Multi-Technique Characterization of Pictorial Organic Binders on XV Century Polychrome Sculptures by Combining Micro- and Non-Invasive Sampling Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Dual-Enzyme In Situ Protocol
2.3. Bligh and Dyer Extraction Protocol
2.4. Enzymatic Digestion
2.5. MALDI-MS(/MS)
2.6. RPLC-ESI-MS(/MS)
2.7. ATR-FTIR
2.8. Data Processing
3. Results and Discussion
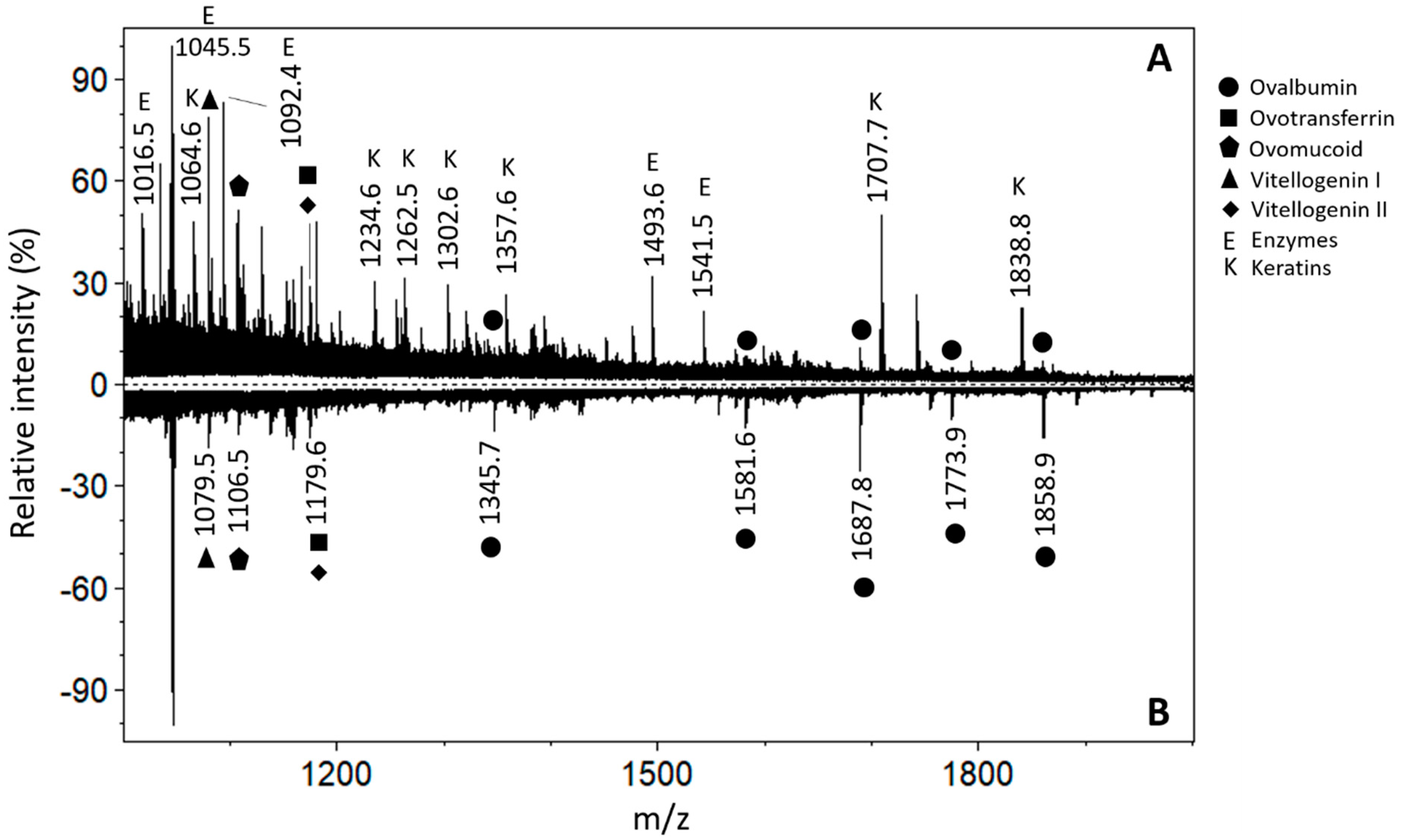
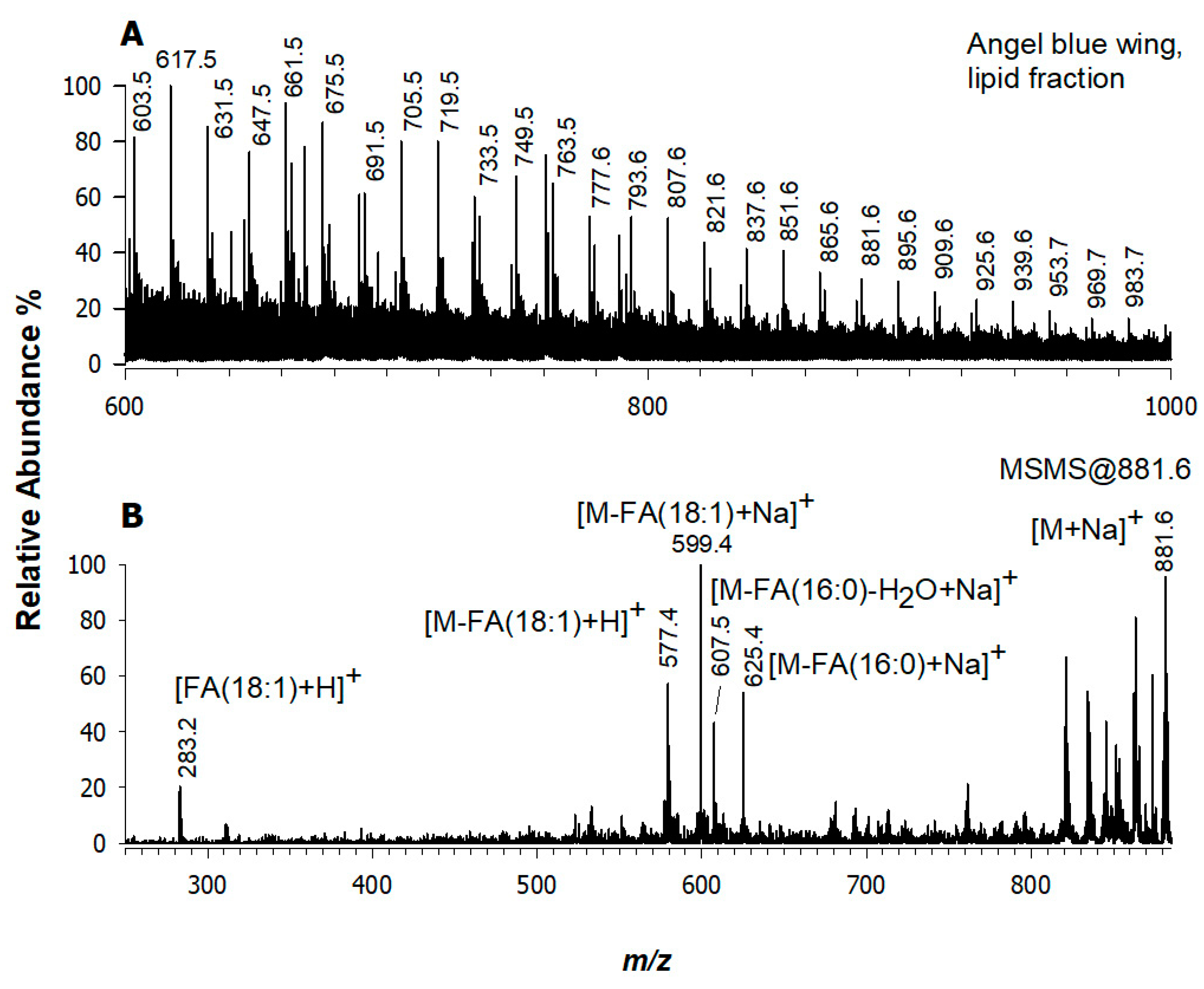
3.1. Angel’s Wing, Blue Layer (Sampled Point 1)
3.2. Angel’s Viola, Brown Layer (Sampled Point 2)
3.3. Angel’s Blouse, Blue Layer (Sampled Point 3)
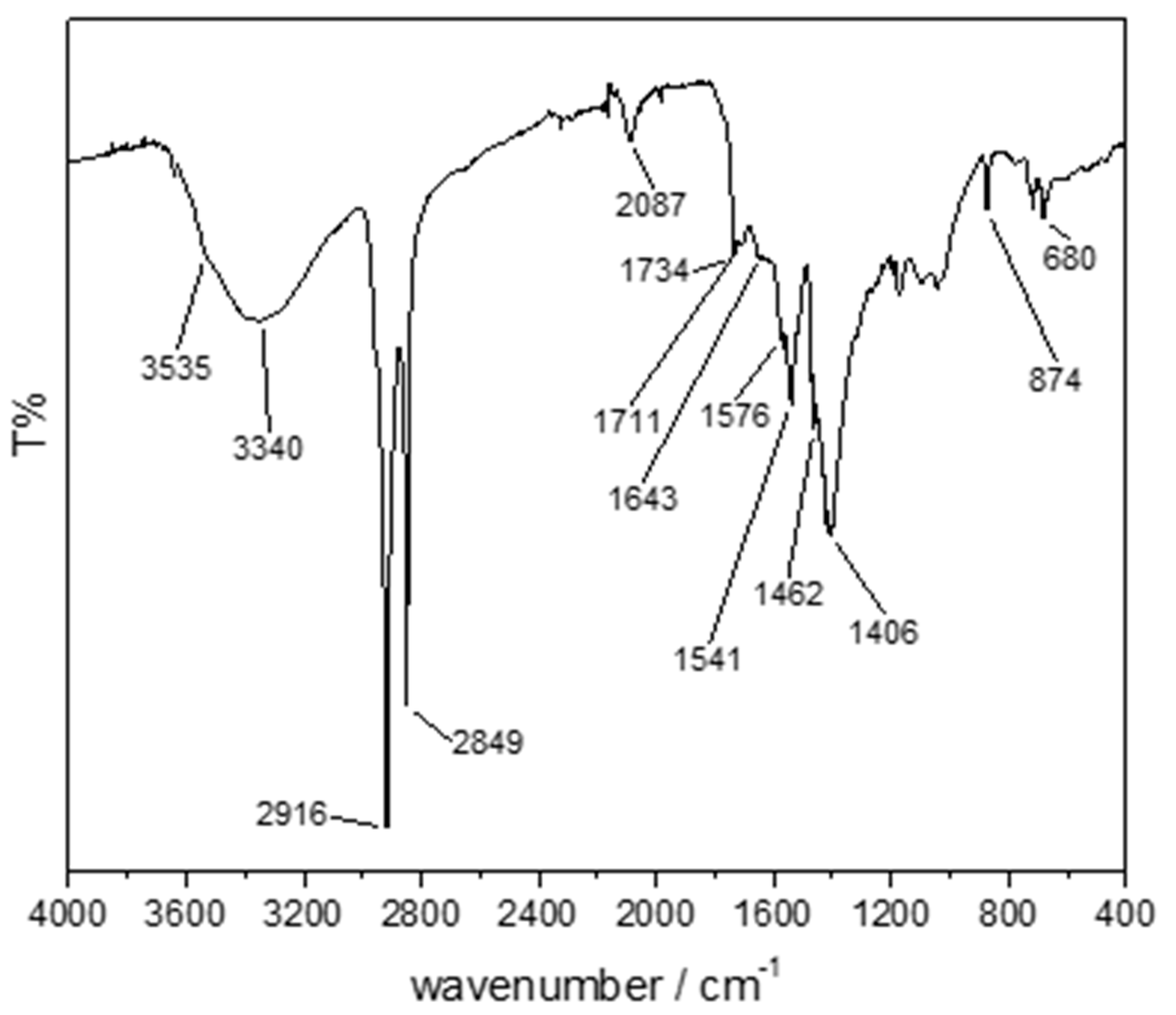
3.4. Lying Lamb (Sampled Point 4)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campanella, L.; Casoli, A.; Colombini, M.P.; Bettolo, R.M.; Matteini, M.; Migneco, L.M.; Montenero, A.; Nodari, L.; Piccioli, C.; Zappalà, M.P.; et al. Chimica per L’arte; Zanichelli: Bologna, Italy, 2007; ISBN 9788808068538. [Google Scholar]
- Previati, G. La Tecnica della Pittura; Sugarco: Milano, Italy, 1905. [Google Scholar]
- Cennini, C. Il Libro Dell’arte, o Trattato della Pittura di Cennino Cennini; F. Le Monnier: Firenze, Italy; ISBN 9788828101482.
