Sewage Sludge Derived Materials for CO2 Adsorption
Abstract
:1. Introduction
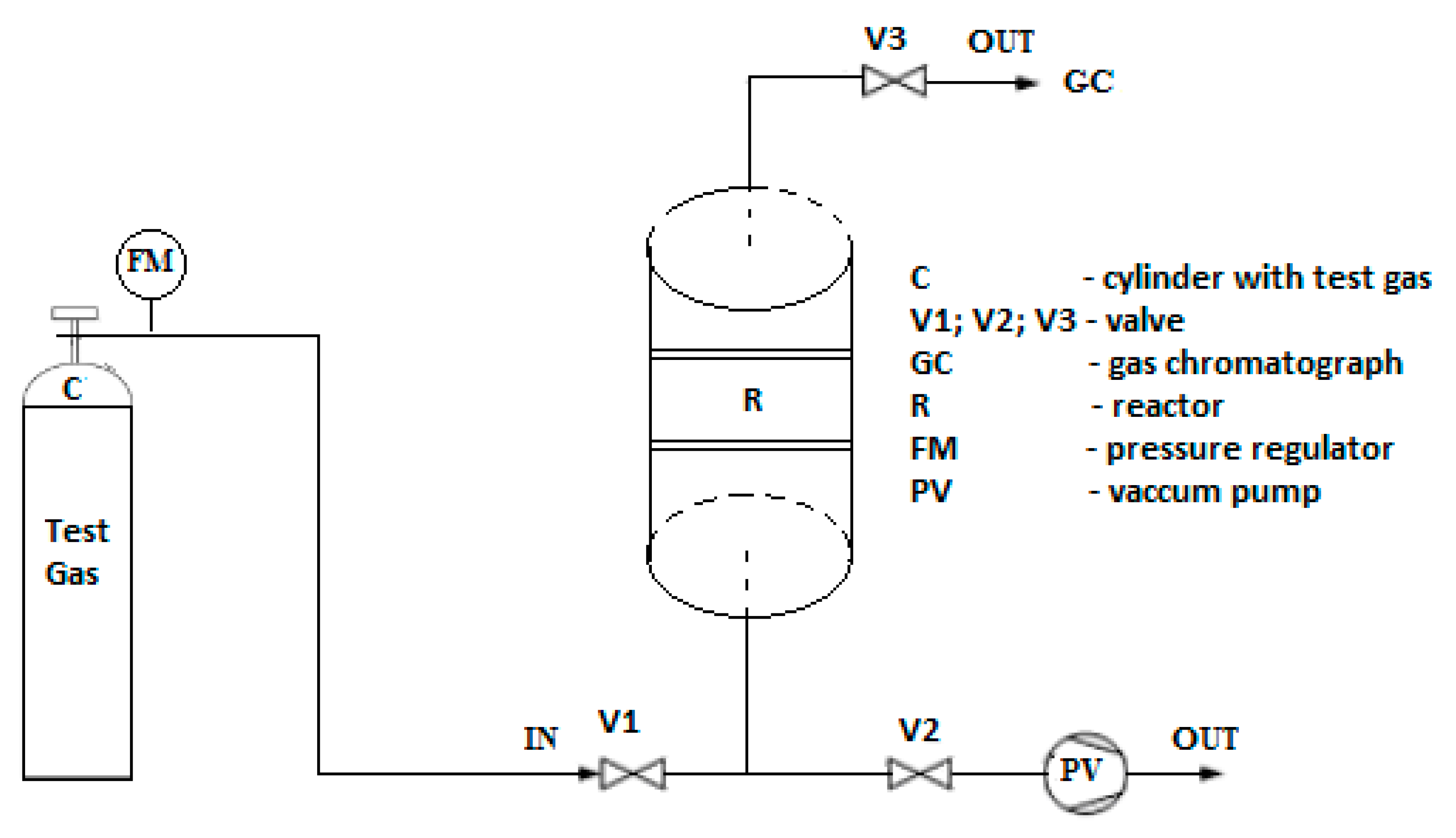
2. Materials and Methods
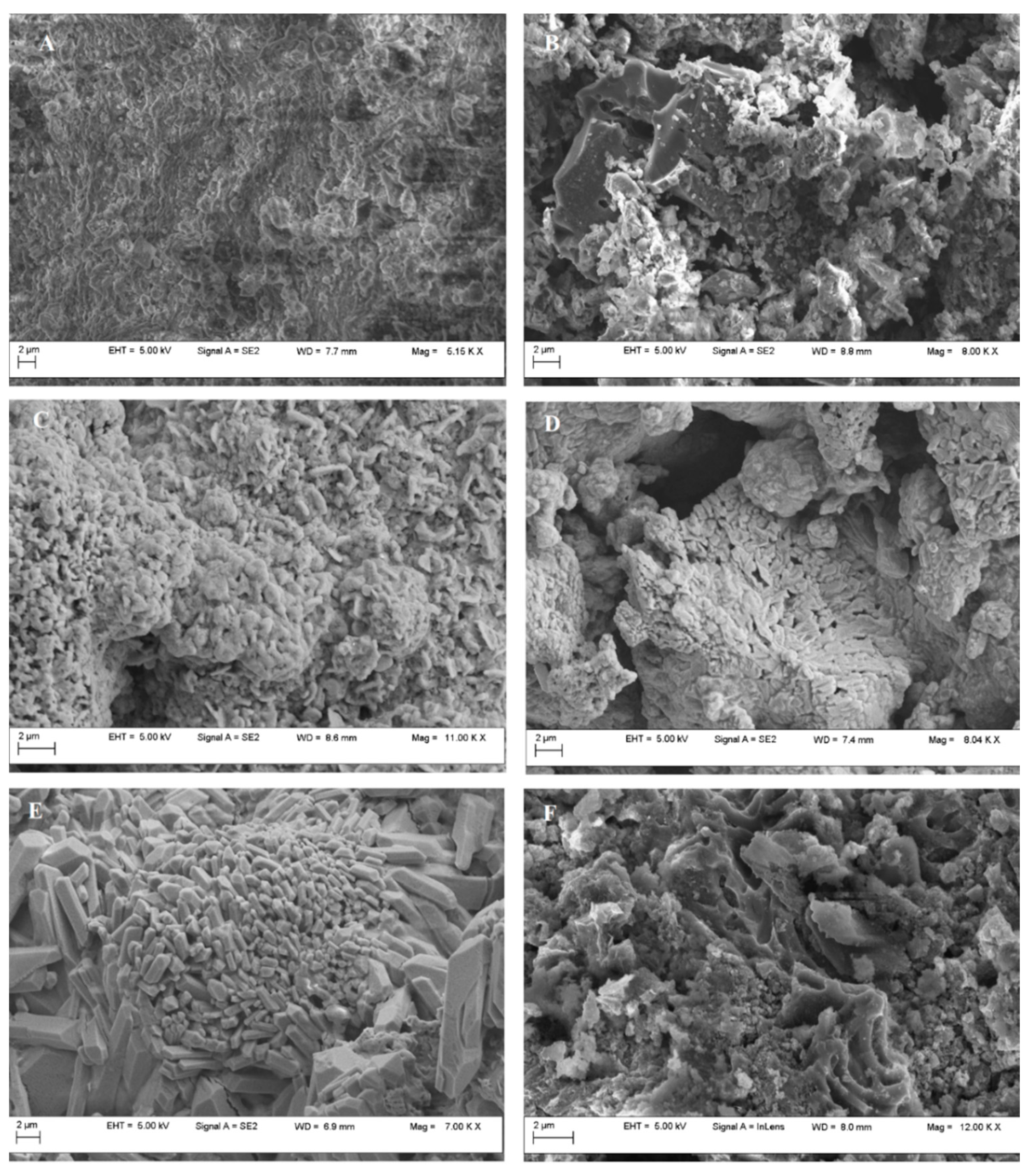
