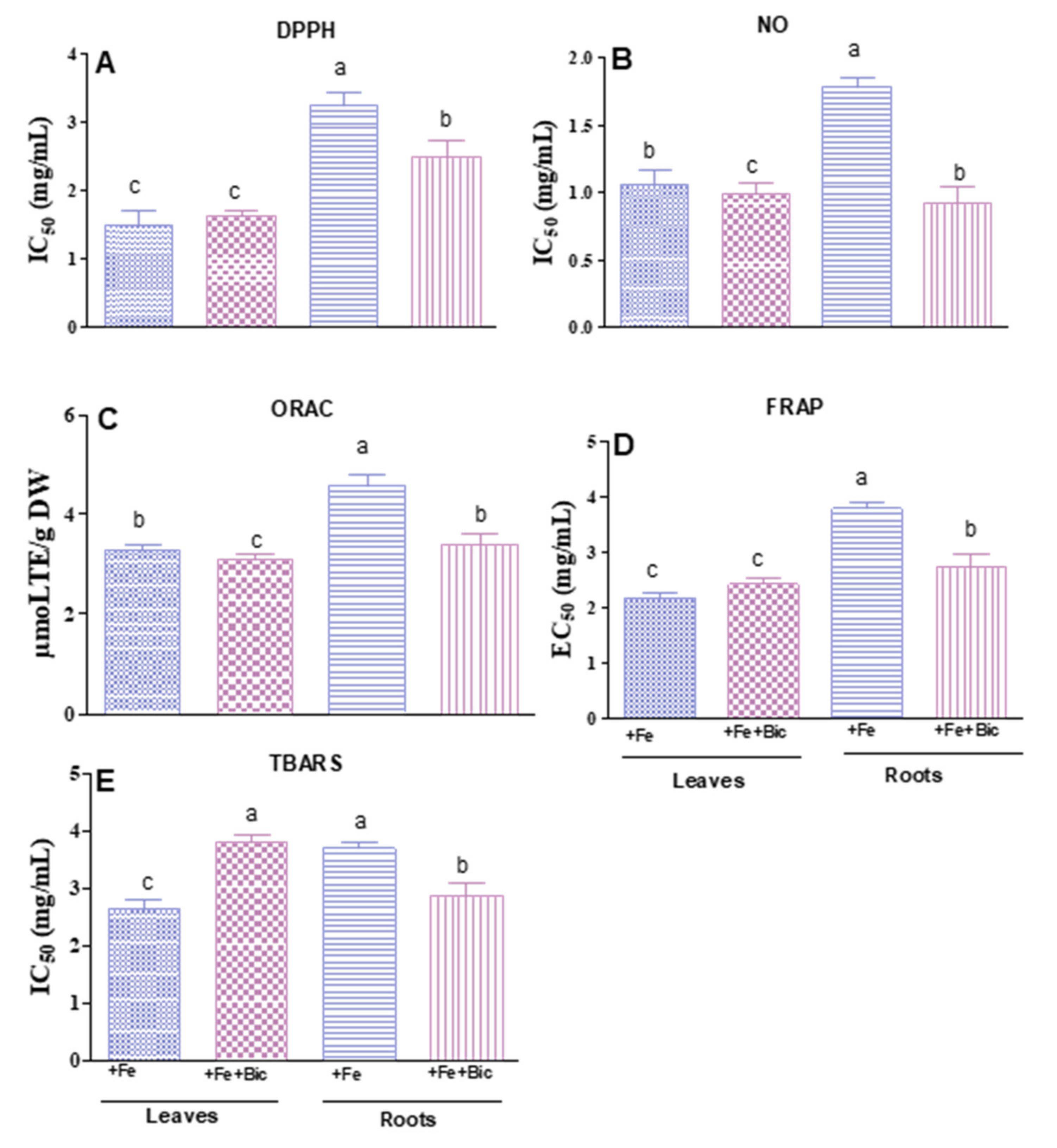
3.3. Effect of HCO3-Induced Fe Deficiency on Antioxidant Abilities
3.4. Effect of Bicarbonate-Induced Fe Deficiency on the Inhibitory Enzymatic Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briat, J.F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Colangelo-Aksoy, E.; Jeong, I.S.; Koiwa, H. Loss of function of Arabidopsis C-terminal domain phosphatase-like1 activates iron deficiency responses at the transcriptional level. Plant Physiol. 2013, 161, 330–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ksouri, R.; Wided, M.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Toselli, M.; Marangoni, B.; Tagliavini, M. Iron content in vegetative and reproductive organs of nectarine trees in calcareous soils during the development of chlorosis. Eur. J. Agron. 2000, 13, 279–286. [Google Scholar] [CrossRef]
- Donnini, S.; Dell’Orto, M.; Zocchi, G. Oxidative stress responses and root lignification induced by Fe deficiency conditions in pear and quince genotypes. Tree Physiol. 2011, 31, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Marschner, H.; Römheld, V. Strategies of plants for acquisition of iron. Plant Soil 1995, 165, 261–274. [Google Scholar] [CrossRef]
- Abadía, J.; López-Millán, A.F.; Rombolà, A.; Abadía, A. Organic acids and Fe deficiency: A review. Plant Soil 2002, 241, 75–86. [Google Scholar] [CrossRef]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Zocchi, G. Metabolic changes in iron-stressed dicotyledonous plants. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Abadía, J., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 359–370. [Google Scholar]
- Rawson, A.; Hossain, M.; Patras, A.; Tuohy, M.; Brunton, N. Effect of boiling and roasting on the polyacetylene and polyphenol content of fennel (Foeniculum vulgare) bulb. Food Res. Int. 2013, 50, 513–518. [Google Scholar] [CrossRef]
- Santayana, M.P.; Tardio, J.; Blanco, E.; Carvalho, A.M.; Lastra, J.J.; San-Miguel, E.; Morales, R. Traditional knowledge of wild edible plants used in the northwest of the Iberian Peninsula (Spain and Portugal): A comparative study. J. Ethnobiol. Ethnomed. 2007, 3, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Heleno, S.A.; Carvalho, A.M.; Ferreira, I.C.F.R. Systematic evaluation of the antioxidant potential of different parts of Foeniculum vulgare Mill. from Portugal. FCT 2009, 47, 2458–2464. [Google Scholar] [CrossRef] [PubMed]
- Novais, M.H.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrábida Natural Park (Portugal). J. Ethnopharmacol. 2013, 93, 183–195. [Google Scholar] [CrossRef]
- Tato, L.; De Nisi, P.; Donnini, S.; Zocchi, G. Low iron availability and phenolic metabolism in a wild plant species (Parietaria judaica L.). Plant Physiol. Biochem. 2013, 72, 145–153. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Grimm, B.; Wobus, U.; Weschke, W. Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plant 2002, 109, 435–442. [Google Scholar] [CrossRef]
- Bettaieb-Rebey, I.; Jabri-Karoui, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Ind. Crop. Prod. 2012, 36, 238–245. [Google Scholar] [CrossRef]
