Seasonal Population Structure of the Copepod Temora turbinata (Dana, 1849) in the Kuroshio Current Edge, Southeastern East China Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Area and Field Study
2.2. Zooplankton Identification and Abundance Measurement
2.3. Data Analysis
3. Results
3.1. Hydrological Conditions
3.2. Variation and Distribution of the Temora turbinata Population
3.3. Sex Ratio Differences of Temora turbinata
3.4. Seasonal Population Structure of Temora turbinata
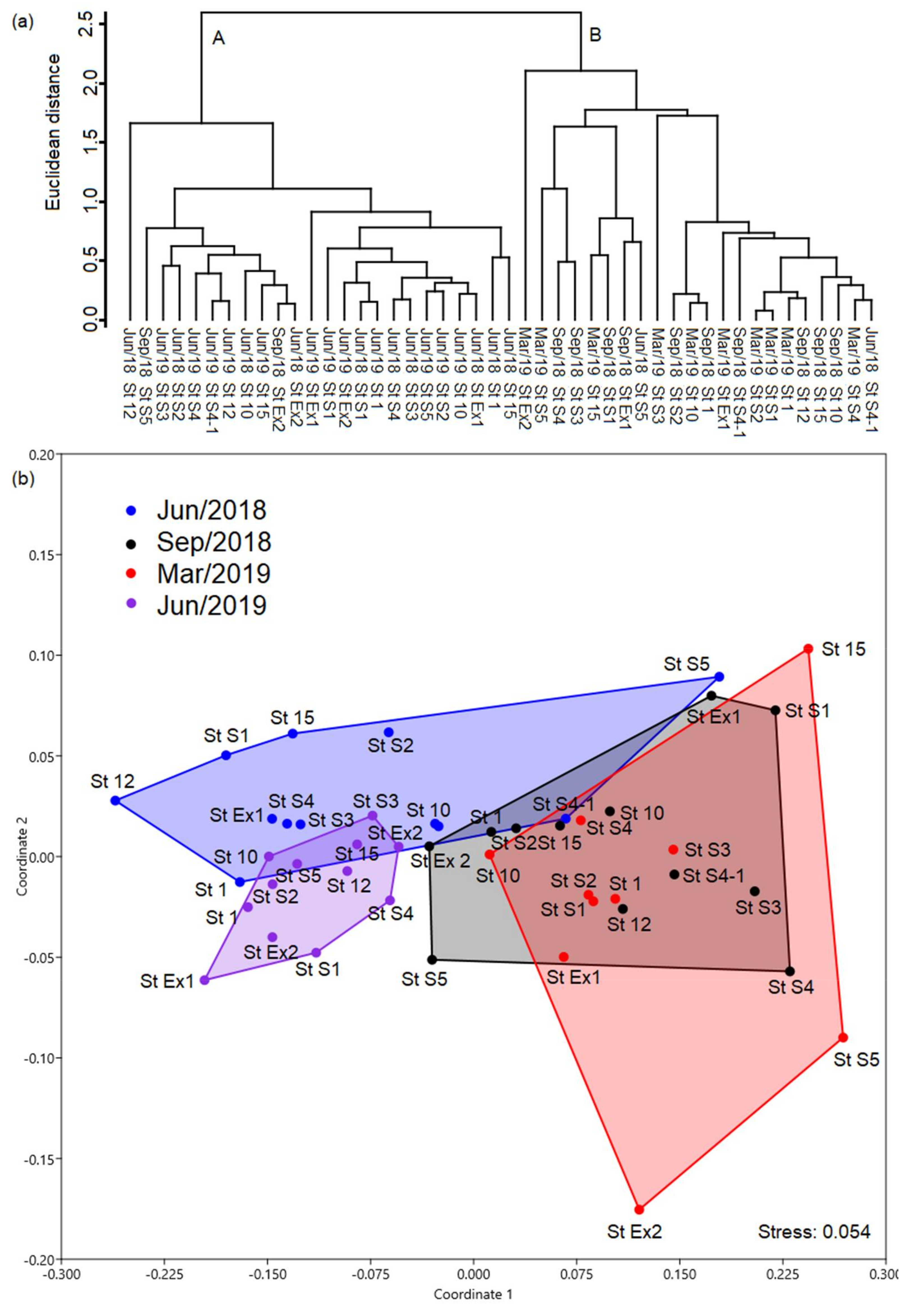
3.5. Statistical Analysis of the Spatiotemporal Distribution of Temora turbinata
4. Discussion
4.1. Hydrography off Northeastern Taiwan Waters
4.2. Biology and Ecology of Temora turbinata
4.3. Sex Ratio of Copepods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiørboe, T. A Mechanistic Approach to Plankton Ecology; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Lahnsteiner, F.; Kletzl, M.; Weismann, T. The risk of parasite transfer to juvenile fishes by live copepod food with the example Triaenophorus crassus and Triaenophorus nodulosus. Aquaculture 2009, 295, 120–125. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Souissi, S.; Tseng, L.-C.; Seurong, L.; Schmitt, F.G.; Fang, L.-S.; Peng, S.-H.; Wu, C.-W.; Hsiao, S.-H.; Twan, W.-H.; et al. A 5-year study of the influence of the northeast and southwest monsoons on copepod assemblages in the boundary coastal waters between the East China Sea and the Taiwan Strait. J. Plankton Res. 2006, 28, 943–985. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.C.; Garcia, T.M.; Neumann-Leitao, S.; Soares, M.O. Ecological indicators and functional groups of copepod assemblages. Ecol. Indic. 2017, 83, 416–426. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Dahms, H.-U.; Tseng, L.-C.; Chen, Q.-C. Intrusions of the Kuroshio Current in the northern South China Sea affects copepod assemblages of the Luzon Strait. J. Exp. Mar. Biol. Ecol. 2007, 352, 12–27. [Google Scholar] [CrossRef]
- Alcaraz, M.; Calbet, A.; Estrada, M.; Marrasé, C.; Saiz, E.; Trepat, I. Physical control of zooplankton communities in the Catalan Sea. Progr. Oceanogr. 2007, 74, 294–312. [Google Scholar] [CrossRef]
- Ka, S.; Hwang, J.-S. Mesozooplankton distribution and composition on the northeastern coast of Taiwan during autumn: Effects of the Kuroshio Current and hydrothermal vents. Zool. Stud. 2011, 50, 155–163. [Google Scholar]
- Hwang, J.-S.; Wong, C.-K. The China Coastal Current as a driving force for transporting Calanus sinicus (Copepoda: Calanoida) from its population centers to waters off Taiwan and Hong Kong during the winter northeast monsoon period. J. Plankton Res. 2005, 27, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Uriarte, I.; Villate, F. Effects of pollution on zooplankton abundance and distribution in two estuaries of the basque coast (Bay of Biscay). Mar. Pollut. Bull. 2004, 49, 220–228. [Google Scholar] [CrossRef]
- Gismervik, I. Top-down impact by copepods on ciliate numbers and persistence depends on copepod and ciliate species composition. J. Plankton Res. 2006, 28, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Piliai, P.P. Post-naupliar development of the calanoid copepod Temora turbinata (Dana), with remarks on the distribution of species of the genus Temora in the Indian Ocean. J. Mar. Biol. Ass. India 1975, 17, 87–95. [Google Scholar]
- Tseng, W.Y. Planktonic copepods from the waters off Tansui (in Chinese, with English abstract). Bull. Taiwan Fish. Res. Inst. 1975, 24, 1–44. [Google Scholar]
- Shih, C.T.; Young, S.S. A checklist of free-living copepods, including those associated with invertebrates, reported from the adjacent seas of Taiwan. Acta Zool. Taiwanica 1995, 6, 65–81. [Google Scholar]
- Hsieh, C.H.; Chiu, T.S. Summer spatial distribution of copepods and fish larvae in relation to hydrography in the northern Taiwan Strait. Zool. Stud. 2002, 41, 85–98. [Google Scholar]
- Hsiao, S.-H.; Lee, C.Y.; Shih, C.T.; Hwang, J.-S. Calanoid copepods of the Kuroshio Current east of Taiwan, with notes on the presence of Calanus jashnovi Hulseman, 1994. Zool. Stud. 2004, 43, 323–331. [Google Scholar]
- Lo, W.T.; Hwang, J.S.; Cheng, Q.C. Spatial variations of copepods in the surface waters of the southeastern Taiwan Strait. Zool. Stud. 2004, 43, 218–228. [Google Scholar]