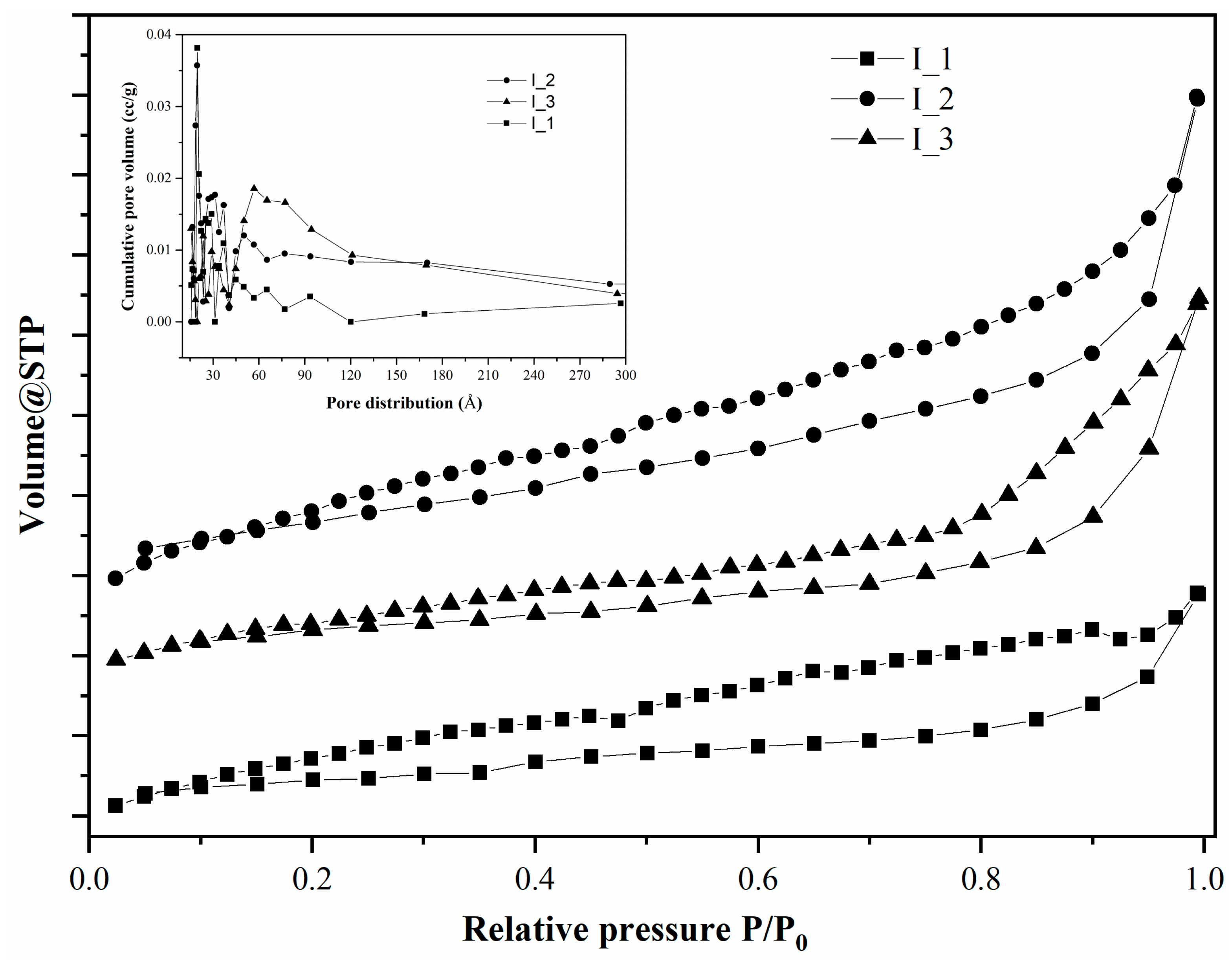
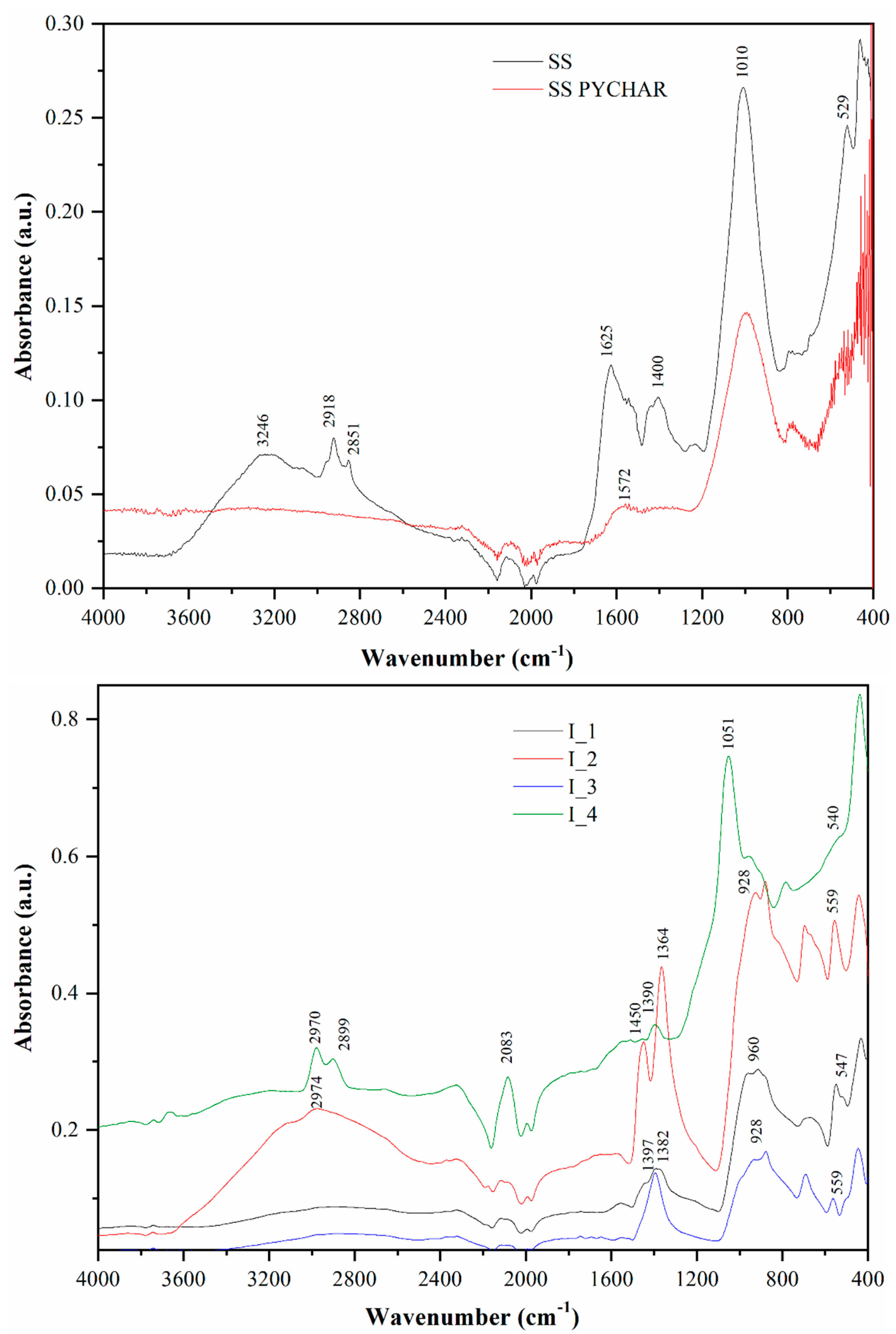
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NOAA. Available online: https://research.noaa.gov/article/ArtMID/587/ArticleID/2742/Despite-pandemic-shutdowns-carbon-dioxide-and-methane-surged-in-2020 (accessed on 17 May 2021).
