A Fast Ubiquitination of UHRF1 Oncogene Is a Unique Feature and a Common Mechanism of Thymoquinone in Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. RNA-Seq, Identification of Differentially Expressed Genes and Bioinformatics Analysis
2.3. Western Blot Analysis
2.4. Cell Proliferation Assay
2.5. Statistical Analysis
3. Results
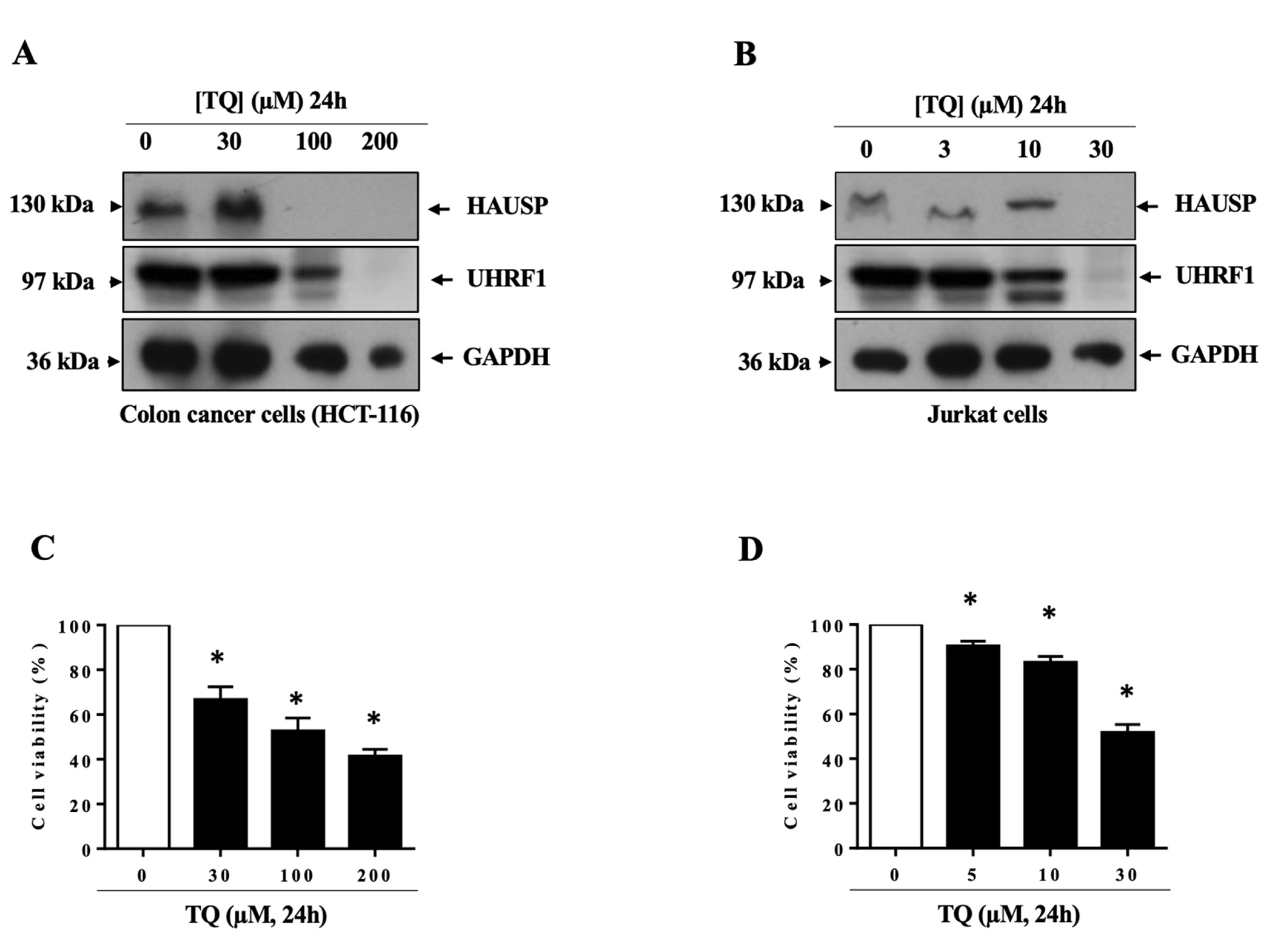
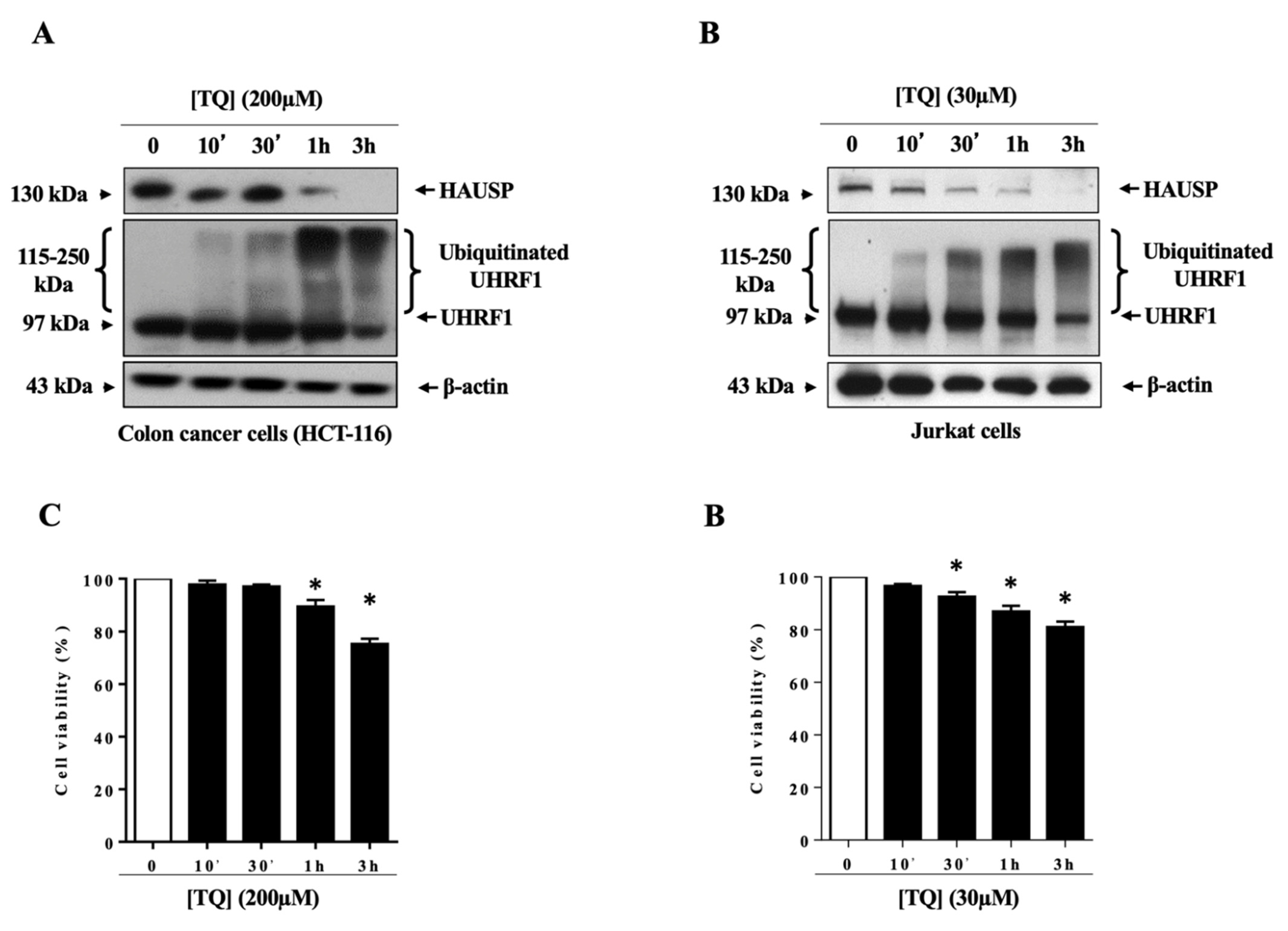
3.1. TQ Induced UHRF1 Ubiquitination Related to HAUSP Downregulation in Human Cancer Cells
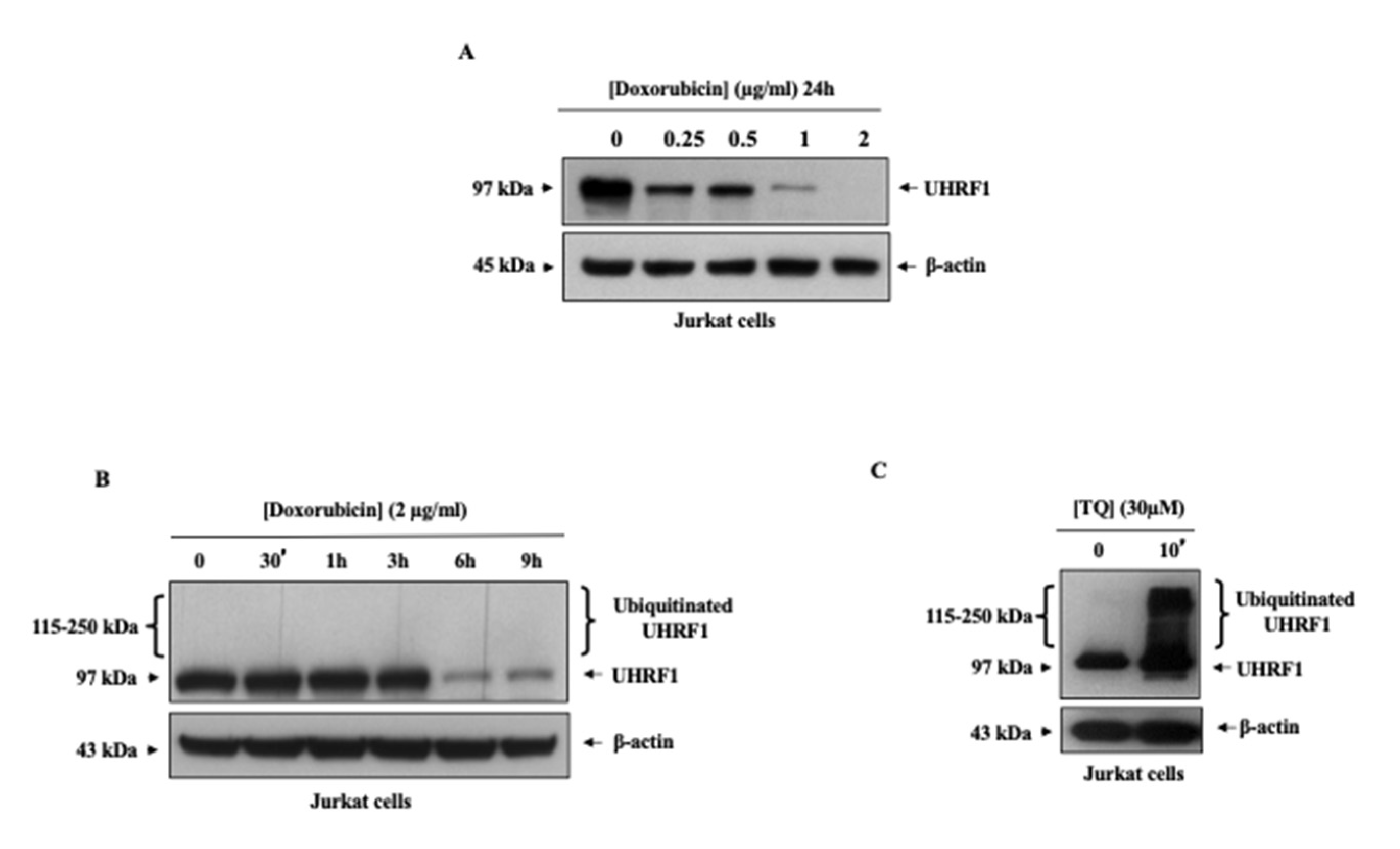
3.2. Doxorubicin Induced Ubiquitination Independently of UHRF1 Downregulation in Cancer Cells
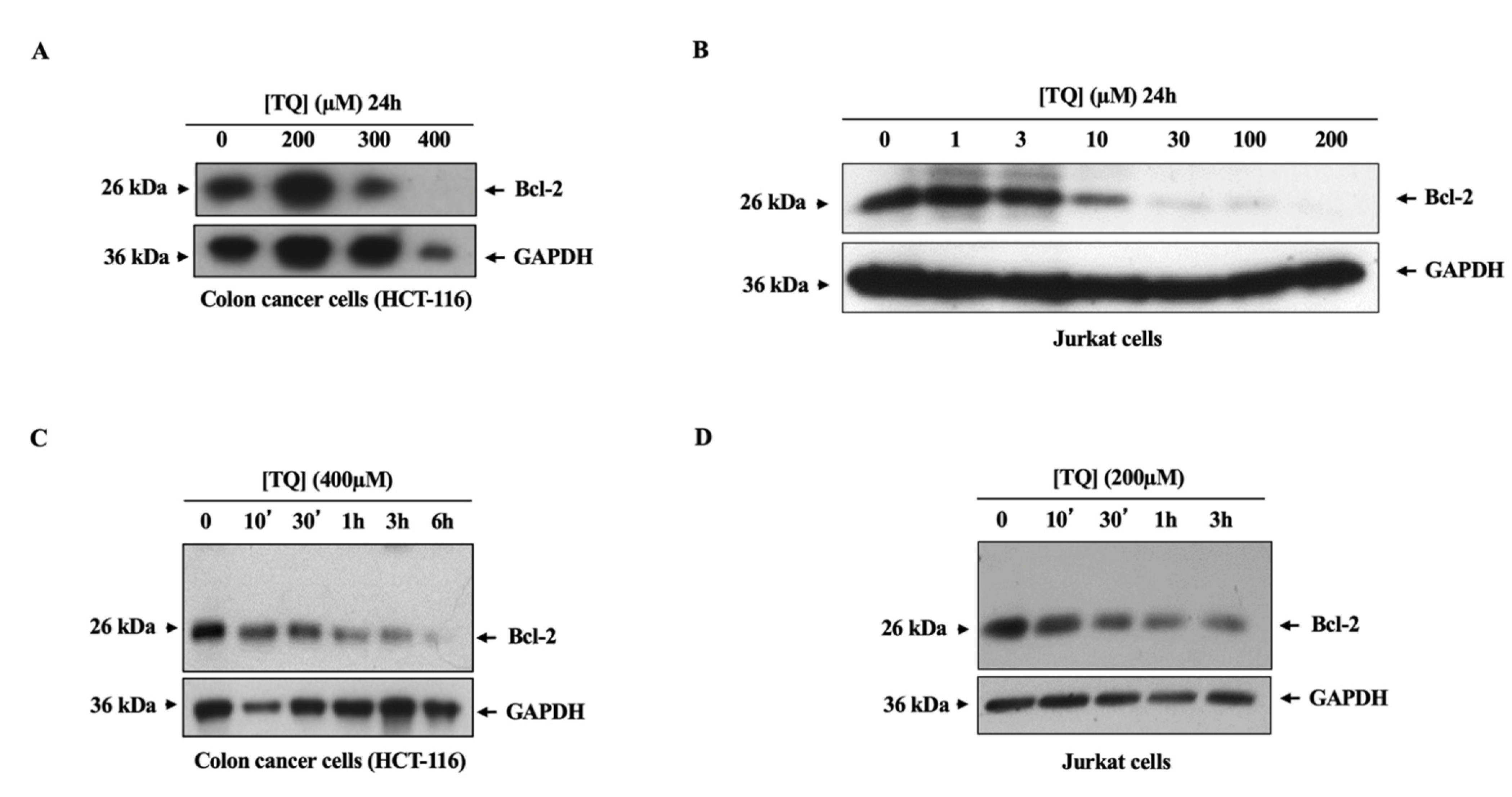
3.3. TQ Induced the Downregulation of the Bcl-2 Oncogene in Cancer Cells through a Ubiquitination-Independent Mechanism
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alhosin, M.; Abusnina, A.; Achour, M.; Sharif, T.; Muller, C.; Peluso, J.; Chataigneau, T.; Lugnier, C.; Schini-Kerth, V.B.; Bronner, C.; et al. Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem. Pharmacol. 2010, 79, 1251–1260. [Google Scholar] [CrossRef] [Green Version]
- Bronner, C.; Krifa, M.; Mousli, M. Increasing role of UHRF1 in the reading and inheritance of the epigenetic code as well as in tumorogenesis. Biochem. Pharmacol. 2013, 86, 1643–1649. [Google Scholar] [CrossRef]
- Zaayter, L.; Mori, M.; Ahmad, T.; Ashraf, W.; Boudier, C.; Kilin, V.; Gavvala, K.; Richert, L.; Eiler, S.; Ruff, M.; et al. A Molecular Tool Targeting the Base-Flipping Activity of Human UHRF1. Chemistry 2019, 25, 13363–13375. [Google Scholar] [CrossRef]
- Achour, M.; Mousli, M.; Alhosin, M.; Ibrahim, A.; Peluso, J.; Muller, C.D.; Schini-Kerth, V.B.; Hamiche, A.; Dhe-Paganon, S.; Bronner, C. Epigallocatechin-3-gallate up-regulates tumor suppressor gene expression via a reactive oxygen species-dependent down-regulation of UHRF1. Biochem. Biophys. Res. Commun. 2013, 430, 208–212. [Google Scholar] [CrossRef]
