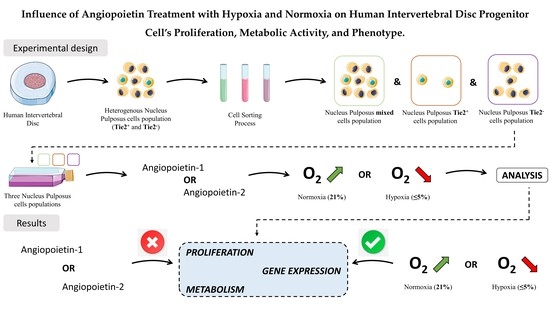
Influence of Angiopoietin Treatment with Hypoxia and Normoxia on Human Intervertebral Disc Progenitor Cell’s Proliferation, Metabolic Activity, and Phenotype
Abstract
:1. Introduction
1.1. Low Back Pain and Intervertebral Disc Degeneration (IVDD)
1.2. Hypoxia vs. Normoxia in the Intervertebral Disc (IVD)
1.3. Nucleus Pulposus Progenitor Cells (also Known as Tie2+ Cells)
1.4. Tie2 Receptor and Its Ligands; Angiopoietin-1 and Angiopoietin-2
1.5. The Role of Tie2/Ang-1 and Ang-2 Signaling Pathway in Human NPCs and NPPCs
1.6. Hypothesis and Aims
2. Materials and Methods
2.1. NPC Isolation
2.2. NPC Expansion
2.3. NPC Sorting
2.4. NPC Seeding and Incubation with Ang-1 or Ang-2
2.5. Resazurin Sodium Salt Cell Activity Assay
2.6. Papain Digestion and DNA Quantification
2.7. RNA Extraction and cDNA Synthesis
2.8. Quantitative Polymerase Chain Reaction (qPCR)
2.9. Statistical Analysis
3. Results
3.1. Effect of Oxygen Tension and Angiopoietin-1/2 on NP Cell’s Proliferation
3.2. Effect of Oxygen Tension and Angiopoietin-1/2 on NP Single Cell’s Metabolism
3.3. Effect of Oxygen Tension and Angiopoietin-1/2 on ECM Related-Genes
3.3.1. Analysis of Aggrecan Relative Gene Expression
3.3.2. Analysis of Collagen Type II Relative Gene Expression
3.4. Effect of Oxygen Tension and Angiopoietin-1/2 on Oxygen Level Related-Genes
Analysis of Hypoxia-Inducible Factor 1-Alpha Relative Gene Expression
3.5. Effect of Oxygen Tension and Angiopoietin-1/2 on NP Progenitor Cells Related-Genes
Analysis of Angiopoietin-1 Receptor Relative Gene Expression
4. Discussion
4.1. Ang-1/2 and Its Effect on DNA Measurement in Normoxic and Hypoxic Conditions
4.2. Ang-1/2 and Its Effect on Metabolic Activity per DNA in Normoxic & Hypoxic Conditions
4.3. Ang-1/2 and Its Effect on the ECM Gene Expression in Normoxia & Hypoxia
4.4. Study Weaknesses and Limitations
4.5. Outlook and Clinical Relevance
5. Conclusions
- To the best of our knowledge, this study cannot provide evidence for stimulation of Ang-1 or Ang-2 or their dose-dependent administration influences on NP cell proliferation.
- Neither on the NP cell’s metabolism, or relative gene expression.