- Hsu, P.K.; Lo, W.T.; Shih, C.T. The coupling of copepod assemblages and hydrography in a eutrophic lagoon in Taiwan: Seasonal and spatial variations. Zool. Stud. 2008, 47, 172–184. [Google Scholar]
- Hwang, J.-S.; Souissi, S.; Dahms, H.-U.; Tseng, L.-C.; Schmitt, F.G.; Chen, Q.-C. Rank-abundance allocations as a tool to analyze planktonic copepod assemblages off the Danshuei river estuary (northern Taiwan). Zool. Stud. 2009, 48, 49–62. [Google Scholar]
- Hwang, J.-S.; Kumar, R.; Hsieh, C.-W.; Kuo, A.Y.; Souissi, S.; Hsu, M.-H.; Wu, J.-T.; Liu, W.-C.; Wang, C.-F.; Chen, Q.-C. Patterns of zooplankton distribution along the marine, estuarine and riverine portions of the Danshuei ecosystem in northern Taiwan. Zool. Stud. 2010, 49, 335–352. [Google Scholar]
- Tseng, L.-C.; Kumar, R.; Chen, Q.-C.; Hwang, J.-S. Faunal shift between two copepod congeners (Temora discaudata and T. turbinata) in the vicinity of two nuclear power plants in southern East China Sea: Spatiotemporal patterns of population trajectories over a decade. Hydrobiologia 2011, 666, 301–315. [Google Scholar] [CrossRef]
- Tseng, L.-C.; Wang, Y.-G.; Lian, G.-S.; Hwang, J.-S. A multi-year investigation of the Temoridae (Copepoda, Calanoida) assemblage succession within the interplay waters of the northern South China Sea. Crustaceana 2020, 93, 519–540. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Tu, Y.-Y.; Tseng, L.-C.; Fang, L.-S.; Souissi, S.; Fang, T.-H.; Lo, W.-T.; Twan, W.-H.; Hsiao, S.-H.; Wu, C.-H.; et al. Taxonomic composition and seasonal distribution of copepod assemblages from waters adjacent to nuclear power plant I and II in Northern Taiwan. J. Mar. Sci. Technol. 2004, 12, 380–391. [Google Scholar]
- Jang, M.C.; Shin, K.; Lee, T.; Noh, I. Feeding selectivity of calanoid copepods on phytoplankton in Jangmok Bay, South coast of Korea. Ocean Sci. J. 2010, 45, 101–111. [Google Scholar] [CrossRef]
- Anna, E.E.S. Remains of the Protozoan Sticholonche zanclea in the faecal pellets of Paracalanus quasimodo, Parvocalanus crassirostris, Temora stylifer and Temora turbinata (Copepoda, Calanoida) in Brazilian coastal waters. Braz. J. Oceanogr. 2013, 61, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.C.; Padmavati, G. Zooplankton production composition and diversity in the coastal waters of Goa. Indian J. Geo-Mar. Sci. 1996, 25, 91–97. [Google Scholar]
- Lopez-Salgado, I.; Suarez-Morales, E. Copepod assemblages in surface waters of the western Gulf of Mexico. Crustaceana 1998, 71, 312–330. [Google Scholar]
- Ara, K. Temporal variability and production of Temora turbinata (Copepoda: Calanoida) in the Cananeia Lagoon estuarine system, Sao Paulo, Brazil. Sci. Mar. 2002, 66, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Chang, W.B.; Fang, L.S. Temporal and spatial variations in species composition, distribution, and abundance of copepods in Kaohsiung Harbor, Taiwan. Zool. Stud. 2004, 43, 454–463. [Google Scholar]
- Fang, T.-H.; Hwang, J.-S.; Shiao, S.-H.; Chen, H.-Y. Trace metals in seawater and copepods in the ocean outfall area off the northern Taiwan coast. Mar. Environ. Res. 2006, 61, 224–243. [Google Scholar] [CrossRef]
- Tseng, L.-C.; Souissi, S.; Dahms, H.-U.; Chen, Q.-C.; Hwang, J.-S. Copepod communities related to water masses in the southwest East China Sea. Helgol. Mar. Res. 2008, 62, 153–165. [Google Scholar] [CrossRef] [Green Version]
- Dunbar, F.N.; Webber, M.K. Zooplankton distribution in the eutrophic Kingston Harbour, Jamaica. Bull. Mar. Sci. 2003, 73, 343–359. [Google Scholar]
- Lester, K.M.; Heil, C.A.; Neely, M.B.; Spence, D.N.; Murasko, S.; Hopkins, T.L.; Sutton, T.T.; Burghart, S.E.; Bohrer, R.N.; Remsen, A.W.; et al. Zooplankton and Karenia brevis in the Gulf of Mexico. Cont. Shelf Res. 2008, 28, 99–111. [Google Scholar] [CrossRef]
- Turner, J.T. Zooplankton feeding ecology: Contents of fecal pellets of the copepods Temora turbinata and T. stylifera from continental shelf and slope waters near the mouth of the Mississippi River. Mar. Biol. 1984, 82, 73–83. [Google Scholar] [CrossRef]
- Chang, K.H.; Doi, H.; Nishibe, Y.; Nam, G.S.; Nakano, S.I. Feeding behavior of the copepod Temora turbinata: Clearance rate and prey preference on the diatom and microbial food web components in coastal areas. J. Ecol. Environ. 2014, 37, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-H.; Dahms, H.-U.; Buskey, E.J.; Strickler, J.R.; Hwang, J.-S. Behavioral interactions of the copepod Temora turbinata with potential ciliate prey. Zool. Stud. 2010, 49, 157–168. [Google Scholar]
