Evaluation of Anti-Obesity Activity of an Herbal Formulation (F2) in DIO Mice Model and Validation of UPLC-DAD Method for Quality Control
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of F2
2.2. UPLC-DAD Analysis of F2 and Method Validation
2.2.1. Linearity, LOD and LOQ
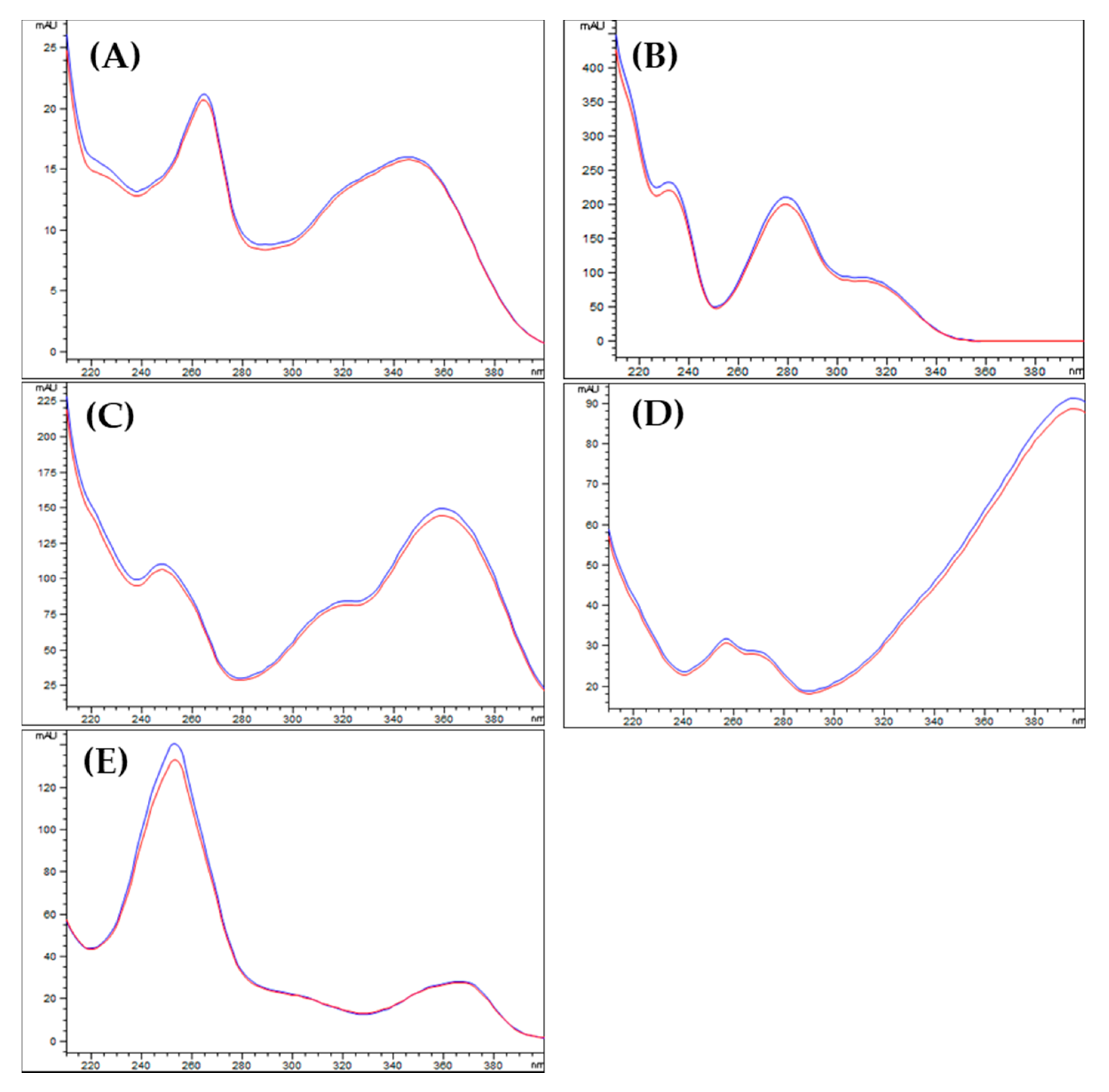
2.2.2. Specificity
2.2.3. Precision
2.3. Quantification of Marker Compounds in F2
2.4. Experimental Animals
2.5. Real-Time PCR Analysis
2.6. Statistical Analysis
3. Results
3.1. Optimization of Chromatographic Condition
3.2. Method Validation for Quantitative Analysis
3.3. Quality Assurance of F2
3.4. Induction of Diet-Induced Obesity (DIO) on C57BL/6J Mice
3.5. Regulation of Body Weight Gain by F2 on Obese Mice
3.6. Effect of F2 on Serum Lipid Profile
3.7. Effect of F2 on Fasting Blood Glucose, Serum Insulin, and HOMA-IR
3.8. Effect of F2 on mRNA Expression of Adipogenic Transcription Factors on White Adipose Tissue
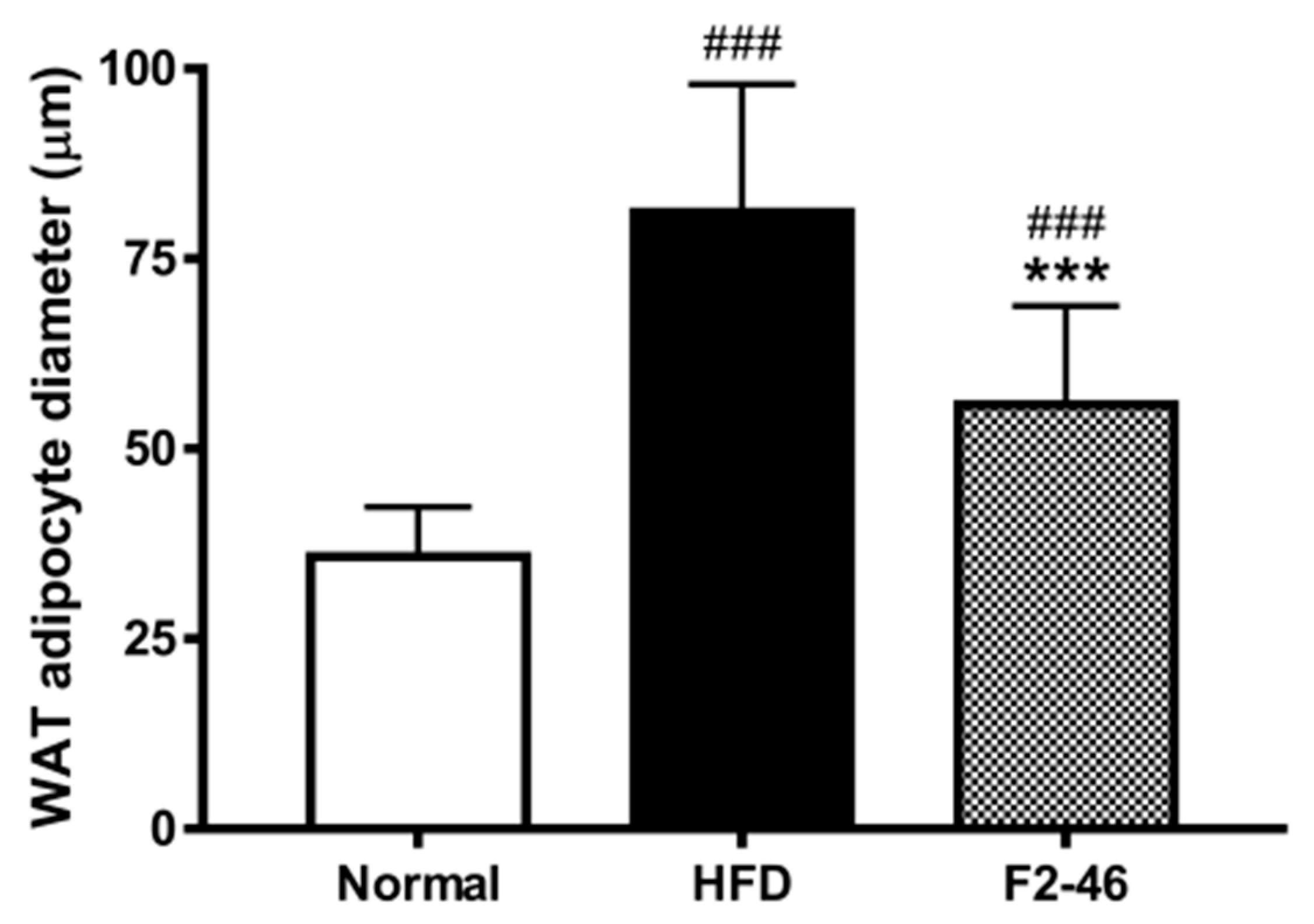
3.9. Effect of F2 on Visceral Organ Weights and Histological Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananthakumar, T.; Jones, N.R.; Hinton, L.; Aveyard, P. Clinical encounters about obesity: Systematic review of patients’ perspectives. Clin. Obes. 2020, 10, e12347. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.-H.; Kim, W.K.; Lee, S.C. Involvement of protein tyrosine phosphatases in adipogenesis: New anti-obesity targets? BMB Rep. 2012, 45, 700. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M. Adipocyte differentiation: From fibroblast to endocrine cell. Exp. Biol. Med. 2001, 226, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabet. Metab. Synd. Obes. Targets Ther. 2021, 14, 67. [Google Scholar] [CrossRef]
- Jones, J.R.; Barrick, C.; Kim, K.-A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef]
- Barrett, P.; Mercer, J.G.; Morgan, P.J. Preclinical models for obesity research. Dis. Model. Mech. 2016, 9, 1245–1255. [Google Scholar] [CrossRef]
- Nilsson, C.; Raun, K.; Yan, F.-F.; Larsen, M.O.; Tang-Christensen, M. Laboratory animals as surrogate models of human obesity. Acta Pharmacol. Sin. 2012, 33, 173–181. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Method Mol. Biol. 2012, 821, 421–433. [Google Scholar]