- Moreover, we also demonstrated a higher NP cell metabolism if cultured in hypoxia.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakai, D.; Nakamura, Y.; Nakai, T.; Mishima, T.; Kato, S.; Grad, S.; Alini, M.; Risbud, M.V.; Chan, D.; Cheah, K.S.; et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat. Commun. 2012, 3, 1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgartner, L.; Wuertz-Kozak, K.; Le Maitre, C.L.; Wignall, F.; Richardson, S.M.; Hoyland, J.; Ruiz Wills, C.; Gonzalez Ballester, M.A.; Neidlin, M.; Alexopoulos, L.G.; et al. Multiscale regulation of the intervertebral disc: Achievements in experimental, in silico, and regenerative research. Int. J. Mol. Sci. 2021, 22, 703. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Evans, E.H.; Kletsas, D.; Jaffray, D.C.; Eisenstein, S.M. Senescence in human intervertebral discs. Eur. Spine J. 2006, 15 (Suppl. 3), S312–S316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-based strategies for ivd repair: Clinical progress and translational obstacles. Nat. Rev. Rheumatol. 2021, 17, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Frauchiger, D.A.; Tekari, A.; May, R.D.; Dzafo, E.; Chan, S.C.W.; Stoyanov, J.; Bertolo, A.; Zhang, X.; Guerrero, J.; Sakai, D.; et al. Fluorescence-activated cell sorting is more potent to fish intervertebral disk progenitor cells than magnetic and beads-based methods. Tissue Eng. Part C Methods 2019, 25, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Gerges, I.; Tamplenizza, M.; Lenardi, C.; Forsyth, N.R.; Liu, Y. Three-dimensional hypoxic culture of human mesenchymal stem cells encapsulated in a photocurable, biodegradable polymer hydrogel: A potential injectable cellular product for nucleus pulposus regeneration. Acta Biomater. 2014, 10, 3463–3474. [Google Scholar] [CrossRef] [Green Version]
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Kwon, W.K.; Moon, H.J.; Kwon, T.H.; Park, Y.K.; Kim, J.H. The role of hypoxia in angiogenesis and extracellular matrix regulation of intervertebral disc cells during inflammatory reactions. Neurosurgery 2017, 81, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.M.; Fairbank, J.C.; Winlove, C.P.; Urban, J.P. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 1998, 23, 1–7, discussion 8. [Google Scholar] [CrossRef] [Green Version]
- Tejada, S.; Batle, J.M.; Ferrer, M.D.; Busquets-Cortes, C.; Monserrat-Mesquida, M.; Nabavi, S.M.; Del Mar Bibiloni, M.; Pons, A.; Sureda, A. Therapeutic effects of hyperbaric oxygen in the process of wound healing. Curr. Pharm. Des. 2019, 25, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.J.; Cheung, K.M.; Zheng, Z.; Wang, H.; Sakai, D.; Leung, V.Y. Ivd progenitor cells: A new horizon for understanding disc homeostasis and repair. Nat. Rev. Rheumatol. 2019, 15, 102–112. [Google Scholar] [CrossRef]
- Tekari, A.; Chan, S.C.W.; Sakai, D.; Grad, S.; Gantenbein, B. Angiopoietin-1 receptor tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res. Ther. 2016, 7, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, D.; Schol, J. Cell therapy for intervertebral disc repair: Clinical perspective. J. Orthop. Translat. 2017, 9, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Schol, J.; Bach, F.C.; Tekari, A.; Sagawa, N.; Nakamura, Y.; Chan, S.C.W.; Nakai, T.; Creemers, L.B.; Frauchiger, D.A.; et al. Successful fishing for nucleus pulposus progenitor cells of the intervertebral disc across species. JOR Spine 2018, 1, e1018. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.; Hackel, S.; Croft, A.S.; Albers, C.E.; Gantenbein, B. The effects of 3d culture on the expansion and maintenance of nucleus pulposus progenitor cell multipotency. JOR Spine 2021, 4, e1131. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovic, E.; Nguyen, V.P.; Dumont, D.J. Activation of tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J. Cell Sci. 2006, 119, 3551–3560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiedler, U.; Augustin, H.G. Angiopoietins: A link between angiogenesis and inflammation. Trends Immunol. 2006, 27, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Daly, C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb. Perspect. Med. 2012, 2, a006550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakui, S.; Yokoo, K.; Muto, T.; Suzuki, Y.; Takahashi, H.; Furusato, M.; Hano, H.; Endou, H.; Kanai, Y. Localization of ang-1, -2, tie-2, and vegf expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab. Investig. 