- Abusnina, A.; Keravis, T.; Yougbaré, I.; Bronner, C.; Lugnier, C. Anti-proliferative effect of curcumin on melanoma cells is mediated by PDE1A inhibition that regulates the epigenetic integrator UHRF1. Mol. Nutr. Food Res. 2011, 55, 1677–1689. [Google Scholar] [CrossRef]
- Qadi, S.A.; Hassan, M.A.; Sheikh, R.A.; Baothman, O.A.; Zamzami, M.A.; Choudhry, H.; Al-Malki, A.L.; Albukhari, A.; Alhosin, M. Thymoquinone-Induced Reactivation of Tumor Suppressor Genes in Cancer Cells Involves Epigenetic Mechanisms. Epigenetics Insights 2019, 12. [Google Scholar] [CrossRef]
- Alhosin, M.; Sharif, T.; Mousli, M.; Etienne-Selloum, N.; Fuhrmann, G.; Schini-Kerth, V.B.; Bronner, C. Down-regulation of UHRF1, associated with re-expression of tumor suppressor genes, is a common feature of natural compounds exhibiting anti-cancer properties. J. Exp. Clin. Cancer Res. CR 2011, 30, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, Y.; Markovtsov, V.; Lang, W.; Sharma, P.; Pearsall, D.; Warner, J.; Franci, C.; Huang, B.; Huang, J.; Yam, G.C.; et al. Critical role of the ubiquitin ligase activity of UHRF1, a nuclear RING finger protein, in tumor cell growth. Mol. Biol. Cell 2005, 16, 5621–5629. [Google Scholar] [CrossRef] [PubMed]
- Citterio, E.; Papait, R.; Nicassio, F.; Vecchi, M.; Gomiero, P.; Mantovani, R.; Di Fiore, P.P.; Bonapace, I.M. Np95 is a histone-binding protein endowed with ubiquitin ligase activity. Mol. Cell. Biol. 2004, 24, 2526–2535. [Google Scholar] [CrossRef] [Green Version]
- Karagianni, P.; Amazit, L.; Qin, J.; Wong, J. ICBP90, a novel methyl K9 H3 binding protein linking protein ubiquitination with heterochromatin formation. Mol. Cell. Biol. 2008, 28, 705–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.; Alhosin, M.; Papin, C.; Ouararhni, K.; Omran, Z.; Zamzami, M.A.; Al-Malki, A.L.; Choudhry, H.; Mély, Y.; Hamiche, A.; et al. Thymoquinone challenges UHRF1 to commit auto-ubiquitination: A key event for apoptosis induction in cancer cells. Oncotarget 2018, 9, 28599–28611. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Brooks, C.L.; Kon, N.; Gu, W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell 2004, 13, 879–886. [Google Scholar] [CrossRef]
