From Exercise to Cognitive Performance: Role of Irisin
Abstract
:Featured Application
Abstract
1. Introduction
2. Cognitive Functions Memory and Aging
3. Irisin
3.1. Irisin Structure
3.2. Irisin Functions
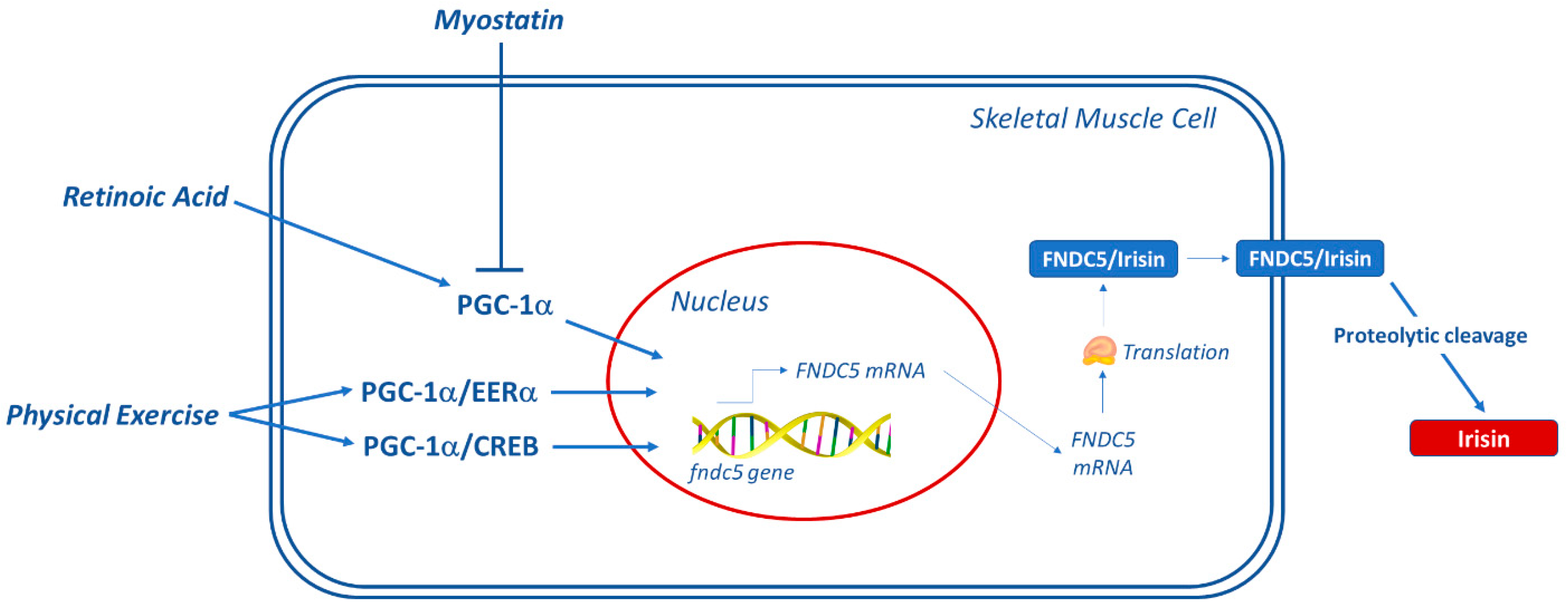
3.3. Expression of FNDC5/Irisin
4. Skeletal Muscle
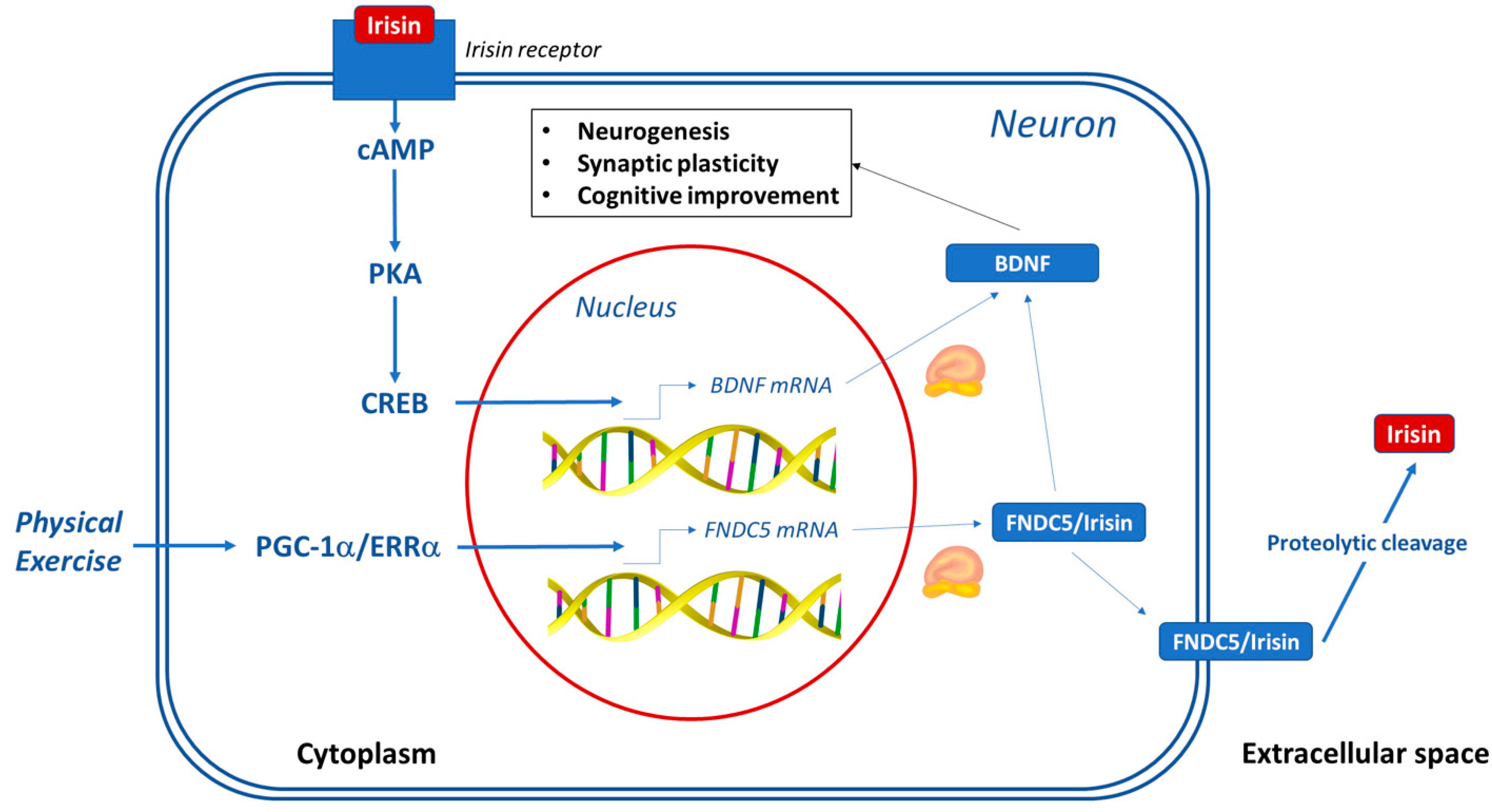
5. Brain
6. Irisin: A New Bridge between Exercise and Cognitive Functions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muller, P.; Taubert, M.; Muller, N.G. Physical exercise as personalized medicine for dementia prevention? Front. Physiol. 2019, 10, 672. [Google Scholar] [CrossRef]
- Silverman, M.N.; Deuster, P.A. Biological mechanisms underlying the role of physical fitness in health and resilience. InterfaceFocus 2014, 4, 20140040. [Google Scholar] [CrossRef] [Green Version]
- Corsaro, A.; Paludi, D.; Villa, V.; D’Arrigo, C.; Chiovitti, K.; Thellung, S.; Russo, C.; Di Cola, D.; Ballerini, P.; Patrone, E.; et al. Conformation dependent pro-apoptotic activity of the recombinant human prion protein fragment 90-231. Int. J. Immunopathol. Pharmacol. 2016, 19, 339–356. [Google Scholar] [CrossRef]
- Katsanos, G.S.; Anogianaki, A.; Castellani, M.L.; Ciampoli, C.; De Amicis, D.; Orso, C.; Pollice, R.; Vecchiet, J.; Tetè, S.; Salini, V.; et al. Biology of neurotensin: Revisited study. Int. J. Immunopathol. Pharmacol. 2008, 21, 255–259. [Google Scholar] [CrossRef]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Eren, M.N.; Celik, A.; Yilmaz, M.; Kalayci, M.; Sahin, İ.; Gungor, O.; Gurel, A.; et al. Cardiac, skeletal muscle and serum irisin responses to with or without water exercise in young and old male rats: Cardiac muscle produces more irisin than skeletal muscle. Peptides 2014, 52, 68–73. [Google Scholar] [CrossRef]
- Archundia-Herrera, C.; Macias-Cervantes, M.; Ruiz-Munoz, B.; Vargas Ortiz, K.; Kornhauser, C.; Perez-Vazquez, V. Muscle irisin response to aerobic vs HIIT in overweight female adolescents. Diabetol. Metab. Syndr. 2017, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dun, S.L.; Lyu, R.M.; Chen, Y.H.; Chang, J.K.; Luo, J.J.; Dun, N.J. Systemic delivery of recombinant brain derived neurotrophic factor (BDNF) in the R6/2 mouse model of Huntington’s disease. PLoS ONE 2013, 8, e64037. [Google Scholar]
- Aydin, S.; Kuloglu, T.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Cakmak, T.; Albayrak, S.; Gungor, S.; Colakoglu, N.; Ozercan, I.H. A comprehensive immunohistochemical examination of the distribution of the fat-burning protein irisin in biological tissues. Peptides 2014, 61, 130–136. [Google Scholar] [CrossRef]
- Piya, M.K.; Harte, A.L.; Sivakumar, K.; Tripathi, G.; Voyias, P.D.; James, S.; Sabico, S.; Al-Daghri, N.M.; Saravanan, P.; Barber, T.M.; et al. The identification of irisin in human cerebrospinal fluid: Influence of adiposity, metabolic markers, and gestational diabetes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E512–E518. [Google Scholar] [CrossRef]