- Mallare, J.T.; Karabell, A.H.; Velasquez-Mieyer, P.; Stender, S.R.; Christensen, M.L. Current and future treatment of metabolic syndrome and type 2 diabetes in children and adolescents. Diabet. Spectr. 2005, 18, 220–228. [Google Scholar] [CrossRef]
- Kang, J.G.; Park, C.-Y. Anti-obesity drugs: A review about their effects and safety. Diabet. Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. A natural solution for obesity: Bioactives for the prevention and treatment of weight gain. A review. Nutr. Neurosci. 2015, 18, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Possible anti-obesity therapeutics from nature-review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef]
- Parasuraman, S.; Thing, G.S.; Dhanaraj, S.A. Polyherbal formulation: Concept of ayurveda. Pharmacog. Rev. 2014, 8, 73. [Google Scholar] [CrossRef]
- Barik, C.S.; Kanungo, S.K.; Tripathy, N.K.; Panda, J.R.; Padhi, M. A review on therapeutic potential of polyherbal formulations. Int. J. Pharm. Sci. Drug Res. 2015, 7, 211–228. [Google Scholar]
- WHO. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Gao, Q.; Li, J.; Cheung, J.K.H.; Duan, J.; Ding, A.; Cheung, A.W.H.; Zhao, K.; Li, W.Z.; Dong, T.T.; Tsim, K.W.K. Verification of the formulation and efficacy of Danggui Buxue Tang (a decoction of Radix Astragali and Radix Angelicae Sinensis): An exemplifying systematic approach to revealing the complexity of Chinese herbal medicine formulae. Chin. Med. 2007, 2, 12. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Bone, K.; Morgan, M. Herbal products: Active constituents, modes of action and quality control. Nutr. Res. Rev. 2000, 13, 47–77. [Google Scholar] [CrossRef]
- Poudel, A.; Kim, S.-G.; Lamichhane, R.; Kim, Y.-K.; Jo, H.-K.; Jung, H.-J. Quantitative assessment of traditional oriental herbal formulation Samhwangsasim-tang using UPLC technique. J. Chromatogr. Sci. 2014, 52, 176–185. [Google Scholar] [CrossRef][Green Version]