2006, 86, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imanishi, Y.; Hu, B.; Jarzynka, M.J.; Guo, P.; Elishaev, E.; Bar-Joseph, I.; Cheng, S.Y. Angiopoietin-2 stimulates breast cancer metastasis through the alpha(5)beta(1) integrin-mediated pathway. Cancer Res. 2007, 67, 4254–4263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Liu, W.; Song, Y.; Wu, X.; Zhang, Y.; Li, S.; Gao, Y.; Tu, J.; Liu, Y.; Yang, C. The role of angiopoietin-2 in nucleus pulposus cells during human intervertebral disc degeneration. Lab. Investig. 2017, 97, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eklund, L.; Olsen, B.R. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp. Cell Res. 2006, 312, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Makinde, T.O.; Agrawal, D.K. Increased expression of angiopoietins and tie2 in the lungs of chronic asthmatic mice. Am. J. Respir Cell Mol. Biol 2011, 44, 384–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarter, S.D.; Mei, S.H.; Lai, P.F.; Zhang, Q.W.; Parker, C.H.; Suen, R.S.; Hood, R.D.; Zhao, Y.D.; Deng, Y.; Han, R.N.; et al. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am. J. Respir. Crit. Care Med. 2007, 175, 1014–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.; Li, Z.; Lang, C.N.; Schmid, B.; Lang, F.K.; Grad, S.; Alini, M.; Richards, R.G.; Schmal, H.; Sudkamp, N.; et al. The tissue renin-angiotensin system and its role in the pathogenesis of major human diseases: Quo vadis? Cells 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wystrach, L.; Bernstein, A.; Grad, S.; Alini, M.; Richards, R.G.; Kubosch, D.; Sudkamp, N.; Izadpanah, K.; Kubosch, E.J.; et al. The tissue-renin-angiotensin-system of the human intervertebral disc. Eur. Cell Mater. 2020, 40, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.; Thiede, T.; Aebli, N.; Baur, M.; Ferguson, S.J.; Stoyanov, J.V. Human mesenchymal stem cell co-culture modulates the immunological properties of human intervertebral disc tissue fragments in vitro. Eur. Spine J. 2011, 20, 592–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gantenbein, B.; Calandriello, E.; Wuertz-Kozak, K.; Benneker, L.M.; Keel, M.J.; Chan, S.C. Activation of intervertebral disc cells by co-culture with notochordal cells, conditioned medium and hypoxia. BMC Musculoskelet. Disord. 2014, 15, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Leung, V.Y.; Long, D.; Chan, D.; Lu, W.W.; Cheung, K.M.; Zhou, G. Coupling of small leucine-rich proteoglycans to hypoxic survival of a progenitor cell-like subpopulation in rhesus macaque intervertebral disc. Biomaterials 2013, 34, 6548–6558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risbud, M.V.; Shapiro, I.M. Notochordal cells in the adult intervertebral disc: New perspective on an old question. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasen, M.; Fei, Q.; Hutton, W.C.; Zhang, J.; Dong, J.; Jiang, X.; Zhang, F. Changes of number of cells expressing proliferation and progenitor cell markers with age in rabbit intervertebral discs. Acta Biochim. Biophys. Sin. 2013, 45, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Yang, P.; Wu, Y.; Tang, Y.; Zhao, Y.; Wu, J.; Wang, D.; He, Q.; Ruan, D. Comparison of biological characteristics of nucleus pulposus mesenchymal stem cells derived from non-degenerative and degenerative human nucleus pulposus. Exp. Ther. Med. 2017, 13, 3574–3580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Jia, Z.; Huang, S.; Wu, Y.; Liu, L.; Lin, L.; Wang, D.; He, Q.; Ruan, D. Age-related changes in nucleus pulposus mesenchymal stem cells: An in vitro study in rats. Stem Cells Int. 2017, 2017, 6761572. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Kang, L.; Liu, W.; Song, Y.; Wu, X.; Zhang, Y.; Hua, W.; Zhao, K.; Li, S.; Tu, J.; et al. Angiopoietin-2 promotes extracellular matrix degradation in human degenerative nucleus pulposus cells. Int. J. Mol. Med. 2018, 41, 3551–3558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, G.; Li, L.; Liu, H.; Song, Y.; Huang, F.; Tu, C.; Shen, B.; Gong, Q.; Li, T.; Liu, L.; et al. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3d scaffolds. Osteoarthr. Cartil. 2013, 21, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwale, F.; Ciobanu, I.; Giannitsios, D.; Roughley, P.; Steffen, T.; Antoniou, J. Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine 2011, 36, E131–E138. [Google Scholar] [CrossRef]