- Liu, Y.; Song, Y.; Zhou, J.; Zhang, X. Modified polyether glycols supported ionic liquids for CO2 adsorption and chemical fixation. Mol. Catal. 2020, 492, 111008. [Google Scholar] [CrossRef]
- Wahono, S.K.; Stalin, J.; Addai-Mensah, J.; Skinner, W.; Vinu, A.; Vasilev, K. Physico-chemical modification of natural mordenite-clinoptilolite zeolites and their enhanced CO2 adsorption capacity. Microporous Mesoporous Mater. 2020, 294, 109871. [Google Scholar] [CrossRef]
- Ünveren, E.E.; Monkul, B.Ö.; Sarıoğlan, Ş.; Karademir, N.; Alper, E. Solid amine sorbents for CO2 capture by chemical adsorption: A review. Petroleum 2017, 3, 37–50. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Song, C.; Sun, Y.; Fan, Z.; Liu, Q.; Ji, N.; Kitamura, Y. Parametric study of a novel cryogenic-membrane hybrid system for efficient CO2 separation. Int. J. Greenh. Gas Control. 2018, 72, 74–81. [Google Scholar] [CrossRef]
- Hosseini, Y.; Najafi, M.; Khalili, S.; Jahanshahi, M.; Peyravi, M. Assembly of amine-functionalized graphene oxide for efficient and selective adsorption of CO2. Mater. Chem. Phys. 2021, 270, 124788. [Google Scholar] [CrossRef]
- Haghighat, M.; Majidian, N.; Hallajisani, A.; Samipourgiri, M. Production of bio-oil from sewage sludge: A review on the thermal and catalytic conversion by pyrolysis. Sustain. Energy Technol. Assess. 2020, 42, 100870. [Google Scholar] [CrossRef]
- Policicchio, A.; Zhao, Y.; Zhong, Q.; Agostino, R.G.; Bandosz, T.J. Cu-BTC/aminated graphite oxide composites as high efficiency CO2 capture media Cu-BTC/aminated graphite oxide composites as high efficiency CO2 capture media. ACS Appl. Mat. Interfaces 2013, 6, 101–108. [Google Scholar] [CrossRef]
- Flores-Flores, M.; Luévano-Hipólito, E.; Torres-Martínez, L.M.; Do, T.-O. CO2 adsorption and photocatalytic reduction over Mg(OH)2/CuO/Cu2O under UV-Visible light to solar fuels. Mater. Chem. Phys. 2019, 227, 90–97. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, H.; Qin, G. Utilization of zeolite as a potential multi-functional proppant for CO2 enhanced shale gas recovery and CO2 sequestration: A molecular simulation study of the impact of water on adsorption in zeolite and organic matter. Fuel 2021, 292, 120312. [Google Scholar] [CrossRef]
- Başaran, K.; Topçubaşı, B.U.; Davran-Candan, T. Theoretical investigation of CO2 adsorption mechanism over amine-functionalized mesoporous silica. J. CO2 Util. 2021, 47, 101492. [Google Scholar] [CrossRef]
- Othman, F.E.C.; Yusof, N.; Samitsu, S.; Abdullah, N.; Hamid, M.F.; Nagai, K.; Abidin, M.N.Z.; Azali, M.A.; Ismail, A.F.; Jaafar, J.; et al. Activated carbon nanofibers incorporated metal oxides for CO2 adsorption: Effects of different type of metal oxides. J. CO2 Util. 2021, 45, 101434. [Google Scholar] [CrossRef]
