Alkylimidazolium Ionic Liquids Absorption and Diffusion in Wood
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
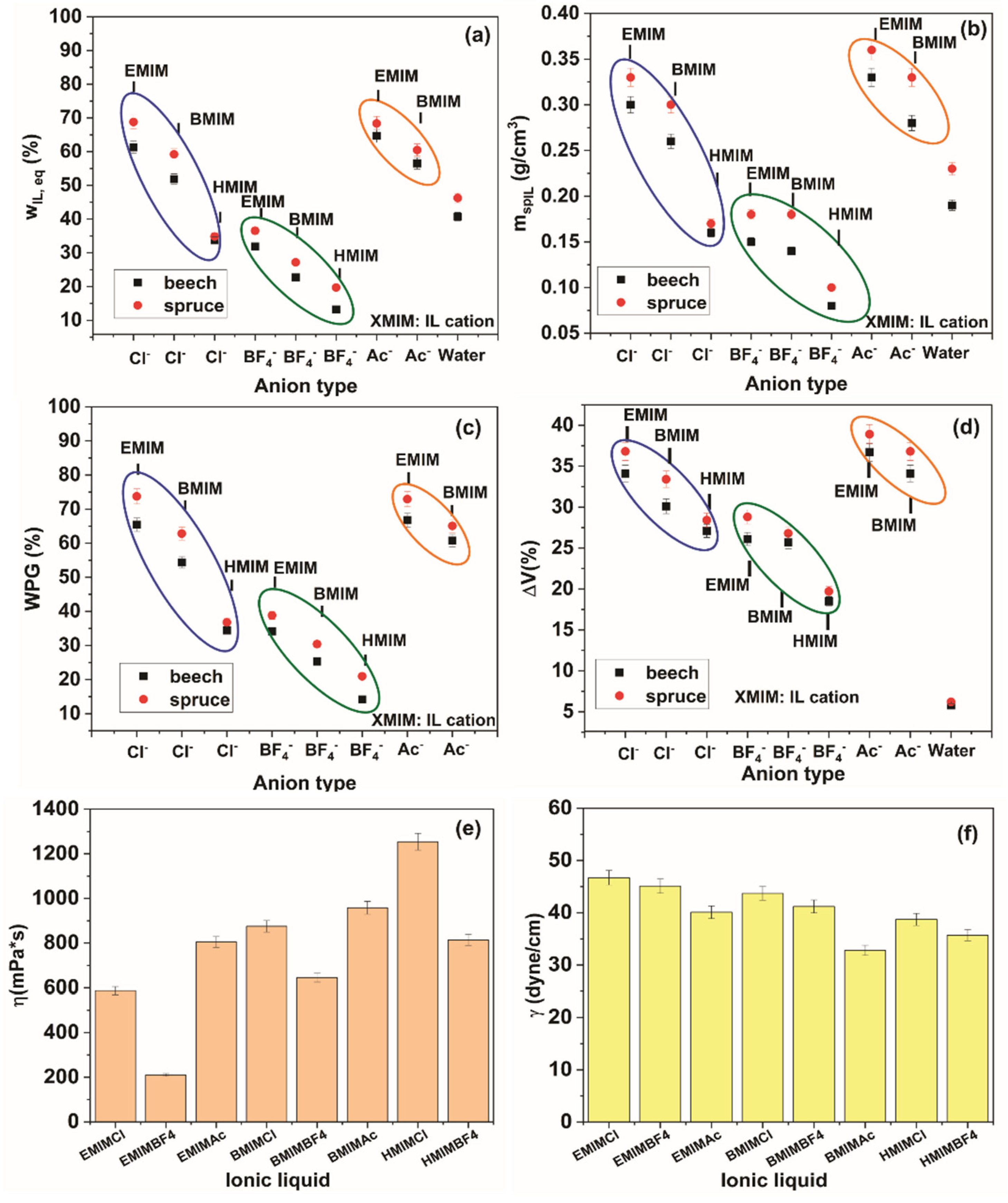
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavia, K.J. A Review of Double-Diffusion Wood Preservation Suitable for Alaska; USDA Forest Service: Washington, DC, USA, 2006; Volume 676, pp. 1–23.