- Bennett, H. The Genetical Theory of Natural Selection–A Complete Variorum Edition; Fisher, R.A., Ed.; Oxford University Press: Oxford, UK, 1999; p. 318. [Google Scholar]
- Irigoien, X.; Obermüller, B.; Head, R.N.; Harris, R.P.; Rey, C.; Hansen, B.W.; Hygum, B.H.; Heath, M.R.; Durbin, E.G. The effect of food on the determination of sex ratio in Calanus spp.: Evidence from experimental studies and field data. ICES J. Mar. Sci. 2000, 57, 1752–1763. [Google Scholar] [CrossRef] [Green Version]
- Timofeev, S.F. Sex ratios in the population of Thysanoessa raschii (M. Sars, 1864) (Euphausiacea) in the Barents Sea (with some notes on the sex ratios in the order Euphausiacea). Crustaceana 2002, 75, 937–956. [Google Scholar] [CrossRef]
- Mauchline, J. The biology of calanoid copepods. Adv. Mar. Biol. 1998, 33, 1–710. [Google Scholar]
- Kiørboe, T. Sex, sex-ratios, and the dynamics of pelagic copepod populations. Oecologia 2006, 148, 40–50. [Google Scholar] [CrossRef]
- Volkman-Rocco, B. The effect of delayed fertilization in some species of the genus Tisbe (Copepoda, Harpacticoida). Biol. Bull. 1972, 142, 520–529. [Google Scholar] [CrossRef]
- Gophen, M. Sex ratio in Mesocyclops leuckarti (Claus) Populations in lake Kinneret (Israel). Hydrobiologia 1979, 66, 41–43. [Google Scholar] [CrossRef]
- Gusmao, L.F.M.; Mckinnon, A.D.; Richardson, A.J. No evidence of predation causing female-biased sex ratios in mating pelagic copepods. Mar. Ecol. Prog. Ser. 2013, 482, 279–298. [Google Scholar] [CrossRef] [Green Version]
- Shayegan, M.; Fereidouni, A.E.; Agh, N.; Khalili, K.J. Effects of salinity on egg and fecal pellet production, development and survival, adult sex ratio and total life span in the calanoid copepod, Acartia tonsa: A laboratory study. Chin. J. Oceanol. Limnol. 2016, 34, 709–718. [Google Scholar] [CrossRef]
- Legrand, J.J.; Legrand-Hamelin, E.; Juchault, P. Sex determination in Crustacea. Biol. Rev. 1987, 62, 439–470. [Google Scholar] [CrossRef]
- Korpelainen, H. Sex ratio and conditions required for environmental sex determination in animals. Biol. Rev. 1990, 65, 147–184. [Google Scholar] [CrossRef] [PubMed]
- Kouwenberg, J.H.M. Sex ratio of Calanoid copepods in relation to population composition in the northwestern Mediterranean. Crustaceana 1993, 64, 281–299. [Google Scholar] [CrossRef]
- Roff, J.C.; Turner, J.T.; Webber, M.K.; Hopcroft, R.R. Bacterivory by tropical copepod nauplii: Extent and possible significance. Aquat. Microbial Ecol. 1995, 9, 165–175. [Google Scholar] [CrossRef]
- Hopcroft, R.R.; Roff, J.C. Zooplankton growth rates: The influence of female size and resources on egg production of tropical marine copepodites. Mar. Biol. 1998, 132, 79–86. [Google Scholar] [CrossRef]
- Hopcroft, R.R.; Roff, J.C. Zooplankton growth rates: The influence of size in nauplii of tropical marine copepodites. Mar. Biol. 1998, 132, 87–96. [Google Scholar] [CrossRef]
- Chisholm, L.A.; Roff, J.C. Size-weight relationships and biomass of tropical neritic copepods off Kingston, Jamaica. Mar. Biol. 1990, 106, 71–77. [Google Scholar] [CrossRef]
- Chisholm, L.A.; Roff, J.C. Abundance, growth rate, and production of tropical neritic copepods off Kingston, Jamaica. Mar. Biol. 1990, 106, 79–89. [Google Scholar] [CrossRef]
- Ara, K. Variabilidade Temporal e Produção dos Copépodos no Complexo Estuarino-Lagunar de Cananéia. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 1998. [Google Scholar]
- Ara, K. Length-weight relationships and chemical content of the planktonic copepods in the Cananéia Lagoon estuarine system, São Paulo, Brazil. Plankton Biol. Ecol. 2001, 48, 121–127. [Google Scholar]
- Tseng, L.-C.; Hung, J.-J.; Chen, Q.-C.; Hwang, J.-S. Seasonality of the copepod assemblages associated with interplay waters off northeastern Taiwan. Helgol. Mar. Res. 2013, 67, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Qiu, B.; Lukas, R. Seasonal and interannual variability of the North Equatorial Current, the Mindanao Current and the Kuroshio along the Pacific western boundary. J. Geophys. Res. 1996, 101, 12315–12330. [Google Scholar] [CrossRef]
- Qu, T.; Lukas, R. The bifurcation of the North Equatorial Current in the Pacific. J. Phys. Oceanogr. 2003, 33, 5–18. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, S. Interannual-to-decadal variability in the bifurcation of the North Equatorial Current off the Philippines. J. Phys. Oceanogr. 2010, 40, 2525–2538. [Google Scholar] [CrossRef]