- Canning, M.; Boutell, C.; Parkinson, J.; Everett, R.D. A RING finger ubiquitin ligase is protected from autocatalyzed ubiquitination and degradation by binding to ubiquitin-specific protease USP7. J. Biol. Chem. 2004, 279, 38160–38168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, Y.M.; Yoo, S.J.; Seol, J.H. Deubiquitination of Chfr, a checkpoint protein, by USP7/HAUSP regulates its stability and activity. Biochem. Biophys. Res. Commun. 2007, 357, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Felle, M.; Joppien, S.; Németh, A.; Diermeier, S.; Thalhammer, V.; Dobner, T.; Kremmer, E.; Kappler, R.; Längst, G. The USP7/Dnmt1 complex stimulates the DNA methylation activity of Dnmt1 and regulates the stability of UHRF1. Nucleic Acids Res. 2011, 39, 8355–8365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Liu, S.; Xu, G.; Zhou, S.; Luo, Z. Dihydroartemisinin induces cell apoptosis through repression of UHRF1 in prostate cancer cells. Anticancer Drugs 2021. [Google Scholar] [CrossRef]
- Alhosin, M.; Omran, Z.; Zamzami, M.A.; Al-Malki, A.L.; Choudhry, H.; Mousli, M.; Bronner, C. Signalling pathways in UHRF1-dependent regulation of tumor suppressor genes in cancer. J. Exp. Clin. Cancer Res. CR 2016, 35, 174. [Google Scholar] [CrossRef] [Green Version]
- Unoki, M. Current and potential anticancer drugs targeting members of the UHRF1 complex including epigenetic modifiers. Recent Pat. Anti-Cancer Drug Discov. 2011, 6, 116–130. [Google Scholar] [CrossRef]
- Kim, M.Y.; Park, S.J.; Shim, J.W.; Yang, K.; Kang, H.S.; Heo, K. Naphthazarin enhances ionizing radiation-induced cell cycle arrest and apoptosis in human breast cancer cells. Int. J. Oncol. 2015, 46, 1659–1666. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.Y.; Hong, D.; Jeong, S.Y.; Kim, J.H. Shikonin causes apoptosis by up-regulating p73 and down-regulating ICBP90 in human cancer cells. Biochem. Biophys. Res. Commun. 2015, 465, 71–76. [Google Scholar] [CrossRef]