- Gur, F.M.; Timurkaan, S.; Yalcin, M.H.; Girgin, A.; GencerTarakci, B. Immunohistochemical localization of irisin in mole rats (Spalaxleucodon). Biotech. Histochem. 2017, 92, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pan, J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem. Biophys. Res. Commun. 2016, 474, 22–28. [Google Scholar] [CrossRef]
- Li, D.J.; Li, Y.H.; Yuan, H.B.; Qu, L.F.; Wang, P. The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 2017, 68, 31–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.S.; Dincer, F.; Mantzoros, C.S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 2013, 62, 1131–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forouzanfar, M.; Rabiee, F.; Ghaedi, K.; Beheshti, S.; Tanhaei, S.; ShoarayeNejati, A.; JodeiriFarshbaf, M.; Baharvand, H.; Nasr-Esfahani, M.H. Fndc5 overexpression facilitated neural differentiation of mouse embryonic stem cells. CellBiol. Int. 2015, 39, 629–637. [Google Scholar] [CrossRef]
- Xia, D.Y.; Huang, X.; Bi, C.F.; Mao, L.L.; Peng, L.J.; Qian, H.R. PGC-1α or FNDC5 Is Involved in Modulating the Effects of Aβ1-42 Oligomers on Suppressing the Expression of BDNF, a Beneficial Factor for Inhibiting Neuronal Apoptosis, Aβ Deposition and Cognitive Decline of APP/PS1 Tg Mice. Front. Aging Neurosci. 2017, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Michalski, B.; Fahnestock, M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Mol. Brain Res. 2003, 111, 148–154. [Google Scholar] [CrossRef]
- Arancibia, S.; Silhol, M.; Mouliere, F. Protective effect of BDNF against beta- amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008, 31, 316–326. [Google Scholar] [CrossRef]
- Kirk-Sanchez, N.J.; McGough, E.L. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2013, 9, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Im, K.; Kwon, H.; Seo, S.W.; Ye, B.S.; Cho, H.; Noh, Y.; Lee, J.M.; Kim, S.T.; Park, S.E.; et al. Higher Physical Activity Is Associated with Increased Attentional Network Connectivity in the Healthy Elderly. Front. Aging Neurosci. 2016, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Archer, J.; Wong, C.K.; Chen, S.H.; Qiu, A. Age-related decline in associative learning in healthy Chinese adults. PLoS ONE 2013, 8, e80648. [Google Scholar] [CrossRef] [Green Version]
- Jawabri, K.H.; Cascella, M. Physiology, Explicit Memory; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cascella, M.; Al Khalili, Y. Short Term Memory Impairment; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Spaniol, J. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia 2009, 47, 1765–1779. [Google Scholar] [CrossRef]
- Chang, W.T.; Langella, S.K.; Tang, Y.; Ahmad, S.; Zhang, H.; Yap, P.T.; Giovanello, K.S.; Lin, W. Brain wide functional networks associated with anatomically- and functionally-defined hippocampal subfields using ultrahigh-resolution fMRI. Sci. Rep. 2021, 11, 10835. [Google Scholar] [CrossRef]
- Cowan, N. What are the differences between long-term, short-term, and working memory? Prog. Brain Res. 2008, 169, 323–338. [Google Scholar] [PubMed] [Green Version]
- Norris, D. Short-term memory and long-term memory are still different. Psychol. Bull. 2017, 143, 992–1009. [Google Scholar] [CrossRef] [Green Version]