- Chang, C.-W.; Kao, Y.-H.; Shen, P.-H.; Kang, P.-C.; Wang, C.-Y. Nanoconfinement of metal oxide MgO and ZnO in zeolitic imidazolate framework ZIF-8 for CO2 adsorption and regeneration. J. Hazard. Mater. 2020, 400, 122974. [Google Scholar] [CrossRef] [PubMed]
- Angin, D. Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 2014, 115, 804–811. [Google Scholar] [CrossRef]
- Manyà, J.J.; Gonzalez, B.; Azuara, M.; Arner, G. Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity. Chem. Eng. J. 2018, 345, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Serafin, J.; Baca, M.; Biegun, M.; Mijowska, E.; Kaleńczuk, R.J.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Direct conversion of biomass to nanoporous activated biocarbons for high CO2 adsorption and supercapacitor applications. Appl. Surf. Sci. 2019, 497, 143722. [Google Scholar] [CrossRef]
- Hadoun, H.; Sadaoui, Z.; Souami, N.; Sahel, D.; Toumert, I. Characterization of mesoporous carbon prepared from date stems by H3PO4 chemical activation. Appl. Surf. Sci. 2013, 280, 1–7. [Google Scholar] [CrossRef]
- Choma, J.; Marszewski, M.; Osuchowski, L.; Jagiello, J.; Dziura, A.; Jaroniec, M. Adsorption Properties of Activated Carbons Prepared from Waste CDs and DVDs. ACS Sustain. Chem. Eng. 2015, 3, 733–742. [Google Scholar] [CrossRef]
- Olivares-Marin, M.; Maroto-Valer, M.M. Development of adsorbents for CO2 capture from waste materials: A review. Greenh. Gases Sci. Technol. 2012, 2, 20–35. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J. Environ. Manag. 2017, 188, 322–336. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Ngo, T.N.L.T.; Chiang, K.-Y. Enhanced hydrogen production in co-gasification of sewage sludge and industrial wastewater sludge by a pilot-scale fluidized bed gasifier. Int. J. Hydrogen Energy 2021, 46, 14083–14095. [Google Scholar] [CrossRef]
- Fedorov, A.; Dubinin, Y.; Yeletsky, P.; Fedorov, I.; Shelest, S.; Yakovlev, V. Combustion of sewage sludge in a fluidized bed of catalyst: ASPEN PLUS model. J. Hazard. Mater. 2021, 405, 124196. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Barbarias, I.; Bilbao, J.; Olazar, M. Sewage sludge valorization by flash pyrolysis in a conical spouted bed reactor. Chem. Eng. J. 2015, 273, 173–183. [Google Scholar] [CrossRef]
- Kargbo, D.M. Biodiesel Production from Municipal Sewage Sludges. Energy Fuels 2010, 24, 2791–2794. [Google Scholar] [CrossRef]
- Balat, M. Mechanisms of Thermochemical Biomass Conversion Processes. Part 1: Reactions of Pyrolysis. Energy Sour. Part A Recover. Util. Environ. Eff. 2008, 30, 620–635. [Google Scholar] [CrossRef]
- Xie, L.-P.; Li, T.; Gao, J.-D.; Fei, X.-N.; Wu, X.; Jiang, Y.-G. Effect of moisture content in sewage sludge on air gasification. J. Fuel Chem. Technol. 2010, 38, 615–620. [Google Scholar] [CrossRef]
