Elemental Carbon and Its Fractions during Evolved Gas Analysis with Respect to Pyrolytic Carbon and Split Time
Abstract
:1. Introduction
2. Materials and Methods
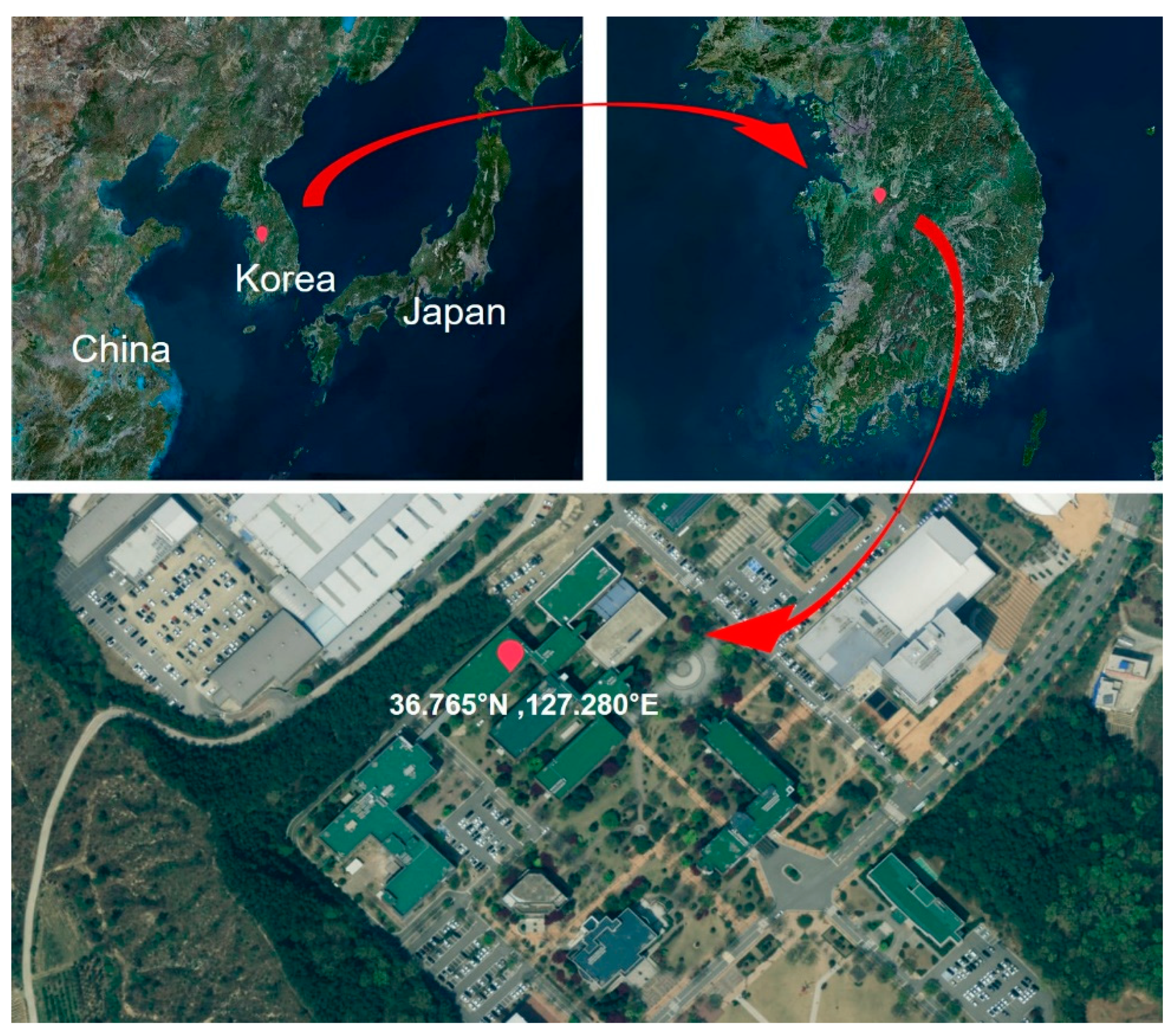
2.1. Sampling of PM2.5
2.2. eBC Measurement
2.3. EC Measurement
3. Results and Discussion
3.1. Oxidation Quantity vs. Oxidation Temperature
3.2. Comparison of EC with eBC
3.3. Various EC Fractions
3.4. PyC, Split Time and EC Concentration
3.5. Limitations of this Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jacob, D.J.; Winner, D.A. Effect of climate change on air quality. Atmos. Environ. 2009, 43, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Laden, F.; Schwartz, J.; Speizer, F.E.; Dockery, D. Reduction in Fine Particulate Air Pollution and Mortality. Am. J. Respir. Crit. Care Med. 2006, 173, 667–672. [Google Scholar] [CrossRef]
- Pope, C.A.; Muhlestein, J.B.; May, H.T.; Renlund, D.G.; Anderson, J.L.; Horne, B.D. Ischemic Heart Disease Events Triggered by Short-Term Exposure to Fine Particulate Air Pollution. Circulation 2006, 114, 2443–2448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohmann, U.; Feichter, J. Global indirect aerosol effects: A review. Atmos. Chem. Phys. Discuss. 2005, 5, 715–737. [Google Scholar] [CrossRef] [Green Version]
- Slaughter, J.C.; Kim, E.; Sheppard, L.; Sullivan, J.H.; Larson, T.V.; Claiborn, C. Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J. Expo. Sci. Environ. Epidemiol. 2004, 15, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, Y.J.; Tanré, D.; Boucher, O. A satellite view of aerosols in the climate system. Nature 2002, 419, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Bae, M.S.; Schauer, J.J.; Ryu, S.Y.; Kim, Y.J.; Cho, S.Y.; Kim, S.J. Evaluation of the TMO and TOT methods for OC and EC measurements and their characteristics in PM2.5 at an urban site of Korea during ACE-Asia. Atmos. Environ. 2005, 39, 5101–5112. [Google Scholar] [CrossRef]
- Schmid, H.; Laskus, L.; Jürgen Abraham, H.; Baltensperger, U.; Lavanchy, V.; Bizjak, M.; Burba, P.; Cachier, H.; Crow, D.; Chow, J.; et al. Results of the “carbon conference” international aerosol carbon round robin test stage I. Atmos. Environ. 2001, 35, 2111–2121. [Google Scholar] [CrossRef]
- Birch, M.E. Analysis of carbonaceous aerosols: Interlaboratory comparison. Analyst 1998, 123, 851–857. [Google Scholar] [CrossRef]
- Birch, M.E.; Cary, R.A. Elemental Carbon-Based Method for Monitoring Occupational Exposures to Particulate Diesel Exhaust. Aerosol Sci. Technol. 1996, 25, 221–241. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Pritchett, L.C.; Pierson, W.R.; Frazier, C.A.; Purcell, R.G. The dri thermal/optical reflectance carbon analysis system: Description, evaluation and applications in U.S. Air quality studies. Atmos. Environ. Part A Gen. Top. 1993, 27, 1185–1201. [Google Scholar] [CrossRef]