- Stoyanov, J.V.; Gantenbein-Ritter, B.; Bertolo, A.; Aebli, N.; Baur, M.; Alini, M.; Grad, S. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur. Cell Mater. 2011, 21, 533–547. [Google Scholar] [CrossRef]
- Guehring, T.; Wilde, G.; Sumner, M.; Grunhagen, T.; Karney, G.B.; Tirlapur, U.K.; Urban, J.P. Notochordal intervertebral disc cells: Sensitivity to nutrient deprivation. Arthritis Rheum 2009, 60, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Chamboredon, S.; Ciais, D.; Desroches-Castan, A.; Savi, P.; Bono, F.; Feige, J.J.; Cherradi, N. Hypoxia-inducible factor-1alpha mrna: A new target for destabilization by tristetraprolin in endothelial cells. Mol. Biol. Cell 2011, 22, 3366–3378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guerrero, J.; Croft, A.S.; Albers, C.E.; Hackel, S.; Gantenbein, B. Spheroid-like cultures for expanding angiopoietin receptor-1 (aka. Tie2) positive cells from the human intervertebral disc. Int. J. Mol. Sci. 2020, 21, 9423. [Google Scholar] [CrossRef] [PubMed]
- Kanda, S.; Miyata, Y.; Mochizuki, Y.; Matsuyama, T.; Kanetake, H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res. 2005, 65, 6820–6827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, T.; Wu, Y.; Boudreau, N.; Li, J.; Matsumoto, M.; Young, W. Regulation of tie2 expression by angiopoietin--potential feedback system. Endothelium 2004, 11, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, J.H.; Moon, S.O.; Kwak, H.J.; Kim, N.G.; Koh, G.Y. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3’-kinase/akt signal transduction pathway. Oncogene 2000, 19, 4549–4552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.; Kim, H.G.; So, J.N.; Kim, J.H.; Kwak, H.J.; Koh, G.Y. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/akt signal transduction pathway. Circ. Res. 2000, 86, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Donor Number | Sex | Age | IVD Level (Indication for Surgery) | Cell’s Passage |
|---|---|---|---|---|
| 1 | Female | 26 | Th12/L1 (Trauma) | P3 |
| 2 | Female | 25 | L1/L2 (Trauma) | P6 |
| 3 | Male | 20 | L1/L2 (Trauma) | P3 |
| 4 | Male | 24 | Th12/L1 (Trauma) | P4 |
| 5 | Male | 24 | L3/L4; L4/L5; L5/S1 pooled (Trauma) | P6 |
| 6 | Female | 19 | Th12/L1; L1/L2 pooled (Trauma) | P6 |
| 7 | Female | 20 | Th12/L1; L1/L2 pooled (Trauma) | P2 |
| 8 | Male | 18 | L1/L2 (Trauma) | P2 |
| Gene | Full Gene Name | Primer Nucleotide Sequence from 5′ to 3′ |
|---|---|---|
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | F—ATC TTC CAG GAG CGA GAT R—GGA GGC ATT GCT GAT GAT |
| 18S | 18S ribosomal RNA | F—CGA TGC GGC GGC GTT ATT R—TCT GTC AAT CCT GTC GTC CGT GTC C |
| TEK | TEK Receptor Tyrosine Kinase | F—TTA GCC AGC TTA GTT CTC TGT GG R—AGC ATC AGA TAC AAG AGG TAG GG |
| COL2 | Collagen Type II | F—AGC AGC AAG AGC AAG GAG AA R—GTA GGA AGG TCA TCT GGA |
| HIF1A | Hypoxia Inducible Factor 1 Subunit Alpha | F—GTC GCT TCG GCC AGT GTG R—GGA AAG GCA AGT CCA GAG GTG |
| ACAN | Aggrecan | F—CAT CAC TGC AGC TGT CAC R—AGC AGC ACT ACC TCC TTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bischof, M.C.; Häckel, S.; Oberli, A.; Croft, A.S.; Oswald, K.A.C.; Albers, C.E.; Gantenbein, B.; Guerrero, J. Influence of Angiopoietin Treatment with Hypoxia and Normoxia on Human Intervertebral Disc Progenitor Cell’s Proliferation, Metabolic Activity, and Phenotype. Appl. Sci. 2021, 11, 7144. https://doi.org/10.3390/app11157144
Bischof MC, Häckel S, Oberli A, Croft AS, Oswald KAC, Albers CE, Gantenbein B, Guerrero J. Influence of Angiopoietin Treatment with Hypoxia and Normoxia on Human Intervertebral Disc Progenitor Cell’s Proliferation, Metabolic Activity, and Phenotype. Applied Sciences. 2021; 11(15):7144. https://doi.org/10.3390/app11157144
Chicago/Turabian StyleBischof, Muriel C., Sonja Häckel, Andrea Oberli, Andreas S. Croft, Katharina A. C. Oswald, Christoph E. Albers, Benjamin Gantenbein, and Julien Guerrero. 2021. "Influence of Angiopoietin Treatment with Hypoxia and Normoxia on Human Intervertebral Disc Progenitor Cell’s Proliferation, Metabolic Activity, and Phenotype" Applied Sciences 11, no. 15: 7144. https://doi.org/10.3390/app11157144
APA StyleBischof, M. C., Häckel, S., Oberli, A., Croft, A. S., Oswald, K. A. C., Albers, C. E., Gantenbein, B., & Guerrero, J. (2021). Influence of Angiopoietin Treatment with Hypoxia and Normoxia on Human Intervertebral Disc Progenitor Cell’s Proliferation, Metabolic Activity, and Phenotype. Applied Sciences, 11(15), 7144. https://doi.org/10.3390/app11157144