- Vasari, G. Le Vite de’ Piú Eccellenti Architetti, Pittori, et Scultori Italiani, da Cimabue Insino a’ Tempi Nostri Nell’edizione per i Tipi di Lorenzo Torrentino; Einaudi: Torino, Italy; ISBN 88-06-59659-4.
- Piqué, F.; Verri, G. Organic Materials in Wall Paintings; Getty Conservation Institute: Los Angeles, CA, USA, 2015; ISBN 9781937433291. [Google Scholar]
- Arie, W.; Hermens, E.; Peek, M. (Eds.) Historical Painting Techniques, Materials, and Studio Practice: Preprints of a Symposium Held at the University of Leiden, the Netherlands, 26–29 June, 1995; Getty Conservation Institute: Marina Del Rey, CA, USA, 1995; ISBN 0892363223. Available online: http://hdl.handle.net/10020/gci_pubs/historical_painting (accessed on 26 August 2021).
- Kuckova, S.; Sandu, I.C.A.; Crhova, M.; Hynek, R.; Fogas, I.; Muralha, V.S.; Sandu, A.V. Complementary cross-section based protocol of investigation of polychrome samples of a 16th century Moravian Sculpture by optical, vibrational and mass spectrometric techniques. Microchem. J. 2013, 110, 538–544. [Google Scholar] [CrossRef]
- Kuckova, S.; Sandu, I.C.A.; Crhova, M.; Hynek, R.; Fogas, I.; Schafer, S. Protein identification and localization using mass spectrometry and staining tests in cross-sections of polychrome samples. J. Cult. Herit. 2013, 14, 31–37. [Google Scholar] [CrossRef]
- Franquelo, M.L.; Duran, A.; Castaing, J.; Arquillo, D.; Perez-Rodriguez, J.L. XRF, μ-XRD and μ-spectroscopic techniques for revealing the composition and structure of paint layers on polychrome sculptures after multiple restorations. Talanta 2012, 89, 462–469. [Google Scholar] [CrossRef]
- Conti, C.; Colombo, C.; Realini, M.; Matousek, P. Subsurface analysis of painted sculptures and plasters using micrometre-scale spatially offset Raman spectroscopy (micro-SORS). J. Raman Spectrosc. 2015, 46, 476–482. [Google Scholar] [CrossRef]
- Egel, E.; Simon, S. Investigation of the painting materials in Zhongshan Grottoes (Shaanxi, China). Herit. Sci. 2013, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Križnar, A.; Muñoz, M.V.; de la Paz, F.; Respaldiza, M.A.; Vega, M. XRF analysis of two terracotta polychrome sculptures by pietro torrigiano. X-ray Spectrom. 2009, 38, 169–174. [Google Scholar] [CrossRef]
- Pinna, D.; Conti, C.; Mazurek, J. Polychrome sculptures of medieval Italian monuments: Study of the binding media and pigments. Microchem. J. 2020, 158, 105100. [Google Scholar] [CrossRef]
- Casoli, A.; Musini, P.C.; Palla, G. A study for the characterization of binding media from medieval polychrome sculptures by gas chromatography-mass spectrometry. Chromatographia 1996, 42, 421–430. [Google Scholar] [CrossRef]
- Wang, X.; Zhen, G.; Hao, X.; Tong, T.; Ni, F.; Wang, Z.; Jia, J.; Li, L.; Tong, H. Spectroscopic investigation and comprehensive analysis of the polychrome clay sculpture of Hua Yan Temple of the Liao Dynasty. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2020, 240. [Google Scholar] [CrossRef]
- Chen, E.; Zhang, B.; Zhao, F. Comprehensive analysis of polychrome grotto relics: A case study of the paint layers from Anyue, Sichuan, China. Anal. Lett. 2020, 53, 1455–1471. [Google Scholar] [CrossRef]