- Pandeya, P.R.; Lamichhane, R.; Lee, K.-H.; Lamichhane, G.; Kim, S.-G.; Jung, H.-J. Efficacy of a Novel Herbal Formulation (F2) on the Management of Obesity: In Vitro and In Vivo Study. Evid. Based Complemen. Altern. Med. 2021, 2021, 8854915. [Google Scholar] [CrossRef]
- Sharma, D.K.; Kim, S.-G.; Lamichhane, R.; Lee, K.-H.; Poudel, A.; Jung, H.-J. Development of UPLC fingerprint with multi-component quantitative analysis for quality consistency evaluation of herbal medicine “Hyangsapyeongwisan”. J. Chromatogr. Sci. 2016, 54, 536–546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.H.; Park, Y.H.; Lee, J.S.; Jeong, H.I.; Lee, K.W.; Kang, T.H. Anti-Obesity Effect of DKB-117 through the Inhibition of Pancreatic Lipase and α-Amylase Activity. Nutrients 2020, 12, 3053. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, K.; Mishra, A.T.; Rao, M.K.; Sarma, K.V.S.; Krishnaraju, A.V.; Trimurtulu, G. Efficacy and tolerability of a novel herbal formulation for weight management in obese subjects: A randomized double blind placebo controlled clinical study. Lipid Health Dis. 2012, 11, 122. [Google Scholar] [CrossRef]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef]
- Rossmeisl, M.; Rim, J.S.; Koza, R.A.; Kozak, L.P. Variation in type 2 diabetes-related traits in mouse strains susceptible to diet-induced obesity. Diabetes 2003, 52, 1958–1966. [Google Scholar] [CrossRef]
- Surwit, R.S.; Kuhn, C.M.; Cochrane, C.; McCubbin, J.A.; Feinglos, M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988, 37, 1163–1167. [Google Scholar] [CrossRef]
- Sclafani, A. Animal models-etiologic classifcation. Int. J. Obes. 1984, 8, 491–508. [Google Scholar]
- Yang, Q.-Q.; Suen, P.K.; Zhang, C.-Q.; Mak, W.S.; Gu, M.-H.; Liu, Q.-Q.; Sun, S.S.-M. Improved growth performance, food efficiency, and lysine availability in growing rats fed with lysine-biofortified rice. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- West, D.B.; Boozer, C.N.; Moody, D.L.; Atkinson, R.L. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 262, R1025–R1032. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-M.; Jang, S.-W.; Ahn, T.-W. Anti-obesity effect of Yangkyuksanwha-tang in high-fat diet-induced obese mice. BMC Complement. Altrn. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, S.-H.; Yuk, H.J.; Lee, G.-J.; Kim, D.-S. Tetragonia tetragonoides (Pall.) Kuntze (New Zealand Spinach) prevents obesity and hyperuricemia in high-fat diet-induced obese mice. Nutrients 2018, 10, 1087. [Google Scholar] [CrossRef]
- Senior, J.R. Intestinal absorption of fats. J. Lipid Res. 1964, 5, 495–521. [Google Scholar] [CrossRef]
- Paccaud, F.; Schlter-Fasmeyer, V.; Wietlisbach, V.; Bovet, P. Dyslipidemia and abdominal obesity: An assessment in three general populations. J. Clin. Epidemiol. 2000, 53, 393–400. [Google Scholar] [CrossRef]
- Qi, L.; Ding, X.; Tang, W.; Li, Q.; Mao, D.; Wang, Y. Prevalence and risk factors associated with dyslipidemia in Chongqing, China. Int. J. Environ. Res. Public Health 2015, 12, 13455–13465. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Yoon, T.; Yang, W.-K.; Kim, S.J.; Kim, H.K. Anti-obesity effects of Geranium thunbergii extract via improvement of lipid metabolism in high-fat diet-induced obese mice. Mol. Med. Rep. 2011, 4, 1107–1113. [Google Scholar] [CrossRef]
- Lee, S.J.; Zhang, G.F.; Sung, N.J. Hypolipidemic and hypoglycemic effects of Orostachys japonicus A. Berger extracts in streptozotocin-induced diabetic rats. Nutr. Res. Pract. 2011, 5, 301–307. [Google Scholar] [CrossRef]
- Kim, S.G.; Poudel, A.; Choi, J.W.; Park, H.J.; Lee, Y.S.; Lee, H.S.; Min, B.S.; Jung, H.J. Anti-obesitic effect of Orostachys japonicus in rats model fed a hyperlipidemic diet. Nat. Prod. Sci. 2011, 17, 117–122. [Google Scholar]
- Liu, J.; Han, L.; Zhu, L.; Yu, Y. Free fatty acids, not triglycerides, are associated with non-alcoholic liver injury progression in high fat diet induced obese rats. Lipid Health Dis. 2016, 15, 27. [Google Scholar] [CrossRef]
- Pandeya, P.R.; Lamichhane, R.; Lee, K.-H.; Kim, S.-G.; Lee, D.-H.; Lee, H.-K.; Jung, H.-J. Bioassay-guided isolation of active anti-adipogenic compound from royal jelly and the study of possible mechanisms. BMC Complement. Altern. Med. 2019, 19, 33. [Google Scholar] [CrossRef]