- Almaraz-Espinoza, A.; Grider, M.H. Physiology, Long Term Memory; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Addis, D.R.; Giovanello, K.S.; Vu, M.A.; Schacter, D.L. Age-related changes in prefrontal and hippocampal contributions to relational encoding. NeuroImage 2013, 84, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakesh, G.; Szabo, T.S.; Alexopoulos, S.G.; Zannas, S.A. Strategies for dementia prevention: Latest evidence and implications. Ther. Adv. Chronic Dis. 2017, 8, 121–136. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S. Exercise and sleep: A systematic review of previous meta-analyses. J. Evid. Based Med. 2017, 10, 26–36. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, T.S.; Mottola, M.F. Physical activity throughout pregnancy is key to preventing chronic disease. Reproduction 2020, 160, R111–R118. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Kruger, K.; Nieman, D.C.; Pyne, D.B.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Ji, L.; Steffens, D.C.; Wang, L. Effects of physical exercise on the aging brain across imaging modalities: A meta-analysis of neuroimaging studies in randomized controlled trials. Int. J. Geriatr. Psychiatry 2021, 36, 1148–1157. [Google Scholar] [CrossRef]
- Quindry, J.C.; Franklin, B.A. Exercise Preconditioning as a Cardioprotective Phenotype. Am. J. Cardiol. 2021, 148, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, S.J.; Prakash, R.; McAuley, E. Aerobic Exercise Training Increases Brain Volume in Aging Humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A Review of the Effects of Physical Activity and Exercise on Cognitive and Brain Functions in Older Adults. J. Aging Res. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petzinger, G.M.; Fisher, B.E.; Akopian, G.; Holschneider, D.P.; Wood, R.; Walsh, J.P.; Lund, B.; Meshul, C.; Vuckovic, M.; Jakowec, M.W. The role of exercise in facilitating basal ganglia function in Parkinson’s disease. Neurodegener. Dis. Manag. 2011, 1, 157–170. [Google Scholar] [CrossRef]
- Shah, T.; Verdile, G.; Sohrabi, H.; Campbell, A.; Putland, E.; Cheetham, C. A combination of physical activity and computerized brain training improves verbal memory and increases cerebral glucose metabolism in the elderly. Transl. Psychiatry 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nishiguchi, S.; Yamada, M.; Tanigawa, T.; Sekiyama, K.; Kawagoe, T.; Suzuki, M. A 12-Week Physical and Cognitive Exercise Program Can Improve Cognitive Function and Neural Efficiency in Community-Dwelling Older Adults: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2015, 63, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K.; Akerstrom, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef]
- Pesce, M.; Ballerini, P.; Paolucci, T.; Puca, I.; Farzaei, M.H.; Patruno, A. Irisin and Autophagy: First Update. Int. J. Mol. Sci. 2020, 21, 7587. [Google Scholar] [CrossRef]