- Hernández, J.; Ballesteros, R.; Aranda, G. Characterisation of tars from biomass gasification: Effect of the operating conditions. Energy 2013, 50, 333–342. [Google Scholar] [CrossRef]
- Huang, B.-S.; Chen, H.-Y.; Chuang, K.-H.; Yang, R.-X.; Wey, M.-Y. Hydrogen production by biomass gasification in a fluidized-bed reactor promoted by an Fe/CaO catalyst. Int. J. Hydrogen Energy 2012, 37, 6511–6518. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Lei, Z.; Cheng, G. Co-gasification of wet sewage sludge and forestry waste in situ steam agent. Bioresour. Technol. 2012, 114, 698–702. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Lin, C.-L.; Chang, T.-J.; Weng, W.-C. Waste-gasification efficiency of a two-stage fluidized-bed gasification system. Waste Manag. 2016, 48, 250–256. [Google Scholar] [CrossRef]
- Chiang, K.-Y.; Lu, C.-H.; Liao, C.-K.; Ger, R.H.-R. Characteristics of hydrogen energy yield by co-gasified of sewage sludge and paper-mill sludge in a commercial scale plant. Int. J. Hydrogen Energy 2016, 41, 21641–21648. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Kauppinen, J.; Soini, T.; García-Gutiérrez, L.M.; Pikkarainen, T. Pollutant emissions released during sewage sludge combustion in a bubbling fluidized bed reactor. Waste Manag. 2020, 105, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Tinnirello, M.; Papurello, D.; Santarelli, M.; Fiorilli, S. Thermal Activation of Digested Sewage Sludges for Carbon Dioxide Removal from Biogas. Fuels 2020, 1, 30–46. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. CO2 adsorption on biochar from co-torrefaction of sewage sludge and leucaena wood using microwave heating. Energy Procedia 2019, 158, 4435–4440. [Google Scholar] [CrossRef]
- Ionete, R.E.; Ionete, E.I.; Spiridon, S.I.; Constantinescu, M. Solar Dryer with Humidity Extractor Used for Organic Matter. Patent Application Number RO132880-A0, 30 October 2018. [Google Scholar]
- Constantinescu, M.; Bucura, F.; Ionete, R.-E.; Niculescu, V.-C.; Ionete, E.I.; Zaharioiu, A.; Oancea, S.; Miricioiu, M.G. Comparative Study on Plastic Materials as a New Source of Energy. Mater. Plast. 2019, 56, 41–46. [Google Scholar] [CrossRef]
- De Andrés, J.M.; Orjales, L.; Narros, A.; Fuente, M.D.M.D.L.; Rodríguez, M.E. Carbon dioxide adsorption in chemically activated carbon from sewage sludge. J. Air Waste Manag. Assoc. 2013, 63, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Constantinescu, M.; Bucura, F.; Ionete, E.I.; Ion-Ebrasu, D.; Sandru, C.; Zaharioiu, A.; Marin, F.; Miricioiu, M.; Niculescu, V.; Oancea, S.; et al. From Plastic to Fuel—New Challenges. Mater. Plast. 2019, 56, 721–729. [Google Scholar] [CrossRef]