- Cavalli, F.; Viana, M.; Yttri, K.E.; Genberg, J.; Putaud, J.-P. Toward a standardised thermal-optical protocol for measuring atmospheric organic and elemental carbon: The EUSAAR protocol. Atmos. Meas. Tech. 2010, 3, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Peterson, M.R.; Richards, M.H. Thermal-Optical-Transmittance Analysis for Organic, Elemental, Carbonate, Total Carbon, and OCX2 in PM2.5 by the EPA/NIOSH Method. In Symposium on Air Quality Measurement Methods and Technology-2002; Winegar, E.D., Tropp, R.J., Eds.; Air & Waste Management Association: Pittsburgh, PA, USA, 2002; Volume 83, pp. 1–19. [Google Scholar]
- Lee, J. Performance Test of MicroAeth® AE51 at Concentrations Lower than 2 μg/m3 in Indoor Laboratory. Appl. Sci. 2019, 9, 2766. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; He, K.-B.; Duan, F.-K.; Du, Z.-Y.; Zheng, M.; Ma, Y.-L. Ambient organic carbon to elemental carbon ratios: Influence of the thermal–optical temperature protocol and implications. Sci. Total Environ. 2014, 468–469, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Lee, S.; Lee, J. Measurement of Black Carbon Concentration and Comparison with PM10 and PM2.5 Concentrations Monitored in Chungcheong Province, Korea. Aerosol Air Qual. Res. 2019, 19, 541–547. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.; Kim, K.J. Monitoring of black carbon concentration at an inland rural area including fixed sources in Korea. Chemosphere 2016, 143, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, J.Z. Uncertainties in Charring Correction in the Analysis of Elemental and Organic Carbon in Atmospheric Particles by Thermal/Optical Methods. Environ. Sci. Technol. 2002, 36, 5199–5204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chung, A.; Paulson, S.E. The effect of metal salts on quantification of elemental and organic carbon in diesel exhaust particles using thermal-optical evolved gas analysis. Atmos. Chem. Phys. Discuss. 2010, 10, 11447–11457. [Google Scholar] [CrossRef] [Green Version]
- Birch, M. Elemental carbon (diesel particulate): Method 5040. In NIOSH Manual of Analytical Methods (NMAM); National Institute for Occupational Safety and Health: Washington, DC, USA, 1999. [Google Scholar]
- Xiaowei, L.; Jean-Charles, R.; Suyuan, Y. Effect of temperature on graphite oxidation behavior. Nucl. Eng. Des. 2004, 227, 273–280. [Google Scholar] [CrossRef]
- Oh, S.-H.; Park, D.-J.; Cho, J.-H.; Han, Y.-J.; Bae, M.-S. Intercomparison of Carbonaceous Analytical Results using NIOSH5040, IMPROVE_A, EUSAAR2 Protocols. J. Korean Soc. Atmos. Environ. 2018, 34, 447–456. [Google Scholar] [CrossRef]