- de Sire, A.; Baricich, A.; Renò, F.; Cisari, C.; Fusco, N.; Invernizzi, M. Myostatin as a potential biomarker to monitor sarcopenia in hip fracture patients undergoing a multidisciplinary rehabilitation and nutritional treatment: A preliminary study. Aging Clin. Exp. Res. 2020, 32, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Sun, L.; Cao, J.; Peng, Y.; Collier, L.; Wu, Y.; Creasey, G.; Li, J.; Qin, Y.; Jarvis, J.; et al. The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J. Biol. Chem. 2013, 288, 13511–13521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuma, K.; Yamaguchi, A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. Brain-derived neurotrophic factor: Regulation, effects, and potential clinical relevance. Neurology 2015, 84, 1693–1704. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenia--The search for emerging biomarkers. Ageing Res. Rev. 2015, 22, 58–71. [Google Scholar] [CrossRef]
- Erickson, K.I.; Prakash, R.S.; Voss, M.W.; Chaddock, L.; Heo, S.; McLaren, M.; Pence, B.D.; Martin, S.A.; Vieira, V.J.; Woods, J.A.; et al. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 2010, 30, 5368–5375. [Google Scholar] [CrossRef] [Green Version]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szuhany, K.L.; Otto, M.W. Assessing BDNF as a mediator of the effects of exercise on depression. J. Psychiatr. Res. 2020, 123, 114–118. [Google Scholar] [CrossRef]
- Ferrer-Martinez, A.; Ruiz-Lozano, P.; Chien, K.R. Mouse PeP: A novel peroxisomal protein linked to myoblast differentiation and development. Dev. Dyn. 2002, 22, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Teufel, A.; Malik, N.; Mukhopadhyay, M.; Westphal, H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene 2002, 297, 79–83. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit beta-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef] [Green Version]
- Nie, Y.; Liu, D. N-Glycosylation is required for FDNC5 stabilization and irisin secretion. Biochem. J. 2017, 474, 3167–3177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, E.; Schering, L.; Buck, F.; Vlach, K.; Schober, H.C.; Drevon, C.A. Irisin: Still chasing shadows. Mol. Metab. 2020, 34, 124–135. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium Human genomics. The genotype-tissue expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Ando, D.; Takamatsu, K.; Goto, K. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism 2015, 64, 1042–1050. [Google Scholar] [CrossRef]
- Murawska-Cialowicz, E.; Wolanski, P.; Zuwala-Jagiello, J.; Feito, Y.; Petr, M.; Kokstejn, J.; Stastny, P.; Goliński, D. Effect of HIIT with Tabata Protocol on Serum Irisin, Physical Performance, and Body Composition in Men. Int. J. Environ. Res. Public Health 2020, 17, 3589. [Google Scholar] [CrossRef]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, S.; Wong, M.D.; Tan, C.S.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J. Diabetes Complicat. 2014, 28, 208–213. [Google Scholar] [CrossRef]
- Zybek-Kocik, A.; Sawicka-Gutaj, N.; Wrotkowska, E.; Sowi’nski, J.; Ruchała, M. Time-dependent irisin concentration changes in patients affected by overt hypothyroidism. Endokrynol. Pol. 2016, 67, 476–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, M.C.; Vella, J.P.; Cafeo, F.R.; Affonso Fonseca, F.L.; Bacci, M.R. Association between irisin and major chronic diseases: A review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4072–4077. [Google Scholar]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S. The myokine irisin is released in response to saturated fatty acids and promotes pancreatic betacell survival and insulin secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [Green Version]