- Rudnick, L.D.; Jan, S.; Centurioni, L.; Lee, C.; Lien, R.C.; Wang, J.; Lee, D.K.; Tseng, R.S.; Kim, Y.Y.; Chern, C.S. Seasonal and mesoscale variability of the Kuroshio near its origin. Oceanography 2011, 24, 52–63. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F. Spreading and salinity change of North Pacific tropical waters in the Philippine Sea. J. Oceanogr. 2012, 68, 439–452. [Google Scholar] [CrossRef]
- Gordon, A.L.; Flament, P.; Villanoy, C.; Centurioni, L. The nascent Kuroshio of Lamon Bay. J. Geophys. Res. Ocean. 2014, 119, 4251–4263. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.S. The Impact of Land-Use Change on the Watershed Runoff: Lan-Yang River Catchment as an Example. Ph.D. Thesis, National Central University, Taoyuan, Taiwan, 2010. [Google Scholar]
- LC–FRD (Library of Congress–Federal Research Division). Country Profile: Taiwan; Library of Congress–Federal Research Division: Washington, DA, USA, 2005; pp. 1–29. [Google Scholar]
- Dahms, H.-U.; Tseng, L.-C.; Hsiao, S.-H.; Chen, Q.-C.; Kim, B.-R.; Hwang, J.-S. Biodiversity of planktonic copepods in the Lanyang River (northeastern Taiwan), a typical watershed of Oceania. Zool. Stud. 2012, 51, 160–174. [Google Scholar]
- Dahms, H.-U.; Tseng, L.-C.; Hsiao, S.-H.; Chen, Q.-C.; Hwang, J.-S. A model study for an oceania watershed: Spatio-temporal changes of mesozooplankton in riverine and estuarine parts of the Lanyang River in Taiwan. Ecol. Res. 2013, 28, 345–357. [Google Scholar] [CrossRef]
- Tseng, L.-C.; Hsiao, S.-H.; Sarkar, S.K.; Bhattacharya, B.D.; Chen, Q.-C.; Hwang, J.-S. Influence of Kuroshio water on the annual copepod community structure in an estuary in the northwest Pacific Ocean. Cont. Shelf Res. 2016, 118, 165–176. [Google Scholar] [CrossRef]
- Tseng, L.-C.; Hung, J.-J.; Molinero, J.C.; Chen, Q.-C.; Hwang, J.-S. Indicator species and seasonal succession of planktonic copepod assemblages driven by the interplay of subtropical and temperate waters in the southern East China Sea. Crustaceana 2015, 88, 96–112. [Google Scholar] [CrossRef]
- Chen, Q.-C. Zooplankton of China Seas; Science Press: Beijing, China, 1992; Volume 1, pp. 1–87. ISBN 7-03-002599-7. [Google Scholar]
- Chen, Q.-C.; Zhang, S.-Z. The planktonic copepods of the Yellow Sea and the East China Sea. I. Calanoida. Stud. Mar. Sin. 1965, 7, 20–31. (In Chinese) [Google Scholar]
- Chihara, M.; Murano, M. An Illustrated Guide to Marine Plankton in Japan; Tokai University Press: Tokyo, Japan, 1997. [Google Scholar]
- Lian, G.-S.; Wang, Y.-G.; Sun, R.X.; Hwang, J.-S. Species Diversity of Marine Planktonic Copepods in China’s Seas; China Ocean Press: Beijing, China, 2019; p. 868. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Box, G.E.P.; Cox, D.R. Analysis of transformations (with discussion). J. R. Stat. Soc. Ser. 1964, 26, 211–246. [Google Scholar]
- Sun, X.P. Analysis of the surface path of the Kuroshio in the East China Sea. In Essays on the Investigation of the Kuroshio; Sun, X.P., Ed.; China Ocean Press: Beijing, China, 1987; pp. 1–14. [Google Scholar]
- Yang, Y.J.; Jan, S.; Chang, M.H.; Wang, J.; Mensah, V.; Kuo, T.H.; Tsai, C.J.; Lee, C.Y.; Andres, M.; Centurioni, L.R. Mean Structure and Fluctuations of the Kuroshio East of Taiwan from In Situ and Remote Observations. Oceanography 2015, 28, 74–83. [Google Scholar] [CrossRef]
- Chao, S.Y. Circulation of the East China Sea, a numerical study. J. Oceanogr. 1991, 46, 273–295. [Google Scholar] [CrossRef]
- Hsueh, Y.; Chern, C.S.; Wang, J. The intrusion of the Kuroshio across the continental slope northeast of Taiwan. J. Geophys. Res. 1992, 97, 14323–14330. [Google Scholar] [CrossRef]
- Liu, C.T. As the Kuroshio turns, (I) Characteristics of the current. Acta Oceanogr. Taiwanica 1983, 14, 88–95. [Google Scholar]
- Liu, C.T.; Pai, S.C. As the Kuroshio turns, (II) The oceanic front north of Taiwan. Acta Oceanogr. Taiwanica 1987, 18, 49–61. [Google Scholar]
- Hsieh, H.; Lo, W. Mesoscale distribution and assemblage structure of fish larvae in the Kuroshio waters off eastern Taiwan. Mar. Biodivers. 2019, 49, 1971–1986. [Google Scholar] [CrossRef]
- Vinod, S.; Santhosh, B.; Rani, M.G.; Muhammed, A.F.; Aneesh, K.S.; Mijo, V.A.; Unnikrishnan, C. Biological information and culture techniques of Temora turbinata (Dana, 1849). In Culture Techniques of Marine Copepods; ICAR-Central Marine Fisheries Research Institute: Kochi, India, 2018; pp. 44–51. [Google Scholar]