- Morris, P.I.; Byrne, A.; Mackay, J.F.G.; Mcfarling, S.M. The effect of steaming prior to pressure treatment on the penetration of borates into western hemlock. For. Prod. J. 1997, 47, 62–65. [Google Scholar]
- Kartal, S.N.; Terzi, E.; Yoshimura, T. Performance of fluoride and boron compounds against drywood and subterranean termites and decay and mold fungi. J. For. Res. 2019, 31, 1425–1434. [Google Scholar] [CrossRef]
- Samani, A.; Ganguly, S.; Kanyal, R.; Tripathi, S. Effect of microwave pre-treatment on preservative retention and treatability of Melia composita wood. J. For. Sci. 2019, 65, 391–396. [Google Scholar] [CrossRef]
- Ela, R.C.A.; Chipkar, S.H.; Bal, T.L.; Xie, X.; Ong, R.G. Lignin–Propiconazole Nanocapsules are an Effective Bio-Based Wood Preservative. ACS Sustain. Chem. Eng. 2021, 9, 2684–2692. [Google Scholar] [CrossRef]
- Jeeshma, V.J.; Anoop, E.V.; Dhamodaran, T.K.; Vidyasagaran, K.; Kunhamu, T.K.; Gopakumar, S. Diffusion treatment of coconut palm wood using organic preservatives. J. Trop. Agric. 2018, 56, 69–72. [Google Scholar]
- Persi, E.; Petaccia, G.; Sibilla, S.; Bentivoglio, R.; Armanini, A. A One-Way Coupled Hydrodynamic Advection-Diffusion Model to Simulate Congested Large Wood Transport. Hydrology 2021, 8, 21. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Houtman, C.J.; Hirth, K.; Lacher, S.; Lorenz, L.; Thybring, E.E.; Hunt, C.G. The Effect of Acetylation on Iron Uptake and Diffusion in Water Saturated Wood Cell Walls and Implications for Decay. Forests 2020, 11, 1121. [Google Scholar] [CrossRef]
- Davis, K.A.; Van Hise, J.R. Comment on “Predicting boron diffusion in wood from surface sorption”. For. Prod. J. 2006, 56, 38–39. [Google Scholar]
- Ra, J.B.; Barnes, H.M.; Conners, T.E. Predicting boron diffusion in wood from surface sorption. For. Prod. J. 2002, 52, 67–70. [Google Scholar]
- Rabbi, M.F.; Islam, M.M.; Rahman, A.N.M. Wood Preservation: Improvement of Mechanical Properties by Vacuum Pressure Process. Int. J. Eng. Appl. Sci. 2017, 2, 75–79. [Google Scholar]
- Kilpeläinen, I.; Xie, H.; King, A.; Granstrom, M.; Heikkinen, S.; Argyropoulos, D. Dissolution of Wood in Ionic Liquids. J. Agric. Food Chem. 2007, 55, 9142–9148. [Google Scholar] [CrossRef]
- Croitoru, C.; Roata, I.C. Ionic Liquids as Antifungal Agents for Wood Preservation. Molecules 2020, 25, 4289. [Google Scholar] [CrossRef] [PubMed]
- Höhne, P.; Tauer, K. Studies on swelling of wood with water and ionic liquids. Wood Sci. Technol. 2016, 50, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Croitoru, C.; Patachia, S.; Porzsolt, A.; Friedrich, C. Effect of alkylimidazolium based ionic liquids on the structure of UV-irradiated cellulose. Cellulose 2011, 18, 1469–1479. [Google Scholar] [CrossRef]
- Croitoru, C.; Patachia, S.; Lunguleasa, A. New Method of Wood Impregnation with Inorganic Compounds Using Ethyl Methylimidazolium Chloride as Carrier. J. Wood Chem. Technol. 2014, 35, 113–128. [Google Scholar] [CrossRef]