- Yan, B.; Shi, X.; Zhang, H. Association of serum irisin with metabolic syndrome in obese Chinese adults. PLoS ONE 2014, 9, e94235. [Google Scholar] [CrossRef] [Green Version]
- Al-Daghri, N.M.; Alkharfy, K.M.; Rahman, S. Irisin as a predictor of glucose metabolism in children: Sexually dimorphic effects. Eur. J. Clin. Investig. 2014, 44, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Pardo, M.; Crujeiras, A.B.; Amil, M. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int. J. Endocrinol. 2014, 2014, 857270. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, Y.; Kurose, S.; Shinno, H. Relationships between serum irisin levels and metabolic parameters in Japanese patients with obesity. Obes. Sci. Pract. 2016, 2, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Yang, L.; Wang, T.; Zhang, J.; Li, T.; Ren, Y.; Wang, M.; Chen, X.; Lv, Y.; Wu, R. Irisin Improves Autophagy of Aged Hepatocytes via Increasing Telomerase Activity in Liver Injury. Oxid. Med. Cell. Longev. 2020, 2020, 6946037. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Lu, C. Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial infarction. Aging 2020, 12, 4474–4488. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Handschin, C.; Spiegelman, B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 2008, 454, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Küster, O.C.; Laptinskaya, D.; Fissler, P.; chnack, C.; Zügel, M.; Nold, V.; Thurm, F.; Pleiner, S.; Karabatsiakis, A.; von Einem, B. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J. Alzheimer’s Dis. 2017, 59, 1097–1111. [Google Scholar] [CrossRef]
- De Oliveira Bristot, V.J.; de Bem Alves, A.C.; Cardoso, L.R.; da Luz Scheffer, D.; Aguiar, A.S., Jr. The Role of PGC-1/UCP2 Signaling in the Beneficial Effects of Physical Exercise on the Brain. Front. Neurosci. 2019, 13, 292. [Google Scholar] [CrossRef] [Green Version]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belen Crujeiras, A. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef] [Green Version]
- Ellefsen, S.; Vikmoen, O.; Slettalokken, G.; Whist, J.E.; Nygaard, H.; Hollan, I. Irisin and FNDC5: Effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur. J. Appl. Physiol. 2014, 114, 1875–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiano, J.P.; Springer, D.A.; Rane, S.G. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha) during exercise. J. Biol. Chem. 2015, 290, 7671–7684. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.Y.; Wu, Z.; Boss, O. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibers. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Buroker, N.E.; Ning, X.H.; Portman, M. Cardiac PPARalpha protein expression is constant as alternate nuclear receptors and PGC-1 coordinately increase during the postnatal metabolic transition. PPAR Res. 2008, 2008, 279531. [Google Scholar] [CrossRef] [PubMed]