- Sabahat, H.K. Adults and Copepodite Stages of Temora turbinata (Copepoda: Calanoida) from the Indian Ocean. Pakistan J. Zool. 2006, 38, 201–205. [Google Scholar]
- Muxagata, E.; Gloeden, I.M. Ocorrenacia de Temora turbinata Dana 1849 (Crustacea: Copepoda) no estuãrio da Lagoa dos Patos, RS, Brasil. Nauplius 1995, 3, 163–164. [Google Scholar]
- Hsieh, C.H.; Chiu, T.S.; Shih, C.T. Copepod diversity and composition as indicators of the intrusion of the Kuroshio Branch Current into the northern Taiwan Strait in spring 2000. Zool. Stud. 2004, 43, 393–403. [Google Scholar]
- Lan, Y.C.; Shih, C.T.; Lee, M.A.; Shieh, H.Z. Spring distribution of copepods in relation to water masses in the northern Taiwan Strait. Zool. Stud. 2004, 43, 332–343. [Google Scholar]
- Tseng, L.-C.; Kumar, R.; Dahms, H.-U.; Chen, Q.-C.; Hwang, J.-S. Monsoon driven seasonal succession of copepod assemblages in the coastal waters of the northeastern Taiwan Strait. Zool. Stud. 2008, 47, 46–60. [Google Scholar]
- Lo, W.-T.; Hwang, J.-S.; Chen, Q.-C. Identity and abundance of surface-dwelling, coastal copepods of southwestern Taiwan. Crustaceana 2001, 74, 1139–1157. [Google Scholar] [CrossRef]
- Dur, G.; Hwang, J.-S.; Souissi, S.; Tseng, L.-C.; Wu, C.-H.; Hsiao, S.-H.; Chen, Q.-C. An overview of the influence of hydrodynamics on the spatial and temporal patterns of calanoid copepod communities around Taiwan. J. Plank. Res. 2007, 29, 97–116. [Google Scholar] [CrossRef] [Green Version]
- Longhurst, A.R. Relationship between diversity and the vertical structure of the upper ocean. Deep Sea Res. 1985, 32, 1535–1570. [Google Scholar] [CrossRef]
- McKinnon, A.D.; Duggan, S. Community ecology of pelagic copepods in tropical waters. In Copepods: Diversity, Habitat and Behavior; Nova Science Publishers: New York, NY, USA, 2014; pp. 25–49. [Google Scholar]
- Polunia, J.J.; Lange, E.K.; Krechik, V.A. Structure and Distribution of Autumn Zooplankton in the Southeastern Baltic Sea in 2015. Oceanology 2019, 59, 66–74. [Google Scholar] [CrossRef]
- Sewell, R.B.S. Notes on the surface living Copepoda of the Bay of Bengal I and II. Rec. Indian Mus. 1912, 7, 313–382. [Google Scholar] [CrossRef]
- Hirst, A.G.; Kiørboe, T. Mortality of marine planktonic copepods: Global rates and patterns. Mar. Ecol. Prog. Ser. 2002, 230, 195–209. [Google Scholar] [CrossRef] [Green Version]
- Schnack, S.B. Seasonal change of zooplankton in Kiel Bay III. Calanoid copepods. Kiel. Meeresforsch 1978, 4, 201–209. [Google Scholar]
- Hirst, A.G.; Sheader, M.; Williams, J.A. Annual pattern of calanoid copepod abundance, prosome length and minor role in pelagic carbon flux in the Solent, UK. Mar. Ecol. Prog. Ser. 1999, 177, 133–146. [Google Scholar] [CrossRef]
- Marshall, S. On the biology of the small copepods in Loch Striven. J. Mar. Biol. Assoc. UK 1949, 28, 45–122. [Google Scholar] [CrossRef] [Green Version]
- Digby, P.S.B. The Biology of the Small Planktonic Copepods of Plymouth. J. Mar. Biol. Assoc. UK 1950, 29, 393. [Google Scholar] [CrossRef] [Green Version]
- Mcalice, W.Y.L.J. Seasonal Succession and Breeding Cycles of Three Species of Acartia (Copepoda: Calanoida) in a Marine Estuary. Estuaries 1979, 2, 228–235. [Google Scholar]
- Norrbin, M.F. Seasonal Patterns in gonad maturation, sex-ratio and size in some small, high latitude copepods—Implications for overwintering tactics. J. Plankton Res. 1994, 16, 115–131. [Google Scholar] [CrossRef]
- Liang, D.; Uye, S.-I. Population dynamics and production of the planktonic copepods in a eutrophic inlet of the Inland Sea of Japan. II. Acartia omorii. Mar. Biol. 1996, 125, 109–117. [Google Scholar] [CrossRef]
- Digby, P.S.B. The biology of the marine planktonic copepods of Scoresby Sound, East Greenland. J. Anim. Ecol. 1954, 23, 298–338. [Google Scholar] [CrossRef]
- Gibbons, S.G. Calanus finmarchicus and Other Copepods in Scottish Waters in 1933; Fishery Board for Scotland (Scientific Investigations): Edinburgh, Scotland, 1936; pp. 3–37. [Google Scholar]
- Wiborg, K.F. The production of zooplankton in the Oslo Fjord in 1933–1934. Hvalrådets Skr. 1940, 21, 1–87. [Google Scholar]
- Moraitou-Apostolopoulou, M. Variability of some morpho-ecological factors in six pelagic copepods from the Aegean Sea. Mar. Biol. 1969, 3, 1–3. [Google Scholar] [CrossRef]