- Estaji, A.; Roosta, H.R.; Rezaei, S.A.; Hosseini, S.S.; Niknam, F. Morphological, physiological and phytochemical response of different Satureja hortensis L. accessions to salinity in a greenhouse experiment. J. Appl. Res. Med. Aromat. Plants 2018, 10, 25–33. [Google Scholar] [CrossRef]
- M’sehli, W.; Houmani, H.; Graziano, D.; Abdelly, C.; Gharsalli, M. Iron deficiency tolerance at leaf level in Medicago ciliaris Plants. Am. J. Plant Sci. 2014, 5, 2541–2553. [Google Scholar] [CrossRef] [Green Version]
- Houmani, H.; Jelali, N.; Abdelly, C.; Gharsalli, M. Mineral elements bioavailability in the halophyte species Suaeda fruticosa. J. Biol. Res. Thessalon. 2012, 17, 113–120. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Saada, M.; Falleh, H.; Jellali, I.; Snoussi, M.; Ksouri, R. Phenolic profile, biological activities and fraction analysis of the medicinal halophyte Retama raetam. S. Afr. J. Bot. 2014, 94, 114–121. [Google Scholar]
- Wasli, H.; Jelali, N.; Silva, A.M.S.; Ksouri, R.; Cardoso, S.M. Variation of polyphenolic composition, antioxidants and physiological characteristics of dill (Anethum graveolens L.) as affected by bicarbonate-induced iron deficiency conditions. Ind. Crop. Prod. 2018, 126, 466–467. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Antioxidant and anti-inflammatory activities of Geranium robertianum decoctions. Food Funct. 2017, 17, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescentprobe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Majdoub, N.; El-Guendouz, S.; Rezgui, M.; Carlier, J.; Costa, C.; Bettaieb-Ben Kaaba, L.; Miguel, M.G. Growth, photosynthetic pigments, phenolic content and biological activities of Foeniculum vulgare Mill., Anethum graveolens L. and Pimpinella anisum L. (Apiaceae) in response to zinc. Ind. Crop. Prod. 2017, 109, 627–636. [Google Scholar] [CrossRef]
- Pereira, O.R.; Catarino, M.D.; Afonso, A.F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis decoctions: Antioxidant activities and inhibition of carbohydrate and lipid metabolic enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef] [Green Version]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Antunes, M.D.; Faleiro, M.L.; Miguel, M.G. Anti-acetylcholinesterase, antidiabetic, anti-inflammatory, antityrosinase and antixanthine oxidase activities of Moroccan propolis. Int. J. Food Sci. Tech. 2016, 51, 1762–1773. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.F.; Liu, Y.Z.; Du, W.; Fu, L.N.; Hussain, S.B.; Peng, S.A. Physiological and transcriptional analysis reveals pathways involved in iron deficiency chlorosis in fragrant citrus. Tree Genet. Genomes 2017, 85, 38–49. [Google Scholar] [CrossRef]
- Donnini, S.; De Nisi, P.; Gabotti, D.; Tato, L.; Zocchi, Z. Adaptive strategies of Parietaria diffusa (M.&K.) to calcareous habitat with limited iron availability. Plant Cell Environ. 2012, 35, 1171–1184. [Google Scholar]
- Dell’Orto, M.; De Nisi, P.; Pontiggia, A.; Zocchi, G. Fe deficiency responses in Parietaria diffusa: A calcicole plant. J. Plant Nutr. 2003, 26, 2057–2068. [Google Scholar] [CrossRef]