- Katsouri, L.; Lim, Y.M.; Blondrath, K.; Eleftheriadou, I.; Lombardero, L.; Birch, A.M. PPARgamma-coactivator-1alpha gene transfer reduces neuronal loss and amyloid-beta generation by reducing beta-secretase in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. USA 2016, 113, 12292–12297. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.Y.; Tse, M.C.L.; Hu, X.; Jia, W.H.; Du, G.H.; Chan, C.B. Interaction of CREB and PGC-1alpha induces fibronectin type III domain containing protein 5 expression in C2C12 myotubes. Cell. Physiol. Biochem. 2018, 50, 1574–1584. [Google Scholar] [CrossRef]
- Berdeaux, R.; Hutchins, C. Anabolic and pro-metabolic functions of CREB-CRTC in skeletal muscle: Advantages and obstacles for type 2 diabetes and cancer cachexia. Front. Endocrinol. 2019, 10, 535. [Google Scholar] [CrossRef]
- Popov, D.V.; Makhnovskii, P.A.; Shagimardanova, E.I.; Gazizova, G.R.; Lysenko, E.A.; Gusev, O.A. Contractile activity-specific transcriptome response to acute endurance exercise and training in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E605–E614. [Google Scholar] [CrossRef] [PubMed]
- le Maire, A.; Alvarez, S.; Shankaranarayanan, P.; Lera, A.R.; Bourguet, W.; Gronemeyer, H. Retinoid receptors and therapeutic applications of RAR/RXR modulators. Curr. Top. Med. Chem. 2012, 12, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Garcia-Carrizo, F.J.; Arreguin, A.; Musinovic, H.; Granados, N.; Palou, A. Retinoic acid increases fatty acid oxidation and irisin expression in skeletal muscle cells and impacts irisin in vivo. Cell. Physiol. Biochem. 2018, 46, 187–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baar, K.; Wende, A.R.; Jones, T.E.; Marison, M.; Nolte, L.A.; Chen, M. Adaptations of skeletal muscle to exercise: Rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002, 16, 1879–1886. [Google Scholar] [CrossRef]
- Ge, X.; Sathiakumar, D.; Lua, B.J.; Kukreti, H.; Lee, M.; McFarlane, C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int. J. Obes. 2017, 41, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-Rodriguez, B.M.; Pena-Bello, L.; Juiz-Valina, P.; Vidal-Bretal, B.; Cordido, F.; Sangiao-Alvarellos, S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci. Rep. 2016, 6, 29898. [Google Scholar] [CrossRef] [Green Version]
- Invernizzi, M.; de Sire, A.; Carda, S.; Venetis, K.; Renò, F.; Cisari, C.; Fusco, N. Bone Muscle Crosstalk in Spinal Cord Injuries: Pathophysiology and Implications for Patients’ Quality of Life. Curr. Osteoporos. Rep. 2020, 18, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, M.; de Sire, A.; Renò, F.; Cisari, C.; Runza, L.; Baricich, A.; Carda, S.; Fusco, N. Spinal Cord Injury as a Model of Bone-Muscle Interactions: Therapeutic Implications From in vitro and in vivo Studies. Front. Endocrinol. 2020, 11, 204. [Google Scholar] [CrossRef]