- Vazquez-Vela, M.E.F.; Torres, N.; Tovar, A.R. White adipose tissue as endocrine organ and its role in obesity. Arch. Med. Res. 2008, 39, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar] [PubMed]
- Noguchi, R.; Kubota, H.; Yugi, K.; Toyoshima, Y.; Komori, Y.; Soga, T.; Kuroda, S. The selective control of glycolysis, gluconeogenesis and glycogenesis by temporal insulin patterns. Mol. Syst. Biol. 2013, 9, 664. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Schwab, U.S. Relationship of dietary fat to glucose metabolism. Atherosclerosis 2000, 150, 227–243. [Google Scholar] [CrossRef]
- Sinitskaya, N.; Gourmelen, S.; Schuster-Klein, C.; Guardiola-Lemaitre, B.; Pevet, P.; Challet, E. Increasing the fat-to-carbohydrate ratio in a high-fat diet prevents the development of obesity but not a prediabetic state in rats. Clin. Sci. 2007, 113, 417–425. [Google Scholar] [CrossRef]
- Barbosa-da-Silva, S.; Bringhenti Sarmento, I.; Lonzetti Bargut, T.C.; Souza-Mello, V.; Barbosa Aguila, M.; Mandarim-de-Lacerda, C.A. Animal Models of Nutritional Induction of Type 2 Diabetes Mellitus. Int. J. Morphol. 2014, 32, 279–293. [Google Scholar] [CrossRef]
- Liu, Z.; Patil, I.Y.; Jiang, T.; Sancheti, H.; Walsh, J.P.; Stiles, B.L.; Yin, F.; Cadenas, E. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS ONE 2015, 10, e0128274. [Google Scholar] [CrossRef]
- Eccleston, H.B.; Andringa, K.K.; Betancourt, A.M.; King, A.L.; Mantena, S.K.; Swain, T.M.; Tinsley, H.N.; Nolte, R.N.; Nagy, T.R.; Abrams, G.A. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid. Redox Signal. 2011, 15, 447–459. [Google Scholar] [CrossRef]





| Standard Compounds | Regression Equation * | R2 | SDY-intercept | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| Astragalin | y = 41.092x + 8.9667 | 0.9996 | 72.57 | 5.83 | 17.66 |
| Fustin | y = 43.41x − 27.017 | 0.9999 | 113.61 | 8.64 | 26.17 |
| Fisetin | y = 46.419x − 204.05 | 0.9995 | 51.75 | 3.68 | 11.15 |
| Sulfuretin | y = 37.027x − 28.963 | 0.9997 | 53.76 | 4.79 | 14.52 |
| Ellagic acid | y = 59.779x − 484.89 | 0.9995 | 58.65 | 3.24 | 9.81 |
| Standard Compounds | Concentrations (µg/mL) | Intra-Day Variability (n = 3) | Inter-Day Variability (n = 3) | ||