- Moore, E.A.; Sander, F. Physioecology of tropical copepods. II. Sex ratios. Crustaceana 1983, 44, 113–122. [Google Scholar] [CrossRef]
- Moraitou-Apostolopoulou, M. Sex ratio in the pelagic copepods Temora stylifera Dana and Centropages typicus Krøyer. J. Exp. Mar. Biol. Ecol. 1972, 8, 83–87. [Google Scholar] [CrossRef]
- Hopkins, C.C.E. The breeding biology of Euchaeta norvegica (Boeck) (Copepoda: Calanoida) in Loch Etive, Scotland: Assessment of breeding intensity in terms of seasonal cycles in the sex ratio, spermatophore attachment, and egg-sac production. J. Exp. Mar. Biol. Ecol. 1982, 60, 91–102. [Google Scholar] [CrossRef]
- Liang, D.; Uye, S. Population dynamics and production of the planktonic copepods in a eutrophic inlet of the Inland Sea of Japan. III. Paracalanus sp. Mar. Biol. 1996, 127, 219–227. [Google Scholar] [CrossRef]
- Goswami, S.C. Development stages, growth and sex ratio in Pseudodiaptomus binghami Sewell (Copepoda: Calanoida). Ind. J. Mar. Sci. 1978, 7, 103–109. [Google Scholar]
- Liang, D.; Uye, S. Seasonal reproductive biology of the egg-carrying calanoid copepod Pseudodiaptomus marinus in a eutrophic inlet of the Inland Sea of Japan. Mar. Biol. 1997, 128, 409–414. [Google Scholar] [CrossRef]
- Gusmão, L.F.M.; McKinnon, A.D. Sex ratios, intersexuality and sex change in copepods. J. Plankton Res. 2009, 31, 1101–1117. [Google Scholar] [CrossRef]
- Hirst, A.G.; Bonnet, D.; Conway, D.V.P.; Kiørboe, T. Does predation control adult sex ratios and longevities in marine pelagic copepods? Limnol. Oceanogr. 2010, 55, 2193–2206. [Google Scholar] [CrossRef]
- Sellner, K.G.; Olson, M.M.; Kononen, K. Copepod grazing in a summer cyanobacterial bloom in the Gulf of Finland. Hydrobiologia 1994, 292, 249–254. [Google Scholar] [CrossRef]
- Finiguerra, M.B.; Dam, H.G.; Avery, D.E.; Burris, Z. Sex-specific tolerance to starvation in the copepod Acartia tonsa. J. Exp. Mar. Biol. Ecol. 2013, 446, 17–21. [Google Scholar] [CrossRef]
- Engström-Öst, J.; Brutemark, A.; Vehmaa, A.; Mothwani, N.H.; Katajisto, T. Consequences of a cyanobacteria bloom for copepod reproduction, mortality and sex ratio. J. Plankton Res. 2015, 37, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Avery, D.E.; Altland, K.K.; Dam, H.G. Sex-related differential mortality of a marine copepod exposed to a toxic dinoflagellate. Limnol. Oceanogr. 2008, 53, 2627–2635. [Google Scholar] [CrossRef]
- Carotenuto, Y.; Ianora, A.; Miralto, A. Maternal and neonate diatom diets impair development and sex differentiation in the copepod Temora stylifera. J. Exp. Mar. Biol. Ecol. 2011, 396, 99–107. [Google Scholar] [CrossRef]
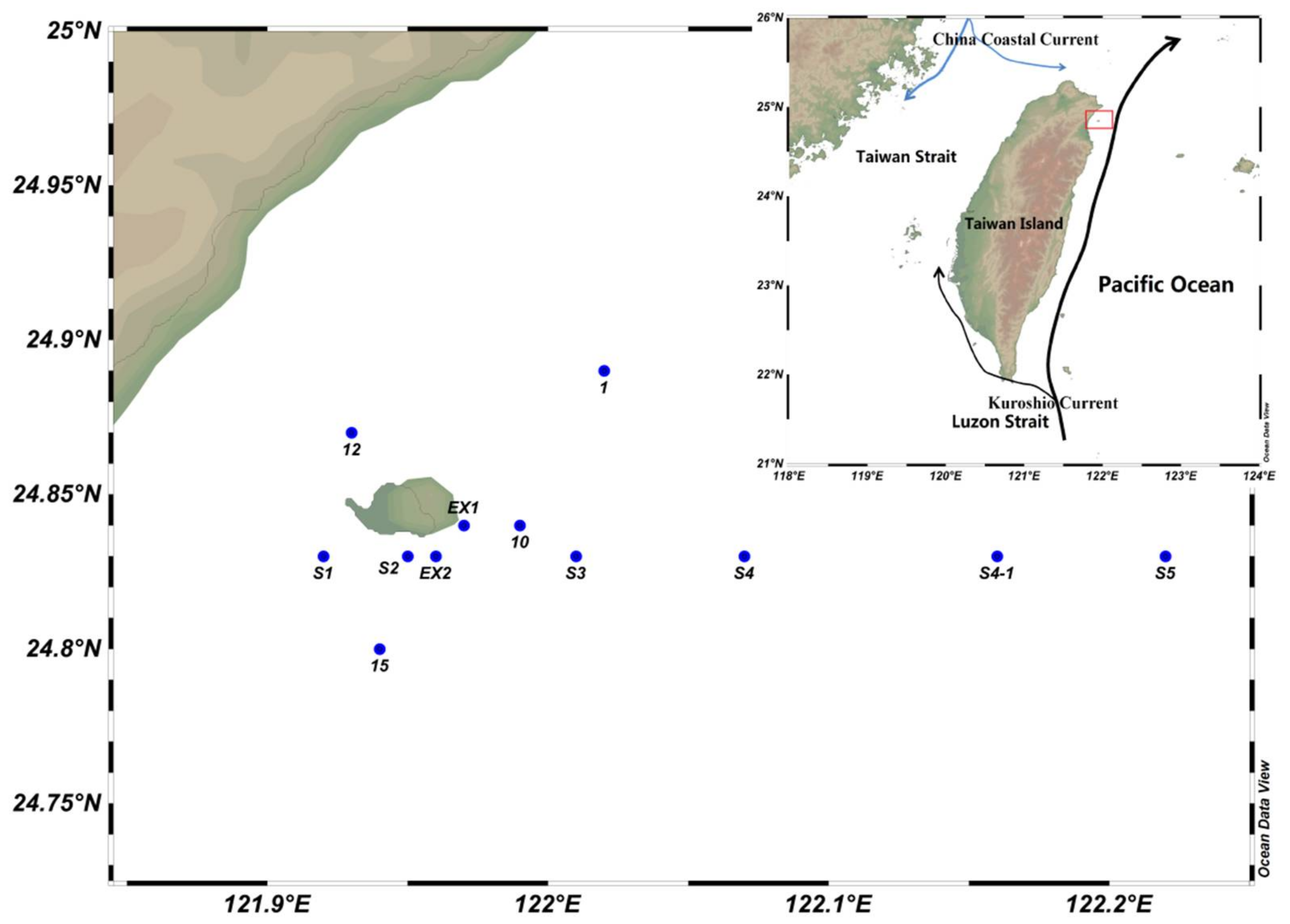





| Station | Longitude (E) | Latitude (N) | Seabed Depth | Grouping |
|---|---|---|---|---|
| St 1 | 24°53.42′ | 122°01.17′ | 454 | DZ |
| St 10 | 24°50.46′ | 121°59.40′ | 210 | MZ |
| St 12 | 24°52.33′ | 121°55.90′ | 130 | SZ |
| St 15 | 24°48.01′ | 121°56.45′ | 273 | MZ |
| St Ex1 | 24°50.57′ | 121°58.02′ | 123 | SZ |
| St Ex2 | 24°49.81′ | 121°57.75′ | 156 | SZ |
| St S1 | 24°50.06′ | 121°54.92′ | 167 | SZ |
| St S2 | 24°49.67′ | 121°57.16′ | 57 | SZ |
| St S3 | 24°49.93′ | 122°00.25′ | 288 | MZ |
| St S4 | 24°50.10′ | 122°04.08′ | 674 | DZ |
| St S4-1 | 24°50.09′ | 122°09.64′ | 536 | DZ |
| St S5 | 24°50.06′ | 122°13.25′ | 878 | DZ |
| Cluster Grouping | Group A | Group B |
|---|---|---|
| Adult male | 736.4 ± 876.08 (29.72%) | 23.05 ± 22.33 (20.19%) |
| Adult female | 588.81 ± 575.61 (23.76%) | 30.11 ± 32.87 (26.37%) |
| Copepodite | 1152.73 ± 1569.72 (46.52%) | 61.03 ± 50.65 (53.45%) |
| All Adult Abundance | Male Abundance | Female Abundance | Copepodite Abundance | Sex Ratio | |
|---|---|---|---|---|---|
| Salinity | −0.014 (0.923) | −0.027 (0.854) | 0.005 (0.972) | 0.197 (0.180) | 0.106 (0.511) |
| Temperature | 0.175 (0.233) | 0.181 (0.219) | 0.157 (0.286) | 0.025 (0.866) | 0.194 (0.223) |
| All adult abundance | 0.983 ** (<0.001) | 0.964 ** (<0.001) | 0.661 ** (<0.001) | 0.405 ** (0.009) | |
| Male abundance | 0.899 ** (<0.001) | 0.569 ** (<0.001) | 0.496 ** (0.001) | ||
| Female abundance | 0.755 ** (<0.001) | 0.247 (0.119) | |||
| Copepodite abundance | 0.196 (0.218) |
| Species | Sex Ratios | Period | Location | References |
|---|---|---|---|---|
| Family Acartiidae | ||||
| Acartia bifilosa | 0.59 | January/1970–October/1971 | Kiel Bay, Baltic | Schnack [94] |