- Elkouni, A.; Rabhi, M.; Ivanov, A.G.; Krol, M.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Huner, N.P.A. Structural and functional integrity of Sulla carnosa photosynthetic apparatus under iron deficiency conditions. Plant. Biol. 2017, 20, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.M.; Norvell, W.A. Growth and nutrient uptake by barley (Hordeum vulgare L. cv Herta): Studies using an N-(2- hydroxyethyl) ethylenedinitrilotriacetic acid-buffered nutrient solution technique. II. Role of zinc in the uptake and root leakage of mineral nutrients. Plant Physiol. 1993, 101, 627–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jelali, N.; Ben salah, I.; M’sehli, W.; Donnini, S.; Zocchi, G.; Gharsalli, M. Comparison of three pea cultivars (Pisum sativum) regarding their responses to direct and bicarbonate- induced iron deficiency. Sci. Hortic. 2011, 129, 548–553. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J.; Tian, G.; Zheng, S. Fe deficiency induces Cu uptake and accumulation in Commelina communis. Plant. Sci. J. 2004, 166, 1371–1377. [Google Scholar] [CrossRef]
- Abdallah, H.B.; Mai, H.J.; Slatni, T.; Fink-Straube, C.; Abdelly, C.; Bauer, P. Natural variation in physiological responses of Tunisian Hedysarum carnosum under iron deficiency. Front. Plant Sci. 2018, 9, 1383. [Google Scholar] [CrossRef] [Green Version]
- Cesco, S.; Neumann, G.; Tomasi, N.; Pinton, R.; Weisskopf, L. Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 2010, 329, 1–25. [Google Scholar] [CrossRef]
- Tomasi, N.; Weisskopf, L.; Renella, G.; Landi, L.; Pinton, R.; Varanini, Z.; Nannipieri, P.; Torrent, J.; Martinoia, E.; Cesco, S. Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol. Biochem. 2008, 40, 1971–1974. [Google Scholar] [CrossRef]
- Boyer, R.F.; Clark, H.M.; Sanchez, S. Solubilization of ferrihydrite iron by plant phenolics: A model for rhizosphere processes. J. Plant Nutr. 1989, 12, 581–592. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2008, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.; Maksimović, J.D.; Maksimović, V.; Shabala, L.; Živanović, B.D.; Yu, T.; Jacobsen, S.E.; Shabala, S. Rutin, a flavonoid with antioxidant activity, improves plant salinity tolerance by regulating K+ retention and Na+ exclusion from leaf mesophyll in quinoa and broad beans. Funct. Plant Biol. 2016, 43, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Jelali, N.; Doninni, S.; Dellorto, M.; Abdelly, C.; Gharsalli, M.; Zocchi, G. Implication of antioxidant defence in tolerance to Fe deficiency of pisum Sativum leaves. J. Appl. Biotechnol. Bioeng. 2017, 2, 1–9. [Google Scholar]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.-O.; Dommes, J. Evaluation of spectrophotometric methods for antioxidant compound measurement in relation to total antioxidant capacity in beverages. Food Chem. 2010, 120, 607–614. [Google Scholar] [CrossRef]