- Teng, H.; Lin, Y.-C.; Hsu, L.-Y. Production of Activated Carbons from Pyrolysis of Waste Tires Impregnated with Potassium Hydroxide. J. Air Waste Manag. Assoc. 2000, 50, 1940–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Rio, S.; LE Coq, L.; Faur, C.; LeComte, D.; Le Cloirec, P. Preparation of Adsorbents from Sewage Sludge by Steam Activation for Industrial Emission Treatment. Process. Saf. Environ. Prot. 2006, 84, 258–264. [Google Scholar] [CrossRef]
- Lin, Q.; Cheng, H.; Chen, G. Preparation and characterization of carbonaceous adsorbents from sewage sludge using a pilot-scale microwave heating equipment. J. Anal. Appl. Pyrolysis 2012, 93, 113–119. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, H.; He, Y. Application and Mechanism of Sludge-Based Activated Carbon for Phenol and Cyanide Removal from Bio-Treated Effluent of Coking Wastewater. Processes 2020, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Majdan, M.; Maryuk, O.; Gładysz-Płaska, A.; Pikus, S.; Kwiatkowski, R. Spectral characteristics of the bentonite loaded with benzyldimethyloctadecylammonium chloride, hexadecyltrimethylammonium bromide and dimethyldioctadecylammonium bromide. J. Mol. Struct. 2008, 874, 101–107. [Google Scholar] [CrossRef]
- Song, X.; Xue, X.; Chen, D.; He, P.; Dai, X. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Droussi, Z.; D’Orazio, V.; Provenzano, M.R.; Hafidi, M.; Ouatmane, A. Study of the biodegradation and transformation of olive-mill residues during composting using FTIR spectroscopy and differential scanning calorimetry. J. Hazard. Mater. 2009, 164, 1281–1285. [Google Scholar] [CrossRef]
- Vǎduva, M.; Stanciu, V. Carbon molecular sieves production and performance assessment in carbon dioxide separation. J. Optoelectron. Adv. Mater. 2007, 9, 2296–2301. [Google Scholar]
- Vǎduva, M.; Stanciu, V. Selective carbon dioxide adsorption from N2-CH 4-CO2 mixture on carbon molecular sieves. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2007, 69, 59–70. [Google Scholar]
- Nascimento, P.F.P.; Sousa, J.F.; Oliveira, J.A.; Possa, R.D.; Santos, L.S.; Carvalho, F.C.; Ruiz, J.A.C.; Pedroza, M.M.; Bezerra, M.B.D. Wood sawdust and sewage sludge pyrolysis chars for CO2 adsorption using a magnetic suspension balance. Can. J. Chem. Eng. 2017, 95, 2148–2155. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- European Emission Allowances. Available online: https://markets.businessinsider.com/commodities/co2-european-emission-allowances (accessed on 28 July 2021).
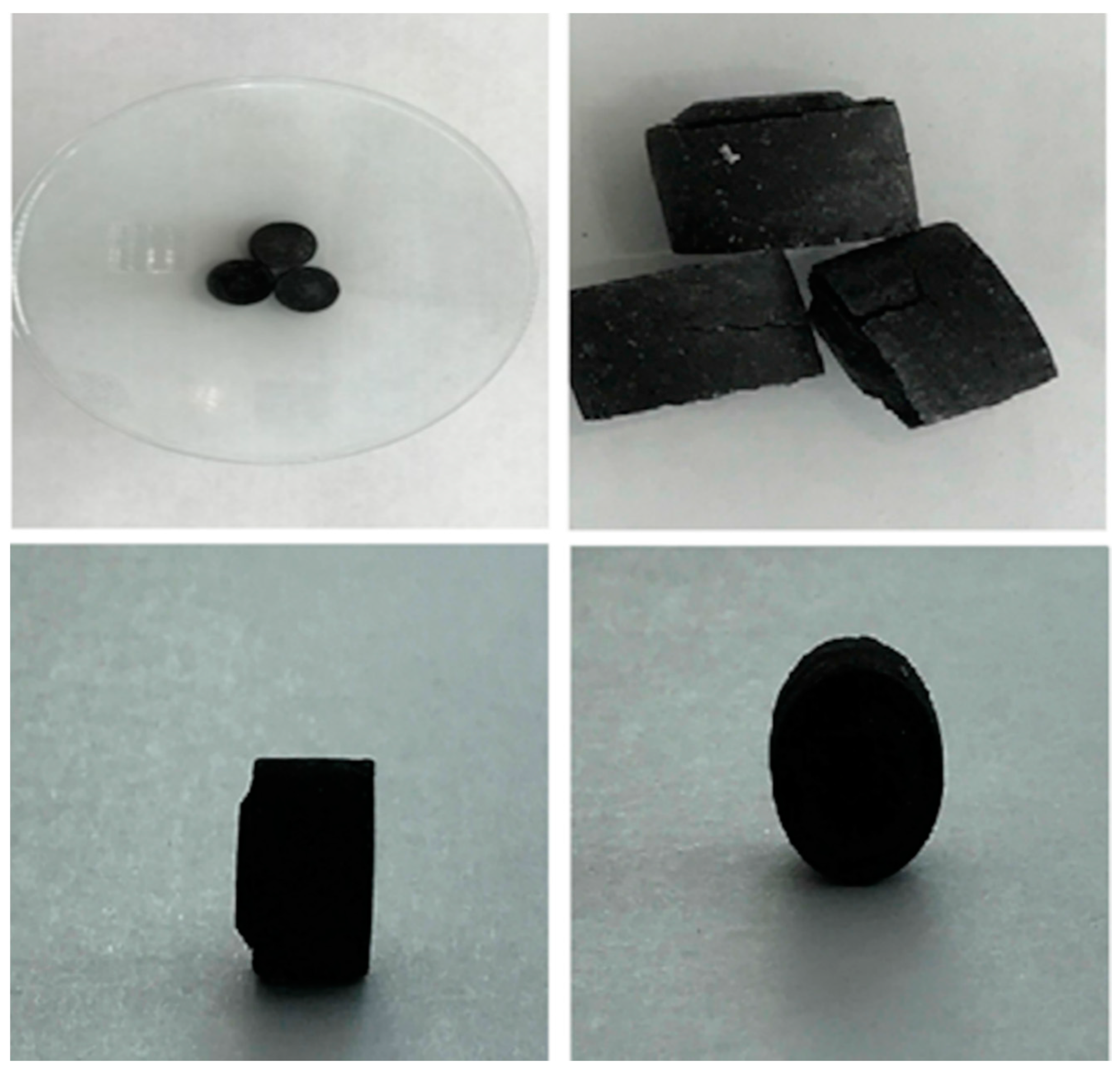

| Shape (Appearance) | Height (mm) | Diameter (mm) | Weight (g) | Colour |
|---|---|---|---|---|
| circular | 11.46 | 12.74 | 0.7 | black |
| Element (wt%) | SS | SSPYCHAR | I_1 | I_2 | I_3 | I_4 |
|---|---|---|---|---|---|---|
| C | 34.51 | 29.46 | 10.85 | 8.87 | 6.02 | 31.93 |
| O | 17.11 | 0.38 | 0.37 | 0.41 | 0.44 | 0.5 |
| N | 6.26 | 4.13 | 1.67 | 1.28 | 0.66 | 0.65 |