- Parashar, G.; Capalash, N. Promoter methylation-independent reactivation of PAX1 by curcumin and resveratrol is mediated by UHRF1. Clin. Exp. Med. 2016, 16, 471–478. [Google Scholar] [CrossRef]
- Krifa, M.; Leloup, L.; Ghedira, K.; Mousli, M.; Chekir-Ghedira, L. Luteolin induces apoptosis in BE colorectal cancer cells by downregulating calpain, UHRF1, and DNMT1 expressions. Nutr. Cancer 2014, 66, 1220–1227. [Google Scholar] [CrossRef]
- Arima, Y.; Hirota, T.; Bronner, C.; Mousli, M.; Fujiwara, T.; Niwa, S.; Ishikawa, H.; Saya, H. Down-regulation of nuclear protein ICBP90 by p53/p21Cip1/WAF1-dependent DNA-damage checkpoint signals contributes to cell cycle arrest at G1/S transition. Genes Cells Devoted Mol. Cell. Mech. 2004, 9, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Alhosin, M.; Ibrahim, A.; Boukhari, A.; Sharif, T.; Gies, J.P.; Auger, C.; Schini-Kerth, V.B. Anti-neoplastic agent thymoquinone induces degradation of alpha and beta tubulin proteins in human cancer cells without affecting their level in normal human fibroblasts. Investig. New Drugs 2012, 30, 1813–1819. [Google Scholar] [CrossRef]
- Abusnina, A.; Alhosin, M.; Keravis, T.; Muller, C.D.; Fuhrmann, G.; Bronner, C.; Lugnier, C. Down-regulation of cyclic nucleotide phosphodiesterase PDE1A is the key event of p73 and UHRF1 deregulation in thymoquinone-induced acute lymphoblastic leukemia cell apoptosis. Cell. Signal. 2011, 23, 152–160. [Google Scholar] [CrossRef]
- Hopfner, R.; Mousli, M.; Jeltsch, J.M.; Voulgaris, A.; Lutz, Y.; Marin, C.; Bellocq, J.P.; Oudet, P.; Bronner, C. ICBP90, a novel human CCAAT binding protein, involved in the regulation of topoisomerase IIalpha expression. Cancer Res. 2000, 60, 121–128. [Google Scholar] [PubMed]
- Bronner, C. Control of DNMT1 abundance in epigenetic inheritance by acetylation, ubiquitylation, and the histone code. Sci. Signal. 2011, 4, pe3. [Google Scholar] [CrossRef] [PubMed]