- Kabir, A.H.; Rahman, M.M.; Haider, S.A.; Paul, N.K. Mechanisms associated with differential tolerance to Fe deficiency in okra (Abelmoschus esculentus Moench). Env. Exp. Bot. 2015, 112, 16–26. [Google Scholar] [CrossRef]
- Msilini, N.; Oueslati, S.; Amdouni, T.; Chebbi, M.; Ksouri, R.; Lachaal, M.; Ouerghi, Z. Variability of phenolic content and antioxidant activity of two lettuce varieties under Fe deficiency. J. Sci. Agric. 2013, 93, 2016–2021. [Google Scholar] [CrossRef]
- Chedea, V.S.; Jisaka, M. Lipoxygenase and carotenoids: A co-oxidation story. Afr. J. Biotechnol. 2013, 12, 2786–2791. [Google Scholar]
- New comer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Pallavi, P.C.; Singh, A.K.; Singh, S.; Singh, N.K. In Silico Structural and Functional Insights into the Lipoxygenase Enzyme of Legume Cajanus Cajan. Int. J. Recent Innov. Trends Comput. Commun. 2014, 5, 87–91. [Google Scholar]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Rahman, U.; Uddin, T.; Choudhary, G.; Iqbal, M. Discovery and molecular docking simulation of 7-hydroxy-6-methoxy- 2H-chromen-2-one as a LOX Inhibitor. Pak. J. Pharm. Sci. 2019, 32, 217–220. [Google Scholar]
- Bae, K.S.; Rahimi, S.; Kim, Y.J.; Renuka Devi, B.S.; Khorolragchaa, A.; Sukweenadhi, J.; Silva, J.; Myagmarjav, D.; Yang, D.C. Molecular characterization of lipoxygenase genes and their expression analysis against biotic and abiotic stresses in Panax ginseng. Eur. J. Plant Pathol. 2016, 145, 331–343. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Neto, R.T.; Silva, A.M.S.; Cardoso, S.M. Health-promoting effects of Thymus herba-barona, Thymus pseudolanuginosus, and Thymus caespititius decoctions. Int. J. Mol. Sci. 2017, 18, 1879. [Google Scholar] [CrossRef]
- Hasbal, G.; Yilmaz-Ozden, T.; Can, A. Antioxidant and antiacetylcholinesterase activities of Sorbus torminalis (L.) Crantz (wild service tree) fruits. JFDA 2014, 23, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Variables | TPC | TFC | LOX | XO | AChE |
|---|---|---|---|---|---|
| TPC | 1 | 0.98 | −0.55 | −0.84 | −0.93 |
| TFC | 0.98 | 1 | −0.47 | −0.79 | −0.98 |
| LOX | −0.55 | −0.47 | 1 | 0.91 | 0.39 |
| XO | −0.84 | −0.79 | 0.91 | 1 | 0.71 |
| AChE | −0.93 | −0.98 | 0.39 | 0.71 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasli, H.; Jelali, N.; Saada, M.; Ksouri, R.; Cardoso, S.M. Insights on the Adaptation of Foeniculum vulgare Mill to Iron Deficiency. Appl. Sci. 2021, 11, 7072. https://doi.org/10.3390/app11157072
Wasli H, Jelali N, Saada M, Ksouri R, Cardoso SM. Insights on the Adaptation of Foeniculum vulgare Mill to Iron Deficiency. Applied Sciences. 2021; 11(15):7072. https://doi.org/10.3390/app11157072
Chicago/Turabian StyleWasli, Hanen, Nahida Jelali, Mariem Saada, Riadh Ksouri, and Susana M. Cardoso. 2021. "Insights on the Adaptation of Foeniculum vulgare Mill to Iron Deficiency" Applied Sciences 11, no. 15: 7072. https://doi.org/10.3390/app11157072
APA StyleWasli, H., Jelali, N., Saada, M., Ksouri, R., & Cardoso, S. M. (2021). Insights on the Adaptation of Foeniculum vulgare Mill to Iron Deficiency. Applied Sciences, 11(15), 7072. https://doi.org/10.3390/app11157072