- Reid, J.P.; Bertram, A.K.; Topping, D.; Laskin, A.; Martin, S.T.; Petters, M.D.; Pope, F.D.; Rovelli, G. The viscosity of atmospherically relevant organic particles. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panteliadis, P.; Hafkenscheid, T.; Cary, B.; Diapouli, E.; Fischer, A.; Favez, O.; Quincey, P.; Viana, M.; Hitzenberger, R.; Vecchi, R.; et al. ECOC comparison exercise with identical thermal protocols after temperature offset correction–instrument diagnostics by in-depth evaluation of operational parameters. Atmos. Meas. Tech. 2015, 8, 779–792. [Google Scholar] [CrossRef] [Green Version]
- Karanasiou, A.; Panteliadis, P.; Perez, N.; Minguillón, M.; Pandolfi, M.; Titos, G.; Viana, M.; Moreno, T.; Querol, X.; Alastuey, A. Evaluation of the Semi-Continuous OCEC analyzer performance with the EUSAAR2 protocol. Sci. Total Environ. 2020, 747, 141266. [Google Scholar] [CrossRef] [PubMed]






| Step/Gas | IMPROVE-A Temp. | NIOSH 5040 Temp./Duration | EUSAAR2 Temp./Duration |
|---|---|---|---|
| OC1/He | 140 °C | 310 °C/80 s | 200 °C/120 s |
| OC2/He | 280 °C | 475 °C/60 s | 300 °C/150 s |
| OC3/He | 480 °C | 615 °C/60 s | 450 °C/180 s |
| OC4/He | 580 °C | 870 °C/90 s | 650 °C/180 s |
| – | Cool down/45 s | Cool down/30 s | |
| EC1/O2+He | 580 °C | 550 °C/45 s | 500 °C/120 s |
| EC2/O2+He | 740 °C | 625 °C/45 s | 550 °C/120 s |
| EC3/O2+He | 840 °C | 700 °C/45 s | 700 °C/70 s |
| EC4/O2+He | 775 °C/45 s | 850 °C/80 s | |
| EC5/O2+He | 850 °C/45 s | ||
| EC6/O2+He | 870 °C/120 s |
| Slope | y-int | R2 | |
|---|---|---|---|
| EC1 vs. EC | 0.047 ± 0.006 | 0.160 ± 0.010 | 0.496 |
| EC2 vs. EC | 0.302 ± 0.039 | −0.009 ± 0.060 | 0.519 |
| EC3 vs. EC | 0.860 ± 0.077 | 0.022 ± 0.119 | 0.688 |
| EC1+EC2 vs. EC | 0.349 ± 0.038 | 1.151 ± 0.059 | 0.601 |
| EC2+EC3 vs. EC | 1.162 ± 0.112 | 0.013 ± 0.173 | 0.657 |
| EC1+EC3 vs. EC | 0.906 ± 0.077 | 0.182 ± 0.118 | 0.715 |
| EC1+EC2+EC3 vs. EC | 1.209 ± 0.111 | 0.174 ± 0.171 | 0.679 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, D.; Lee, J. Elemental Carbon and Its Fractions during Evolved Gas Analysis with Respect to Pyrolytic Carbon and Split Time. Appl. Sci. 2021, 11, 7544. https://doi.org/10.3390/app11167544
Lee J, Kim D, Lee J. Elemental Carbon and Its Fractions during Evolved Gas Analysis with Respect to Pyrolytic Carbon and Split Time. Applied Sciences. 2021; 11(16):7544. https://doi.org/10.3390/app11167544
Chicago/Turabian StyleLee, Juhan, Dohyun Kim, and Jeonghoon Lee. 2021. "Elemental Carbon and Its Fractions during Evolved Gas Analysis with Respect to Pyrolytic Carbon and Split Time" Applied Sciences 11, no. 16: 7544. https://doi.org/10.3390/app11167544
APA StyleLee, J., Kim, D., & Lee, J. (2021). Elemental Carbon and Its Fractions during Evolved Gas Analysis with Respect to Pyrolytic Carbon and Split Time. Applied Sciences, 11(16), 7544. https://doi.org/10.3390/app11167544