- Invernizzi, M.; de Sire, A.; Fusco, N. Rethinking the clinical management of volumetric muscle loss in patients with spinal cord injury: Synergy among nutritional supplementation, pharmacotherapy, and rehabilitation. Curr. Opin. Pharmacol. 2021, 57, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Khan, I.U.; Polo, R.; Zubair, H.; Drummer, C.; Shahab, M. Irisin in the primate hypothalamus and its effect on GnRH in vitro. J. Endocrinol. 2019, 241, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef]
- Yu, K.W.; Wang, C.J.; Wu, Y.; Wang, Y.Y.; Wang, N.H.; Kuang, S.Y. An enriched environment increases the expression of fibronectin type III domain-containing protein 5 and brain-derived neurotrophic factor in the cerebral cortex of the ischemic mouse brain. Neural Regen. Res. 2020, 15, 1671–1677. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.P.; Lin, J.B.; Xiong, Y.; Liao, W.J.; Wan, Q. Effect of enriched environment on angiogenesis and neurological functions in rats with focal cerebral ischemia. Brain Res. 2017, 1655, 176–185. [Google Scholar] [CrossRef]
- Wang, C.J.; Wu, Y.; Zhang, Q.; Yu, K.W.; Wang, Y.Y. An enriched environment promotes synaptic plasticity and cognitive recovery after permanent middle cerebral artery occlusion in mice. Neural Regen. Res. 2019, 14, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Siteneski, A.; Cunha, M.P.; Lieberknecht, V.; Pazini, F.L.; Gruhn, K.; Brocardo, P.S. Central irisin administration affords antidepressant-like effect and modulates neuroplasticity-related genes in the hippocampus and prefrontal cortex of mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Jodeiri Farshbaf, M.; Garasia, S.; Moussoki, D.P.K.; Mondal, A.K.; Cherkowsky, D.; Manal, N. Hippocampal injection of the exercise-induced myokine irisin suppresses acute stress-induced neurobehavioral impairment in a sex-dependent manner. Behav.Neurosci. 2020, 134, 233–247. [Google Scholar] [CrossRef]
- Gawlinska, K.; Gawlinski, D.; Przegalinski, E.; Filip, M. Maternal high-fat diet during pregnancy and lactation provokes depressive-like behavior and influences the irisin/brain-derived neurotrophic factor axis and inflammatory factors in male and female offspring in rats. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, T.; Leung, P.S. Irisin ameliorates glucolipotoxicity associated beta-cell dysfunction and apoptosis via AMPK signaling and antiinflammatory actions. Cell. Physiol. Biochem. 2018, 51, 924–937. [Google Scholar] [CrossRef]
- Kuipers, S.D.; Bramham, C.R. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: New insights and implications for therapy. Curr. Opin. Drug Discov. Dev. 2006, 9, 580–586. [Google Scholar]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J.Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nature reviews Neuroscience 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.T.; Williams, J.S.; Shen, C.-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef]