|---|---|---|---|---|---|
| Mean ± SD | RSD | Mean ± SD | RSD | ||
| (µg/mL) | (%) | (µg/mL) | (%) | ||
| Astragalin | 250 | 249.09 ± 2.16 | 0.87 | 248.60 ± 8.70 | 3.50 |
| 125 | 126.19 ± 1.03 | 0.82 | 127.86 ± 3.48 | 2.72 | |
| 62.5 | 65.04 ± 0.42 | 0.64 | 63.34 ± 1.84 | 2.90 | |
| 31.25 | 29.06 ± 0.35 | 1.21 | 29.68 ± 1.29 | 4.34 | |
| Fustin | 1000 | 1001.69 ± 23.82 | 0.87 | 1000.31 ± 24.69 | 2.47 |
| 500 | 497.52 ± 6.80 | 0.82 | 503.36 ± 13.41 | 2.66 | |
| 250 | 246.18 ± 2.00 | 0.64 | 236.88 ± 2.97 | 1.26 | |
| 125 | 128.48 ± 2.73 | 1.21 | 135.98 ± 3.58 | 2.63 | |
| Fisetin | 250 | 248.50 ± 3.03 | 1.22 | 251.73 ± 4.95 | 1.97 |
| 125 | 128.18 ± 1.52 | 1.18 | 122.69 ± 4.19 | 3.41 | |
| 62.5 | 63.32 ± 0.65 | 1.03 | 59.16 ± 1.15 | 1.95 | |
| 31.25 | 29.05 ± 0.99 | 3.42 | 31.61 ± 0.78 | 2.47 | |
| Sulfuretin | 500 | 502.49 ± 9.77 | 1.95 | 501.72 ± 4.69 | 0.94 |
| 250 | 244.11 ± 2.24 | 0.92 | 249.96 ± 5.10 | 2.04 | |
| 125 | 125.61 ± 1.73 | 1.38 | 115.68 ± 2.22 | 1.92 | |
| 62.5 | 64.50 ± 2.55 | 3.95 | 64.85 ± 1.85 | 2.86 | |
| Ellagic acid | 500 | 503.36 ± 3.37 | 0.67 | 499.81 ± 12.87 | 2.57 |
| 250 | 243.48 ± 9.54 | 3.92 | 247.05 ± 9.30 | 3.76 | |
| 125 | 122.53 ± 1.06 | 0.87 | 133.33 ± 5.76 | 4.32 | |
| 62.5 | 65.07 ± 0.87 | 1.34 | 60.95 ± 2.23 | 3.65 | |
| Marker Compounds | Amount in F2 (mg/g) |
|---|---|
| Astragalin | 3.64 |
| Fustin | 35.83 |
| Fisetin | 5.90 |
| Sulfuretin | 3.22 |
| Ellagic acid | 1.82 |
| Normal | HFD | F2-46 | GC | |
|---|---|---|---|---|
| Initial body weight (g) | 19.35 ± 1.02 | 20.02 ± 1.29 | 20.23 ± 1.03 | 19.67 ± 0.63 |
| Body weight after 5-weeks of obesity induction period (g) | 21.85 ± 1.22 ** | 26.06 ± 1.10 | 26.49 ± 1.84 | 25.84 ± 2.01 |
| Body weight gain in 5-weeks of obesity induction period (g) | 2.51 ± 0.51 *** | 6.04 ± 1.54 | 6.25 ± 1.16 | 6.17 ± 1.70 |
| Final weight after 7-weeks of drug treatment period (g) | 23.97 ± 1.36 *** | 31.70 ± 1.14 | 30.20 ± 2.53 | 29.14 ± 2.00 |
| Weight gain during 7-weeks of drug treatment period (g) | 2.11 ± 0.50 *** | 5.63 ± 1.00 | 3.71 ± 1.29 ** | 3.29 ± 0.28 *** |