- Kofunato, Y.; Kumamoto, K.; Saitou, K.; Hayase, S.; Okayama, H.; Miyamoto, K.; Sato, Y.; Katakura, K.; Nakamura, I.; Ohki, S.; et al. UHRF1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncol. Rep. 2012, 28, 1997–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Yang, Y.Z.; Shi, C.Z.; Zhang, P.; Moyer, M.P.; Zhang, H.Z.; Zou, Y.; Qin, H.L. UHRF1 promotes cell growth and metastasis through repression of p16(ink(4)a) in colorectal cancer. Ann. Surg. Oncol. 2012, 19, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Abbady, A.Q.; Bronner, C.; Trotzier, M.A.; Hopfner, R.; Bathami, K.; Muller, C.D.; Jeanblanc, M.; Mousli, M. ICBP90 expression is downregulated in apoptosis-induced Jurkat cells. Ann. N. Y. Acad. Sci. 2003, 1010, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xing, F.; Tang, Z.; Bronner, C.; Lu, X.; Di, J.; Zeng, S.; Liu, J. Anisomycin suppresses Jurkat T cell growth by the cell cycle-regulating proteins. Pharmacol. Rep. PR 2013, 65, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Mandili, G.; Khadjavi, A.; Gallo, V.; Minero, V.G.; Bessone, L.; Carta, F.; Giribaldi, G.; Turrini, F. Characterization of the protein ubiquitination response induced by Doxorubicin. FEBS J. 2012, 279, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Halim, V.A.; García-Santisteban, I.; Warmerdam, D.O.; van den Broek, B.; Heck, A.J.R.; Mohammed, S.; Medema, R.H. Doxorubicin-induced DNA Damage Causes Extensive Ubiquitination of Ribosomal Proteins Associated with a Decrease in Protein Translation. Mol. Cell. Proteom. MCP 2018, 17, 2297–2308. [Google Scholar] [CrossRef] [Green Version]
- Lang, V.; Aillet, F.; Xolalpa, W.; Serna, S.; Ceccato, L.; Lopez-Reyes, R.G.; Lopez-Mato, M.P.; Januchowski, R.; Reichardt, N.C.; Rodriguez, M.S. Analysis of defective protein ubiquitylation associated to adriamycin resistant cells. Cell Cycle 2017, 16, 2337–2344. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Tian, J. LIN28B promotes the progression of colon cancer by increasing B-cell lymphoma 2 expression. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 103, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Meng, Q.; Tian, A. Expressions of the anti-apoptotic genes Bag-1 and Bcl-2 in colon cancer and their relationship. Am. J. Surg. 2010, 200, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Meterissian, S.H.; Kontogiannea, M.; Al-Sowaidi, M.; Linjawi, A.; Halwani, F.; Jamison, B.; Edwardes, M. Bcl-2 is a useful prognostic marker in Dukes’ B colon cancer. Ann. Surg. Oncol. 