- Gelao, C.; Tragni, B. Il Presepe Pugliese. arte e Folklore; Adda, 2000. [Google Scholar]
- Fabris, D. Presepi scultorei con strumenti musicali del Cinquecento in Puglia. RIdIM/RCMI Newsl. 1991, 16, 8. [Google Scholar]
- Saponaro, M. Il Presepe Cinquecentesco della Cattedrale di Altamura; Torre di Nebbia: Bari, Italy, 2007. [Google Scholar]
- Calvano, C.D.; Rigante, E.; Picca, R.A.; Cataldi, T.R.I.; Sabbatini, L. An easily transferable protocol for in-situ quasi-non-invasive analysis of protein binders in works of art. Talanta 2020, 215, 120882. [Google Scholar] [CrossRef]
- Calvano, C.D.; Rigante, E.C.L.; Cataldi, T.R.I.; Sabbatini, L. In situ hydrogel extraction with dual-enzyme digestion of proteinaceous binders: The key for reliable mass spectrometry investigations of artworks. Anal. Chem. 2020, 92, 10257–10261. [Google Scholar] [CrossRef]
- Bligh, E.G. and Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Van Der Werf, I.D.; Calvano, C.D.; Palmisano, F.; Sabbatini, L. A simple protocol for Matrix Assisted Laser Desorption Ionization- time of flight-mass spectrometry (MALDI-TOF-MS) analysis of lipids and proteins in single microsamples of paintings. Anal. Chim. Acta 2012, 718, 1–10. [Google Scholar] [CrossRef]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative hydrogels based on semi-interpenetrating p(HEMA)/PVP networks for the cleaning of water-sensitive cultural heritage artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef]
- Micheli, L.; Mazzuca, C.; Missori, M.; Teodonio, L.; Mosca Conte, A.; Pulci, O.; Arcadipane, L.; Dominijanni, S.; Palleschi, A.; Palleschi, G.; et al. Interdisciplinary approach to develop a disposable real time monitoring tool for the cleaning of graphic artworks. Application on “le Nozze di Psiche”. Microchem. J. 2018, 138, 369–378. [Google Scholar] [CrossRef]
- Baglioni, P.; Baglioni, M.; Bonelli, N.; Chelazzi, D.; Giorgi, R. Smart Soft Nanomaterials for Cleaning; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128139110. [Google Scholar]
- Mazzuca, C.; Poggi, G.; Bonelli, N.; Micheli, L.; Baglioni, P.; Palleschi, A. Innovative chemical gels meet enzymes: A smart combination for cleaning paper artworks. J. Colloid Interface Sci. 2017, 502, 153–164. [Google Scholar] [CrossRef]
- Leo, G.; Bonaduce, I.; Andreotti, A.; Marino, G.; Pucci, P.; Colombini, M.P.; Birolo, L. Deamidation at asparagine and glutamine as a major modification upon deterioration/aging of proteinaceous binders in mural paintings. Anal. Chem. 2011, 83, 2056–2064. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Doménech-Carbó, M.T.; Osete-Cortina, L.; Donnici, M.; Guasch-Ferré, N.; Gasol-Fargas, R.M.; Iglesias-Campos, M.Á. Electrochemical assessment of pigments-binding medium interactions in oil paint deterioration: A case study on indigo and Prussian blue. Herit. Sci. 2020, 8, 71. [Google Scholar] [CrossRef]
- Fermo, P.; Mearini, A.; Bonomi, R.; Arrighetti, E.; Comite, V. An integrated analytical approach for the characterization of repainted wooden statues dated to the fifteenth century. Microchem. J. 2020, 157, 105072. [Google Scholar] [CrossRef]