| A. bifilosa | 0.35 | January–December/1993 | Southampton Water, UK | Hirst et al. [95] |
| Acartia clausi | 0.72 | April–December/1993 | Southampton Water, UK | Hirst et al. [95] |
| A. clausi | 0.2 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| A. clausi | 0 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| A. clausi | 0.75 | January–October/1933 | Loch Striven, Scotland | Marshall [96] |
| A. clausi | 0.56 | January–November/1947 | Plymouth Area, English Channel | Digby [97] |
| A. clausi | 0.75 | November/1971–December/1972 | Damariscotta River estuary, USA | Lee and McAlice [98] |
| Acartia discaudata | 0.89 | March–December/1993 | Southampton Water, UK | Hirst et al. [95] |
| A. discaudata | 0.45 | January/1970–October/1971 | Kiel Bay, Baltic | Schnack [94] |
| Acartia longiremis | 0.67 | January/1970–October/1971 | Kiel Bay, Baltic | Schnack [94] |
| A. longiremis | 0.18 | October/1985–October/1986 | Balsfjorden, Norway | Norrbin [99] |
| A. longiremis | 0.39 | November/1971–December/1972 | Damariscotta River estuary, USA | Lee and McAlice [98] |
| Acartia omori | 0.79 | November/1986–July/1987 | Fukuyama Harbor, Inland Sea of Japan | Liang and Uye [100] |
| Acartia tonsa | 0.39 | January/1970–October/1971 | Kiel Bay, Baltic | Schnack [94] |
| A. tonsa | 1.44 | November/1971–December/1972 | Damariscotta River estuary, USA | Lee and McAlice [98] |
| Family Calanidae | ||||
| Calanus finmarchicus | 0 | August/1950–August/1961 | Scoresby Sound, East Greenland | Digby [101] |
| C. finmarchicus | 0.23 | February–August/1933 | Scottish Waters | Gibbons [102] |
| Calanus finmarchicus | 0.25 | June/1933–May/1934 | Oslo Fjord, Norway | Wiborg [103] |
| C. helgolandicus | 0.18 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| C. helgolandicus | 0.08 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| C. helgolandicus | 0.04 | 3 seasons | North Aegean Sea | Moraitou-Apostolopoulou [104] |
| Calanus minor | 0.28 | 3 seasons | North Aegean Sea | Moraitou-Apostolopoulou [104] |
| Calanus tenuicornis | 0.01 | 3 seasons | North Aegean Sea | Moraitou-Apostolopoulou [104] |
| Undinula vulgaris | 0.33 | September/1971–August/1973 | St Vincents, Barbados | Moore and Sander [105] |
| Family Candaciidae | ||||
| Candacia armata | 0.39 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| C. armata | 0.75 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| Family Centropagidae | ||||
| Centropages hamatus | 1.78 | March–October/1933 | Loch Striven, Scotland | Marshall [96] |
| C. hamatus | 1.04 | January/1970–October/1971 | Kiel Bay, Baltic | Schnack [94] |
| C. hamatus | 0.37 | March–December/1993 | Southampton Water, UK | Hirst et al. [95] |
| C. hamatus | 1.13 | June/1933–May/1934 | Oslo Fjord, Norway | Wiborg [103] |
| Centropages typicus | 0.81 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| C. typicus | 0.79 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| C. typicus | 0.85 | January–October/1947 | Plymouth Area, English Channel | Digby [97] |
| C. typicus | 0.72 | 3 seasons | North Aegean Sea | Moraitou-Apostolopoulou [104] |
| C. typicus | 0.72 | February/1965–December/1965 | North Aegean Sea | Moraitou-Apostolopoulou [106] |
| Centropages violaceus | 0.59 | 3 seasons | North Aegean Sea | Moraitou-Apostolopoulou [104] |
| Family Clausocalanidae | ||||
| Clausocalanus spp. | 0.15 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| Clausocalanus spp. | 0.18 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| Microcalanus pygmaeus | 1.4 | January–October/1933 | Loch Striven, Scotland | Marshall [96] |
| M. pygmaeus | 0.03 | August/1950–August/1951 | Scoresby Sound, East Greenland | Digby [101] |
| M. pygmaeus | 0.25 | June/1933–May/1934 | Oslo Fjord, Norway | Wiborg [103] |
| Pseudocalanus acuspes | 0.43 | October/1985–October/1986 | Balsfjorden, Norway | Norrbin [99] |
| Pseudocalanus elongatus | 0.27 | January–December/1947 | Plymouth Area, English Channel | Digby [97] |
| P. minutus | 0.28 | January–October/1933 | Loch Striven, Scotland | Marshall [96] |
| P. minutus | 0.03 | August/1950–August/1951 | Scoresby Sound, East Greenland | Digby [101] |
| P. minutus | 0.25 | June/1933–May/1934 | Oslo Fjord, Norway | Wiborg [103] |
| Pseudocalanus sp. | 0.39 | January/1970–October/1971 | Kiel Bay, Baltic | Schnack [94] |
| Family Euchaetidae | ||||