| Food intake during 7-weeks of the drug treatment period (g /mice) | 139.94 | 101.92 | 100.52 | 101.38 |
| Food efficiency ratio (%) | 1.51 ± 0.35 *** | 5.53 ± 0.99 | 3.69 ± 1.28 ** | 3.25 ± 0.28 *** |
| Groups | Serum Insulin (µIU/mL) | Blood Glucose (mmol/L) | HOMA-IR |
|---|---|---|---|
| Normal | 4.12 ± 0.89 ** | 7.78 ± 0.86 | 1.44 ± 0.42 ** |
| HFD | 20.87 ± 12.66 | 8.57 ± 1.17 | 8.09 ± 5.18 |
| F2-46 | 10.18 ± 2.01 * | 8.28 ± 1.05 | 3.68 ± 0.45 * |
| GC | 4.69 ± 2.10 ** | 8.63 ± 1.43 | 1.86 ± 1.12 ** |
| Groups | WAT (g) | Liver (g) | Kidney (g) | Spleen (mg) |
|---|---|---|---|---|
| Normal | 0.39 ± 0.07 *** | 0.93 ± 0.05 *** | 0.29 ± 0.02 | 60.42 ± 6.50 |
| HFD | 2.07 ± 0.12 | 1.06 ± 0.04 | 0.31 ± 0.02 | 62.64 ± 6.84 |
| F2-46 | 1.63 ± 0.29 ** | 0.92 ± 0.04 *** | 0.31 ± 0.02 | 61.70 ± 6.78 |
| GC | 1.32 ± 0.26 *** | 0.82 ± 0.08 *** | 0.29 ± 0.01 * | 58.03 ± 9.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandeya, P.R.; Lee, K.-H.; Lamichhane, R.; Lamichhane, G.; Poudel, A.; Jung, H.-J. Evaluation of Anti-Obesity Activity of an Herbal Formulation (F2) in DIO Mice Model and Validation of UPLC-DAD Method for Quality Control. Appl. Sci. 2021, 11, 7404. https://doi.org/10.3390/app11167404
Pandeya PR, Lee K-H, Lamichhane R, Lamichhane G, Poudel A, Jung H-J. Evaluation of Anti-Obesity Activity of an Herbal Formulation (F2) in DIO Mice Model and Validation of UPLC-DAD Method for Quality Control. Applied Sciences. 2021; 11(16):7404. https://doi.org/10.3390/app11167404
Chicago/Turabian StylePandeya, Prakash Raj, Kyung-Hee Lee, Ramakanta Lamichhane, Gopal Lamichhane, Amrit Poudel, and Hyun-Ju Jung. 2021. "Evaluation of Anti-Obesity Activity of an Herbal Formulation (F2) in DIO Mice Model and Validation of UPLC-DAD Method for Quality Control" Applied Sciences 11, no. 16: 7404. https://doi.org/10.3390/app11167404
APA StylePandeya, P. R., Lee, K.-H., Lamichhane, R., Lamichhane, G., Poudel, A., & Jung, H.-J. (2021). Evaluation of Anti-Obesity Activity of an Herbal Formulation (F2) in DIO Mice Model and Validation of UPLC-DAD Method for Quality Control. Applied Sciences, 11(16), 7404. https://doi.org/10.3390/app11167404