| S | 0.86 | 0.47 | 0 | 0 | 0 | 0 |
| H | 5.01 | 1.17 | 2.07 | 2.76 | 2.95 | 2.15 |
| Sample | Metal (mg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Fe | Mn | Ni | Cu | Zn | K | Na | Pb | Si | |
| SS | 0.19 | 28.95 | 0.14 | 0.02 | 0.36 | 0.11 | - | - | 0.02 | 144.75 |
| SSPYCHAR | 0.45 | 0.09 | 0.26 | 0.23 | 1.64 | 1.52 | 4.70 | 2.65 | 0.06 | 323.32 |
| I_1 | <0.01 | 150.95 | 0.19 | <0.01 | 0.66 | 0.36 | 197.93 | 9.5 | <0.02 | 147.80 |
| I_2 | <0.01 | 9.69 | 0.15 | <0.01 | 0.43 | 0.11 | 169.49 | 6.47 | <0.02 | 146.22 |
| I_3 | <0.01 | 8.15 | 0.11 | <0.01 | 0.13 | 0.11 | 168.99 | 6.06 | <0.02 | 146.00 |
| I_4 | <0.01 | 14.23 | 0.07 | <0.01 | 1.83 | 0.11 | 16.27 | 7.34 | <0.02 | 434.24 |
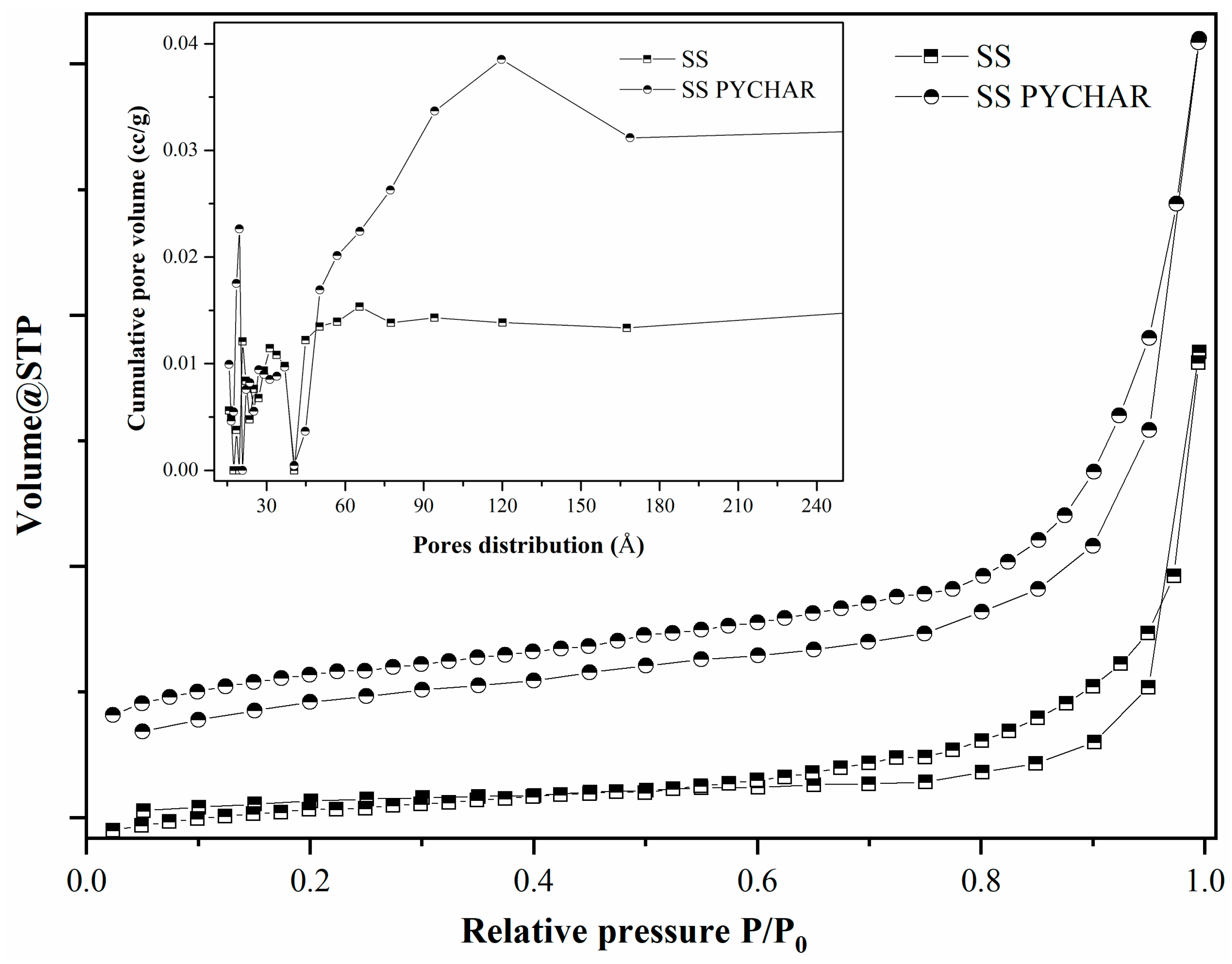
| Material Type | SBET m2/g | V cm3/g | dp Å |
|---|---|---|---|
| SS * | 2.972 | 0.030 | 41.666 |
| SSPYCHAR | 15.608 | 0.042 | 39.250 |
| I_1 | 3.305 | 0.008 | 39.256 |
| I_2 | 6.447 | 0.017 | 39.252 |
| I_3 | 3.618 | 0.014 | 31.308 |
| I_4 | 921.013 | 0.657 | 39.306 |
| Gas Mixture | Injection Number | Time (min) | CO2 Evolution (vol.%) | a (1) (cm3/g) | η (2) (%) | R (3) (%) |
|---|---|---|---|---|---|---|
| 74 Vol % CO2 balance N2 | 1 | 8 | 51.19 | 11.87 | 99.68 | 98.11 |
| 2 | 8 | 38.74 | ||||
| 3 | 8 | 22.23 | ||||
| 4 | 8 | 16.88 | ||||
| 5 | 8 | 6.09 | ||||
| 6 | 8 | 2.12 | ||||
| 7 | 8 | 0.23 | ||||
| 8 | 8 | 9.12 | ||||
| 9 | 8 | 17.46 | ||||
| 10 | 8 | 44.27 | ||||
| 11 | 8 | 67.00 | ||||
| 12 | 8 | 74.05 | ||||
| 0.1 Vol % CO2 balance N2 | 1 | 8 | 0.0076 | 0.92 | 95.00 | - |
| 2 | 8 | >LOD (4) | ||||
| 3 | 8 | >LOD | ||||
| 4 | 8 | >LOD | ||||
| 5 | 8 | >LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miricioiu, M.G.; Zaharioiu, A.; Oancea, S.; Bucura, F.; Raboaca, M.S.; Filote, C.; Ionete, R.E.; Niculescu, V.C.; Constantinescu, M. Sewage Sludge Derived Materials for CO2 Adsorption. Appl. Sci. 2021, 11, 7139. https://doi.org/10.3390/app11157139
Miricioiu MG, Zaharioiu A, Oancea S, Bucura F, Raboaca MS, Filote C, Ionete RE, Niculescu VC, Constantinescu M. Sewage Sludge Derived Materials for CO2 Adsorption. Applied Sciences. 2021; 11(15):7139. https://doi.org/10.3390/app11157139
Chicago/Turabian StyleMiricioiu, Marius Gheorghe, Anca Zaharioiu, Simona Oancea, Felicia Bucura, Maria Simona Raboaca, Constantin Filote, Roxana Elena Ionete, Violeta Carolina Niculescu, and Marius Constantinescu. 2021. "Sewage Sludge Derived Materials for CO2 Adsorption" Applied Sciences 11, no. 15: 7139. https://doi.org/10.3390/app11157139
APA StyleMiricioiu, M. G., Zaharioiu, A., Oancea, S., Bucura, F., Raboaca, M. S., Filote, C., Ionete, R. E., Niculescu, V. C., & Constantinescu, M. (2021). Sewage Sludge Derived Materials for CO2 Adsorption. Applied Sciences, 11(15), 7139. https://doi.org/10.3390/app11157139