| Euchaeta marina/acuta | 0.22 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| E. marina/acuta | 0.19 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| Euchaeta norvegica | 0.12 | September/1971–April/1972 | Loch Etive, Scotland | Hopkins [107] |
| Family Metridiidae | ||||
| Metridia longa | 0 | August/1950–September/1950 | Scoresby Sound, East Greenland | Digby [101] |
| M. longa | 0.54 | June/1933–August/1938 | Oslo Fjord, Norway | Wiborg [103] |
| Pleuromamma gracilis | 0.52 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| P. gracilis | 0.41 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| Family Oithonidae | ||||
| Oithona nana | 0.27 | August–December/1947 | Plymouth Area, English Channel | Digby [97] |
| Oithona similis | 0.18 | January–October/1933 | Loch Striven, Scotland | Marshall [96] |
| O. similis | 0.18 | January–December/1947 | Plymouth Area, English Channel | Digby [97] |
| O. similis | 0.06 | August/1950–August/1951 | Scoresby Sound, East Greenland | Digby [101] |
| Family Oncaeidae | ||||
| Oncaea borealis | 0.45 | August/1950–August/1951 | Scoresby Sound, East Greenland | Digby [101] |
| O. borealis | 0.32 | June/1933–May/1934 | Oslo Fjord, Norway | Wiborg [103] |
| Oncaea mediterranea | 0.75 | September/1971–August/1973 | St Vincents, Barbados | Moore and Sander [105] |
| Family Paracalanidae | ||||
| Paracalanus parvus | 0.15 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| P. parvus | 0.1 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| P. parvus | 0.15 | July–October/1933 | Loch Striven, Scotland | Marshall [96] |
| P. parvus | 0.18 | August/1970–October/1971 | Kiel Bay, Baltic | Schnack [94] |
| P. parvus | 0.1 | January–December/1947 | Plymouth Area, English Channel | Digby [97] |
| Paracalanus sp. | 0.45 | November/1986–October/1987 | Fukuyama Harbor, Inland Sea of Japan | Liang and Uye [108] |
| Family Pseudodiaptomidae | ||||
| Pseudodiaptomus binghami | 0.22 | June–September of 1971–1973 | Mandovi Estuary, India | Goswami [109] |
| P. binghami | 0.22 | June–September of 1971–1973 | Zuari Estuary, India | Goswami [109] |
| P. binghami | 0.2 | June–September of 1971–1973 | Cumbarjua Canal, India | Goswami [109] |
| Pseudodiaptomus marinus | 0.64 | November/1986–November/1987 | Fukuyama Harbor, Inland Sea of Japan | Liang and Uye [110] |
| Family Temoridae | ||||
| Temora longicornis | 1.08 | March–October/1933 | Loch Striven, Scotland | Marshall [96] |
| T. longicornis | 0.75 | February–October/1947 | Plymouth Area, English Channel | Digby [97] |
| T. longicornis | 1.04 | January/1970–October/1971 | Kiel Bay, Baltic | Schnack [94] |
| T. longicornis | 1.63 | March–September/1993 | Southampton Water, UK | Hirst et al. [95] |
| Temora stylifera | 1.27 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| T. stylifera | 0.85 | September/1986–June/1988 | Golfe du Lion, Mediterranean | Kouwenberg [47] |
| T. stylifera | 0.75 | 3 seasons | North Aegean Sea | Moraitou-Apostolopoulou [104] |
| T. stylifera | 0.75 | February/1965–December/1965 | North Aegean Sea | Moraitou-Apostolopoulou [106] |
| T. stylifera | 0.32 | September/1971–August/1973 | St Vincents, Barbados | Moore and Sander [105] |
| Temora turbinata | 1.13 | June/2018 | Kueishan Island | Present study |
| T. turbinata | 0.62 | September/2018 | Kueishan Island | Present study |
| T. turbinata | 0.85 | March/2019 | Kueishan Island | Present study |
| T. turbinata | 1.39 | June/2019 | Kueishan Island | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-G.; Tseng, L.-C.; Xing, B.-P.; Sun, R.-X.; Chen, X.-Y.; Wang, C.-G.; Hwang, J.-S. Seasonal Population Structure of the Copepod Temora turbinata (Dana, 1849) in the Kuroshio Current Edge, Southeastern East China Sea. Appl. Sci. 2021, 11, 7545. https://doi.org/10.3390/app11167545
Wang Y-G, Tseng L-C, Xing B-P, Sun R-X, Chen X-Y, Wang C-G, Hwang J-S. Seasonal Population Structure of the Copepod Temora turbinata (Dana, 1849) in the Kuroshio Current Edge, Southeastern East China Sea. Applied Sciences. 2021; 11(16):7545. https://doi.org/10.3390/app11167545
Chicago/Turabian StyleWang, Yan-Guo, Li-Chun Tseng, Bing-Peng Xing, Rou-Xin Sun, Xiao-Yin Chen, Chun-Guang Wang, and Jiang-Shiou Hwang. 2021. "Seasonal Population Structure of the Copepod Temora turbinata (Dana, 1849) in the Kuroshio Current Edge, Southeastern East China Sea" Applied Sciences 11, no. 16: 7545. https://doi.org/10.3390/app11167545






