Antiviral Filtering Capacity of GO-Coated Textiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Graphene Oxide (GO) Synthesis
2.3. GO Modified Textiles
2.4. GO Characterization
2.4.1. Transmission Electron Microscopy (TEM)/EDX
2.4.2. Atomic Force Microscopy (AFM) Study
2.4.3. X-ray Diffraction Analysis (XRD)
2.4.4. X-ray Photoelectron Spectroscopy (XPS)
2.4.5. Thermo Gravimetric Analysis (TGA)
2.4.6. Raman Spectroscopy
2.4.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.8. Z-Potential
2.4.9. GO Modified Textiles Characterization
2.4.10. VP-SEM Engineered Textiles Characterization Protocol
2.4.11. SEM Characterization Engineered Textiles
2.4.12. Cell Culture, Reagents and Infection
3. Results
3.1. Characterization of GO Nano Sheets
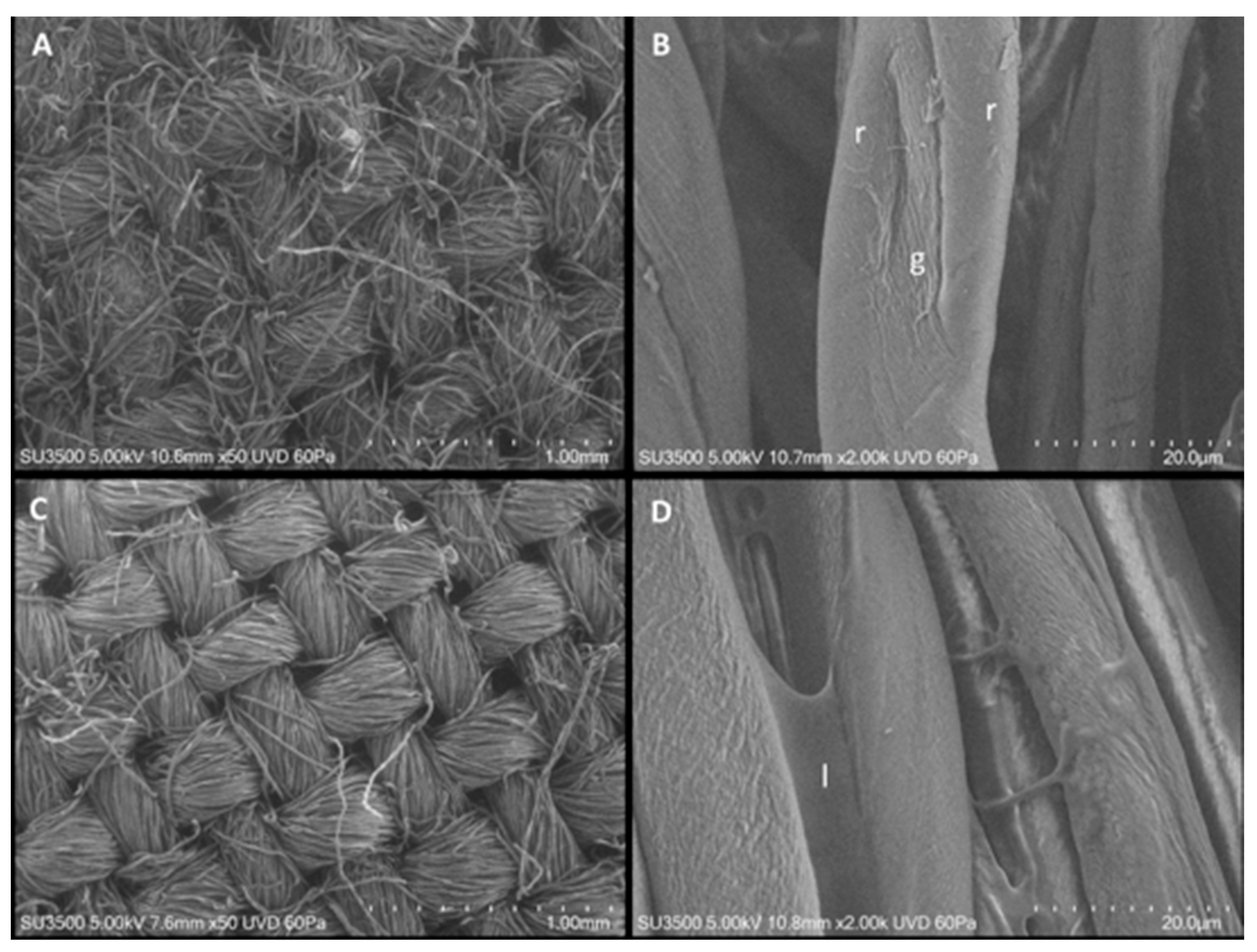
3.2. Characterization of Textiles with and without Coating
3.3. Textiles with Different GO Concentrations (5%, 10%) Show Different Virus Binding Properties
3.4. Anti-Viral Effect of GO Covered Tissues
3.5. Virus Aggregates around GO Sheets
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Valentini, F.; Calcaterra, A.; Ruggiero, V.; Pichichero, E.; Martino, A.; Iosi, F.; Bertuccini, L.; Antonaroli, S.; Mardente, S.; Zicari, A.; et al. Functionalized Graphene Derivatives: Antibacterial Properties and Cytotoxicity. J. Nanomater. 2019. [Google Scholar] [CrossRef] [Green Version]
- Sametband, M.; Kalt, I.; Gedanken, A.; Sarid, R. Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 1228–1235. [Google Scholar] [CrossRef]
- Innocenzi, P.; Stagi, L. Carbon-based antiviral nanomaterials: Graphene, C-dots and fullerenes. A perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef] [PubMed]
- Natacha, S.O.; Tim, J.D.; Jessika, C.; Ronald, W.A.L.; Yvonne, V.D.M.; Leon, C.; Julian, D.; Jutte, J.C.; Marjolein, K.; Montserrat, B.; et al. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef]
- Atzori, M.; Artizzu, F.; Sessini, E.; Marchiò, L.; Loche, D.; Serpe, A.; Deplano, P.; Concas, G.; Pop, F.; Avarvari, N.; et al. Halogen-Bonding in a New Family of tris (haloanilato) metallate (III) Magnetic Molecular Building Blocks. Dalton Trans. 2014, 43, 7006–7019. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Gedanken, A.; Sarid, R. Inhibition of HSV-1 Attachment, Entry, and Cell-to-Cell Spread by Functionalized Multivalent Gold Nanoparticles. Small 2010, 6, 1044–1050. [Google Scholar] [CrossRef]
- Basak, S.; Packirisamy, G. Nano-based antiviral coatings to combat viral infections. Nano-Struct. Nano-Objects 2020, 24, 100620. [Google Scholar] [CrossRef]
- Ziem, B.; Rahn, J.; Donskyi, I.; Silberreis, K.; Cuellar, L.; Dernedde, J.; Keil, G.; Mettenleiter, T.C.; Haag, R. Polyvalent 2D Entry Inhibitors for Pseudorabies and African Swine Fever Virus. Macromol. Biosci. 2017, 17, 1600499. [Google Scholar] [CrossRef]
- Yang, X.X.; Li, C.M.; Li, Y.F.; Wang, J.; Huang, C.Z. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale 2017, 9, 16086–16092. [Google Scholar] [CrossRef]