- Muhammad, N.; Man, Z.; Mutalib, M.I.A.; Bustam, M.A.; Wilfred, C.D.; Khan, A.S.; Ullah, Z.; Gonfa, G.; Nasrullah, A. Dissolution and Separation of Wood Biopolymers Using Ionic Liquids. ChemBioEng Rev. 2015, 2, 257–278. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review on the Partial and Complete Dissolution and Fractionation of Wood and Lignocelluloses Using Imidazolium Ionic Liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Geng, Y.; Simonsen, J.; Li, K. Application of ionic liquids for electrostatic control in wood. Holzforschung 2004, 58, 280–285. [Google Scholar] [CrossRef]
- Abu-Eishah, S.I. Utilization of Ionic Liquids in Wood and Wood-Related Applications—A Review. In Ionic Liquids—Current State of the Art; IntechOpen: London, UK, 2015. [Google Scholar]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic Liquids. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 218–225. [Google Scholar]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, H. Ionic liquids in the pretreatment of lignocellulosic biomass. Acta Innov. 2021, 38, 23–36. [Google Scholar] [CrossRef]
- Croitoru, C.; Patachia, S.; Doroftei, F.; Parparita, E.; Vasile, C. Ionic liquids influence on the surface properties of electron beam irradiated wood. Appl. Surf. Sci. 2014, 314, 956–966. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Xie, Y.; Wang, Q.; Sui, S.; Wolcott, M.P. Thermoplastic deformation of poplar wood plasticized by ionic liquids measured by a nonisothermal compression technique. Holzforschung 2014, 68, 555–566. [Google Scholar] [CrossRef]
- de Meijer, M.; Militz, H. Moisture transport in coated wood. Part 1: Analysis of sorption rates and moisture content profiles in spruce during liquid water uptake. Eur. J. Wood Prod. 2000, 58, 354–362. [Google Scholar] [CrossRef]
- Babiak, M.; Pavlíková, M.; Jokel, J. Modelling of the Transport of Water in Wood. IFAC Proc. Vol. 1986, 19, 139–144. [Google Scholar] [CrossRef]
- Dutta, T.; Shi, J.; Sun, J.; Zhang, X.; Cheng, G.; Simmons, B.; Singh, S. CHAPTER 3. Ionic Liquid Pretreatment of Lignocellulosic Biomass for Biofuels and Chemicals. In Ionic Liquids in the Biorefinery Concept; Green Chemistry Series; Royal Society of Chemistry (RSC): London, UK, 2015; Volume 2016, pp. 65–94. [Google Scholar]
- Ou, R.; Xie, Y.; Wang, Q.; Sui, S.; Wolcott, M.P. Material pocket dynamic mechanical analysis: A novel tool to study thermal transition in wood fibers plasticized by an ionic liquid (IL). Holzforschung 2015, 69, 223–232. [Google Scholar] [CrossRef]
- Baraúna, E.E.P.; Paes, J.B.; Monteiro, T.C.; Moulin, J.C.; Ferreira, G.L.; Silveira, A.G.; Baldin, T.; Junior, C.R.S.; Arantes, M.D.C. Influence of the impregnation with boron compounds on the physical properties of Eucalyptus wood. Sci. For. 2020, 48. [Google Scholar] [CrossRef]
- Fredriksson, M.; Thybring, E.E. On sorption hysteresis in wood: Separating hysteresis in cell wall water and capillary water in the full moisture range. PLoS ONE 2019, 14, e0225111. [Google Scholar] [CrossRef]
- Pop, M.A.; Croitoru, C.; Bedo, T.; Geamăn, V.; Radomir, I.; Zaharia, S.M.; Chicoș, L.A. Influence of Internal Innovative Architecture on the Mechanical Properties of 3D Polymer Printed Parts. Polymers 2020, 12, 1129. [Google Scholar] [CrossRef]
- Simpson, W.; TenWolde, A. Physical properties and moisture relations of wood. In Wood Handbook: Wood as an Engineering Material; Forest Products Laboratory: Madison, WI, USA, 1999. [Google Scholar]