- Belviranl, M.; Okudan, N.; Kabak, B.; Erdoğan, M.; Karanfilci, M. The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. Physician Sports Med. 2016, 44, 290–296. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Hariri, A.R.; Goldberg, T.E.; Mattay, V.S.; Kolachana, B.S.; Callicott, J.H.; Egan, M.F.; Weinberger, D.R. Brain derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003, 23, 6690–6694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Vaynman, S.S.; Ying, Z.; Yin, D.; Gomez-Pinilla, F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006, 1070, 124–130. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [Green Version]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Shimada, H.; Park, H.; Makizako, H.; Doi, T.; Lee, S.; Suzuki, T. Depressive symptoms and cognitive performance in older adults. J. Psychiatr. Res. 2014, 57, 149–156. [Google Scholar] [CrossRef]
- Hashemi, M.S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.H.; Baharvand, H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Ieraci, A.; Madaio, A.I.; Mallei, A.; Lee, F.S.; Popoli, M. Brain-Derived Neurotrophic Factor Val66Met Human Polymorphism Impairs the Beneficial Exercise-Induced Neurobiological Changes in Mice. Neuropsychopharmacology 2016, 41, 3070–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science 2018, 36, eaan8821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Lourenco, M.V.; Ribeiro, F.C.; Sudo, F.K.; Drummond, C.; Assunção, N.; Vanderborght, B.; Tovar-Moll, F.; Mattos, P.; De Felice, F.G.; Ferreira, S.T. Cerebrospinal fluid irisin correlates with amyloid-β, BDNF, and cognition in Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12034. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P.; He, Z.; Yin, S.; Lucia, A. Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach. Front. Physiol. 2018, 9, 1735. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is irisin a human exercise gene? Nature 2012, 488, E9–E10. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Sato, K.; Kurihara, T.; Hasegawa, N.; Fujie, S.; Fujita, S.; Sanada, K.; Hamaoka, T.; Tabata, I.; Iemitsu, M. Endurance training-induced increase in circulating irisin levels is associated with reduction of abdominal visceral fat in middle-aged and older adults. PLoS ONE 2015, 10, e0120354. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesce, M.; La Fratta, I.; Paolucci, T.; Grilli, A.; Patruno, A.; Agostini, F.; Bernetti, A.; Mangone, M.; Paoloni, M.; Invernizzi, M.; et al. From Exercise to Cognitive Performance: Role of Irisin. Appl. Sci. 2021, 11, 7120. https://doi.org/10.3390/app11157120
Pesce M, La Fratta I, Paolucci T, Grilli A, Patruno A, Agostini F, Bernetti A, Mangone M, Paoloni M, Invernizzi M, et al. From Exercise to Cognitive Performance: Role of Irisin. Applied Sciences. 2021; 11(15):7120. https://doi.org/10.3390/app11157120
Chicago/Turabian StylePesce, Mirko, Irene La Fratta, Teresa Paolucci, Alfredo Grilli, Antonia Patruno, Francesco Agostini, Andrea Bernetti, Massimiliano Mangone, Marco Paoloni, Marco Invernizzi, and et al. 2021. "From Exercise to Cognitive Performance: Role of Irisin" Applied Sciences 11, no. 15: 7120. https://doi.org/10.3390/app11157120
APA StylePesce, M., La Fratta, I., Paolucci, T., Grilli, A., Patruno, A., Agostini, F., Bernetti, A., Mangone, M., Paoloni, M., Invernizzi, M., & de Sire, A. (2021). From Exercise to Cognitive Performance: Role of Irisin. Applied Sciences, 11(15), 7120. https://doi.org/10.3390/app11157120