- Donskyi, I.S.; Azab, W.; Cuellar-Camacho, J.L.; Guday, G.; Lippitz, A.; Unger, W.E.S.; Osterrieder, K.; Adeli, M.; Haag, R. Functionalized nanographene sheets with high antiviral activity through synergistic electrostatic and hydrophobic interactions. Nanoscale 2019, 11, 15804–15809. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F.; Compagnini, G.; Patané, G.; Ursini, O.; Angelini, G.; Ribic, P.R.; Margaritondo, G.; Cricenti, A.; Palleschi, G.; Valentini, F. Graphene nanoribbons produced by the oxidative unzipping of single-wall carbon nanotubes. Carbon 2010, 48, 2596–2602. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley Pub. Co.: Reading, MA, USA, 1956. [Google Scholar]
- Romeo, M.A.; Masuelli, L.; Gaeta, A.; Nazzari, C.; Granato, M.; Gilardini Montani, M.S.; Faggioni, A.; Cirone, M. Impact of HHV-6A and HHV-6B lytic infection on autophagy and endoplasmic reticulum stress. J. Gen. Virol. 2019, 100, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, X.; Chang, C.T. Preparation and characterization of graphene oxide. J. Nano Mater. 2014, 2014, 276143. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, I.M. Improved Synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- De Oliveira Costa, M.A.; Mecheri, B.; D’Epifanio, A.; Placidi, E.; Arciprete, F.; Valentini, F.; Perandini, A.; Valentini, V.; Licoccia, S. Graphene oxide nanoplatforms to enhance catalytic performance of iron phthalocyanine for oxygen reduction reaction in bio electrochemical systems. J. Power Sources 2017, 356, 381–388. [Google Scholar] [CrossRef]
- Romeo, M.A.; Gilardini-Montani, M.S.; Gaeta, A.; D’Orazi, G.; Faggioni, A.; Cirone, M. HHV-6A infection dysregulates autophagy/UPR interplay increasing beta amyloid production and tau phosphorylation in astrocytoma cells as well as in primary neurons, possible molecular mechanisms linking viral infection to Alzheimer’s disease. BBA 2020, 1866, 165647. [Google Scholar] [CrossRef]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef]
- Dunn, N.; Kharlamova, N.; Fogdell-Hahn, A. The role of herpesvirus 6A and 6B in multiple sclerosis and epilepsy. Scand. J. Immunol. 2020, 92, e12984. [Google Scholar] [CrossRef]
- Choudhary, S.; Marquez, M.; Alencastro, F.; Spors, F.; Zhao, Y.; Tiwari, V. Herpes Simplex Virus Type-1 (HSV-1) Entry into Human Mesenchymal Stem Cells Is Heavily Dependent on Heparan Sulfate. J. Biomed. Biotechnol. 2011, 2011, 264350. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, X.; Zhu, G.; Nian, Q.; Zhou, H.; Yang, D.; Qin, C.; Tang, R. Virus capture and destruction by label-free graphene oxide for detection and disinfection applications. Small 2015, 11, 1171–1176. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Navaneethaiyer, U.; Mohan, R.; Jehee, L.; Sang-Jae, K. Graphene oxide nanostructures modified multifunctional cotton fabrics. Appl. Nanosci. 2012, 2, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Vaideki, K.; Jayakumar, S.; Thilagavathi, G.; Rajendran, R. A study on the antimicrobial efficacy of RF oxygen plasma and neem extract treated cotton fabrics. Appl. Surf. Sci. 2007, 253, 7323–7329. [Google Scholar] [CrossRef]