- Glass Samuel, V.; Zelinka, S.L. Wood Handbook, Moisture Relations and Physical Properties of Wood. In Wood Handbook: Wood as an Engineering Material; Forest Products Laboratory: Madison, WI, USA, 2010; Chapter 4. [Google Scholar]
- Elaieb, M.T.; Shel, F.; Jalleli, M.; Langbour, P.; Candelier, K. Physical properties of four ring-porous hardwood species: Influence of wood rays on tangential and radial wood shrinkage. Madera y Bosques 2019, 25, e2521695. [Google Scholar] [CrossRef]
- Li, M.; He, B.; Li, J.; Zhao, L. Physico-chemical Characterization and Comparison of Microcrystalline Cellulose from Several Lignocellulosic Sources. BioResources 2019, 14. [Google Scholar] [CrossRef]
- Penttilä, P.A.; Altgen, M.; Carl, N.; Van Der Linden, P.; Morfin, I.; Österberg, M.; Schweins, R.; Rautkari, L. Moisture-related changes in the nanostructure of woods studied with X-ray and neutron scattering. Cellulose 2020, 27, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Onkarappa, H.S.; Prakash, G.K.; Pujar, G.H.; Kumar, C.R.R.; Radha, V.; Latha, M.S.; Betageri, V.S. Synthesis and characterization of nanocellulose using renewable resources through Ionic liquid medium. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 035001. [Google Scholar] [CrossRef]
- Sharma, V.; Yadav, J.; Kumar, R.; Tesarova, D.; Ekielski, A.; Mishra, P.K. On the rapid and non-destructive approach for wood identification using ATR-FTIR spectroscopy and chemometric methods. Vib. Spectrosc. 2020, 110, 103097. [Google Scholar] [CrossRef]
- Lourenço, A.; Araújo, S.; Gominho, J.; Evtuguin, D. Cellulose Structural Changes during Mild Torrefaction of Eucalyptus Wood. Polymers 2020, 12, 2831. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-L.; Li, S.; Sun, Y.-X.; Han, H.-Y.; Zhang, B.-X.; Hu, B.-Z.; Gao, Y.-F.; Hu, X.-M. Ionic liquids as efficient pretreatment solvents for lignocellulosic biomass. RSC Adv. 2017, 7, 47990–47998. [Google Scholar] [CrossRef] [Green Version]


| IL-Related Properties (Independent Variable) | Statistical Analysis Parameters * | Wood-Related Properties (Dependent Variable) | ||||
|---|---|---|---|---|---|---|
| Spruce | Beech | |||||
| wIL,eq | ΔV | wIL,eq | ΔV | |||
| γ | Normality | p | 0.140 | 0.365 | 0.261 | 0.554 |
| Correlation | P | −0.031 | −0.258 | −0.118 | 0.017 | |
| R | 0.953 | 0.621 | 0.824 | 0.890 | ||
| η | Normality | p | 0.145 | 0.332 | 0.282 | 0.483 |
| Correlation | P | 0.099 | −0.306 | 0.018 | −0.368 | |
| r | −0.851 | −0.555 | −0.729 | −0.473 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croitoru, C.; Roata, I.C. Alkylimidazolium Ionic Liquids Absorption and Diffusion in Wood. Appl. Sci. 2021, 11, 7640. https://doi.org/10.3390/app11167640
Croitoru C, Roata IC. Alkylimidazolium Ionic Liquids Absorption and Diffusion in Wood. Applied Sciences. 2021; 11(16):7640. https://doi.org/10.3390/app11167640
Chicago/Turabian StyleCroitoru, Catalin, and Ionut Claudiu Roata. 2021. "Alkylimidazolium Ionic Liquids Absorption and Diffusion in Wood" Applied Sciences 11, no. 16: 7640. https://doi.org/10.3390/app11167640