2001, 8, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Paul-Samojedny, M.; Kokocińska, D.; Samojedny, A.; Mazurek, U.; Partyka, R.; Lorenz, Z.; Wilczok, T. Expression of cell survival/death genes: Bcl-2 and Bax at the rate of colon cancer prognosis. Biochim. Et Biophys. Acta 2005, 1741, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Kleschyov, A.L.; Strand, S.; Schmitt, S.; Gottfried, D.; Skatchkov, M.; Sjakste, N.; Daiber, A.; Umansky, V.; Munzel, T. Dinitrosyl-iron triggers apoptosis in Jurkat cells despite overexpression of Bcl-2. Free Radic. Biol. Med. 2006, 40, 1340–1348. [Google Scholar] [CrossRef]
- Thomson, S.J.; Brown, K.K.; Pullar, J.M.; Hampton, M.B. Phenethyl isothiocyanate triggers apoptosis in Jurkat cells made resistant by the overexpression of Bcl-2. Cancer Res. 2006, 66, 6772–6777. [Google Scholar] [CrossRef] [Green Version]
- Molto, L.; Rayman, P.; Paszkiewicz-Kozik, E.; Thornton, M.; Reese, L.; Thomas, J.C.; Das, T.; Kudo, D.; Bukowski, R.; Finke, J.; et al. The Bcl-2 transgene protects T cells from renal cell carcinoma-mediated apoptosis. Clin. Cancer Res. 2003, 9, 4060–4068. [Google Scholar]
- Breitschopf, K.; Haendeler, J.; Malchow, P.; Zeiher, A.M.; Dimmeler, S. Posttranslational modification of Bcl-2 facilitates its proteasome-dependent degradation: Molecular characterization of the involved signaling pathway. Mol. Cell. Biol. 2000, 20, 1886–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Lin, F.; Wu, X.; Zhao, Q.; Zhao, B.; Lin, P.; Zhang, Y.; Yu, X. Pseudolaric acid B induces apoptosis via proteasome-mediated Bcl-2 degradation in hormone-refractory prostate cancer DU145 cells. Toxicol. Vitr. 2012, 26, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, H.; Capalash, N. UHRF1: The key regulator of epigenetics and molecular target for cancer therapeutics. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhosin, M.; Leon-Gonzalez, A.J.; Dandache, I.; Lelay, A.; Rashid, S.K.; Kevers, C.; Pincemail, J.; Fornecker, L.M.; Mauvieux, L.; Herbrecht, R.; et al. Bilberry extract (Antho 50) selectively induces redox-sensitive caspase 3-related apoptosis in chronic lymphocytic leukemia cells by targeting the Bcl-2/Bad pathway. Sci. Rep. 2015, 5, 8996. [Google Scholar] [CrossRef] [Green Version]
- Sutton, K.M.; Greenshields, A.L.; Hoskin, D.W. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer 2014, 66, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, J.; Cai, W.; Pan, Y.; Li, R.; Li, B. The Effect of Thymoquinone on Apoptosis of SK-OV-3 Ovarian Cancer Cell by Regulation of Bcl-2 and Bax. Int. J. Gynecol. Cancer 2017, 27, 1596–1601. [Google Scholar] [CrossRef]
- Park, E.J.; Chauhan, A.K.; Min, K.J.; Park, D.C.; Kwon, T.K. Thymoquinone induces apoptosis through downregulation of c-FLIP and Bcl-2 in renal carcinoma Caki cells. Oncol. Rep. 2016, 36, 2261–2267. [Google Scholar] [CrossRef] [Green Version]
- Badr, G.; Mohany, M.; Abu-Tarboush, F. Thymoquinone decreases F-actin polymerization and the proliferation of human multiple myeloma cells by suppressing STAT3 phosphorylation and Bcl2/Bcl-XL expression. Lipids Health Dis. 2011, 10, 236. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. J. Cell. Physiol. 2019, 234, 10421–10431. [Google Scholar] [CrossRef]
- Ng, W.K.; Yazan, L.S.; Ismail, M. Thymoquinone from Nigella sativa was more potent than cisplatin in eliminating of SiHa cells via apoptosis with down-regulation of Bcl-2 protein. Toxicol. Vitr. 2011, 25, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Rajendran, P.; Sethi, G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br. J. Pharmacol. 2010, 161, 541–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantino, L.; Barlocco, D. STAT 3 as a target for cancer drug discovery. Curr. Med. Chem. 2008, 15, 834–843. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, X.; Shen, J.; Hua, D. Alpinetin inhibits proliferation and migration of ovarian cancer cells via suppression of STAT3 signaling. Mol. Med. Rep. 2018, 18, 4030–4036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadabadi, M.R.; Mozafari, M.R. Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Pal, R.R.; Rajpal, V.; Singh, P.; Saraf, S.A. Recent Findings on Thymoquinone and Its Applications as a Nanocarrier for the Treatment of Cancer and Rheumatoid Arthritis. Pharmaceutics 2021, 13, 775. [Google Scholar] [CrossRef]
- Abdullah, O.; Omran, Z.; Hosawi, S.; Hamiche, A.; Bronner, C.; Alhosin, M. Thymoquinone Is a Multitarget Single Epidrug That Inhibits the UHRF1 Protein Complex. Genes 2021, 12, 622. [Google Scholar] [CrossRef]

| Gene | logFc* | p-Value |
|---|---|---|
| USP4 | −1.065 | 0.026 |
| USP7 | −0.907 | 0.05 |
| USP8 | −1.003 | 0.037 |
| USP12 | −1.067 | 0.0283 |
| USP15 | −1.19 | 0.012 |
| USP24 | −1.566 | 0.001 |
| USP32 | −1.239 | 0.01 |
| USP34 | −1.9 | 0.0000398 |
| Gene | logFc* | p-Value |
|---|---|---|
| USP27 | 0.207 | 0.84 |
| USP35 | 0.49 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhosin, M.; Abdullah, O.; Kayali, A.; Omran, Z. A Fast Ubiquitination of UHRF1 Oncogene Is a Unique Feature and a Common Mechanism of Thymoquinone in Cancer Cells. Appl. Sci. 2021, 11, 7633. https://doi.org/10.3390/app11167633
Alhosin M, Abdullah O, Kayali A, Omran Z. A Fast Ubiquitination of UHRF1 Oncogene Is a Unique Feature and a Common Mechanism of Thymoquinone in Cancer Cells. Applied Sciences. 2021; 11(16):7633. https://doi.org/10.3390/app11167633
Chicago/Turabian StyleAlhosin, Mahmoud, Omeima Abdullah, Asaad Kayali, and Ziad Omran. 2021. "A Fast Ubiquitination of UHRF1 Oncogene Is a Unique Feature and a Common Mechanism of Thymoquinone in Cancer Cells" Applied Sciences 11, no. 16: 7633. https://doi.org/10.3390/app11167633
APA StyleAlhosin, M., Abdullah, O., Kayali, A., & Omran, Z. (2021). A Fast Ubiquitination of UHRF1 Oncogene Is a Unique Feature and a Common Mechanism of Thymoquinone in Cancer Cells. Applied Sciences, 11(16), 7633. https://doi.org/10.3390/app11167633