- Li, D.; Zhang, W.; Xiaoqing, Y.; Wang, Z.; Su, Z.; Wei, G. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef] [PubMed]
- Ziem, B.; Azab, W.; Gholami, M.F.; Rabe, J.; Osterrieder, P.; Haag, R. Size-dependent Inhibition of Herpesvirus Cellular Entry by Polyvalent Nanoarchitectures. Nanoscale 2017, 9, 3774–3783. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Dwivedi, N.; Dhand, C.; Khan, R.; Sathish, N.; Gupta, M.K.; Kumar, R.; Kumar, S. Potential of graphene-based materials to combat COVID-19: Properties, perspectives, and prospects. Mater. Today Chem. 2020, 18, 100385. [Google Scholar] [CrossRef] [PubMed]
- Donskyi, I.; Drüke, M.; Silberreis, K.; Lauster, D.; Ludwig, K.; Kühne, C.; Unger, W.; Böttcher, C.; Herrmann, A.; Dernedde, J.; et al. Interactions of Fullerene-Polyglycerol Sulfates at Viral and Cellular Interfaces. Small 2018, 14, 1800189. [Google Scholar] [CrossRef]
- Matharu, R.K.; Porwal, H.; Chen, B.; Ciric, L.; Edirisinghe, M. Viral Filtration Using Carbon-Based Materials. Med. Devices Sens. 2020, 3, e10107. [Google Scholar] [CrossRef]






| Samples | AFM Thickness (nm) (N° Layers) | AFM Area (μm2) | XPS_C1s Oxygen Groups (At. %) Peak BE (eV) | FTIR Assigned Bands (ῦ, cm−1) | SEM/EDX Microanalysis |
|---|---|---|---|---|---|
| GO | 1.4 ± 0.6 (2–3) | 5 × 10−2–0.2 | C-O (21.71 At. %) 286.6 (eV) C=O (18.32 At. %) 287.7 (eV) | OH (Strong bands) ῦ = 3413 C(=O) (Medium bands) ῦ = 1722 CO (Weak bands) ῦ = 1076 | Si, S, Ca, Cr, Fe, Co, Ni, Y are absent and not detectable |
| Samples | Z-Potential ζ (mV) |
|---|---|
| GO | −40.5 ± 0.2 |
| human herpesvirus 6A (HHV-6A) | +18.7 ± 0.5 |
| GO/6A (HHV-6A) conjugation | +16.9 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentini, F.; Cirone, M.; Relucenti, M.; Santarelli, R.; Gaeta, A.; Mussi, V.; De Simone, S.; Zicari, A.; Mardente, S. Antiviral Filtering Capacity of GO-Coated Textiles. Appl. Sci. 2021, 11, 7501. https://doi.org/10.3390/app11167501
Valentini F, Cirone M, Relucenti M, Santarelli R, Gaeta A, Mussi V, De Simone S, Zicari A, Mardente S. Antiviral Filtering Capacity of GO-Coated Textiles. Applied Sciences. 2021; 11(16):7501. https://doi.org/10.3390/app11167501
Chicago/Turabian StyleValentini, Federica, Mara Cirone, Michela Relucenti, Roberta Santarelli, Aurelia Gaeta, Valentina Mussi, Sara De Simone, Alessandra Zicari, and Stefania Mardente. 2021. "Antiviral Filtering Capacity of GO-Coated Textiles" Applied Sciences 11, no. 16: 7501. https://doi.org/10.3390/app11167501
APA StyleValentini, F., Cirone, M., Relucenti, M., Santarelli, R., Gaeta, A., Mussi, V., De Simone, S., Zicari, A., & Mardente, S. (2021). Antiviral Filtering Capacity of GO-Coated Textiles. Applied Sciences, 11(16), 7501. https://doi.org/10.3390/app11167501