- Samain, L.; Silversmit, G.; Sanyova, J.; Vekemans, B.; Salomon, H.; Gilbert, B.; Grandjean, F.; Long, G.J.; Hermann, R.P.; Vincze, L.; et al. Fading of modern Prussian blue pigments in linseed oil medium. J. Anal. At. Spectrom. 2011, 26, 930–941. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, R.; Prati, S.; Quaranta, M.; Joseph, E.; Kendix, E.; Galeotti, M. Attenuated total reflection micro FTIR characterisation of pigment-binder interaction in reconstructed paint films. Anal. Bioanal. Chem. 2008, 392, 65–76. [Google Scholar] [CrossRef]
- Calvano, C.D.; Van Der Werf, I.D.; Palmisano, F.; Sabbatini, L. Fingerprinting of egg and oil binders in painted artworks by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis of lipid oxidation by-products. Anal. Bioanal. Chem. 2011, 400, 2229–2240. [Google Scholar] [CrossRef]
- Picariello, G.; Paduano, A.; Sacchi, R.; Addeo, F. MALDI-TOF mass spectrometry profiling of polar and nonpolar fractions in heated vegetable oils. J. Agric. Food Chem. 2009, 57, 5391–5400. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Zubiaguirre, L.; Ribechini, E.; Degano, I.; La Nasa, J.; Carrero, J.A.; Iñañez, J.; Olivares, M.; Castro, K. GC–MS and HPLC-ESI-QToF characterization of organic lipid residues from ceramic vessels used by Basque whalers from 16th to 17th centuries. Microchem. J. 2018, 137, 190–203. [Google Scholar] [CrossRef]
- Herrera Cubero, L.; Potvin, M.A.; Melanson, J.E. Quantitative analysis of positional isomers of triacylglycerols via electrospray ionization tandem mass spectrometry of sodiated adducts. Rapid Commun. Mass Spectrom. 2010, 24, 2745–2752. [Google Scholar] [CrossRef] [Green Version]
- Balgoma, D.; Guitton, Y.; Evans, J.J.; Le Bizec, B.; Dervilly-Pinel, G.; Meynier, A. Modeling the fragmentation patterns of triacylglycerides in mass spectrometry allows the quantification of the regioisomers with a minimal number of standards. Anal. Chim. Acta 2019, 1057, 60–69. [Google Scholar] [CrossRef]
- Fremout, W.; Kuckova, S.; Crhova, M.; Sanyova, J.; Saverwyns, S.; Hynek, R.; Kodicek, M.; Vandenabeele, P.; Moens, L. Classification of protein binders in artist’s paints by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry: An evaluation of principal component analysis (PCA) and soft independent modelling of class analogy (SIMCA). Rapid Commun. Mass Spectrom. 2011, 25, 1631–1640. [Google Scholar] [CrossRef]
- Cicatiello, P.; Ntasi, G.; Rossi, M.; Marino, G.; Giardina, P.; Birolo, L. Minimally invasive and portable method for the identification of proteins in ancient paintings. Anal. Chem. 2018, 90, 10128–10133. [Google Scholar] [CrossRef]
- Dallongeville, S.; Koperska, M.; Garnier, N.; Reille-Taillefert, G.; Rolando, C.; Tokarski, C. Identification of animal glue species in artworks using proteomics: Application to a 18th century gilt sample. Anal. Chem. 2011, 83, 9431–9437. [Google Scholar] [CrossRef]
- van der Werf, I.D.; Calvano, C.D.; Germinario, G.; Cataldi, T.R.I.; Sabbatini, L. Chemical characterization of medieval illuminated parchment scrolls. Microchem. J. 2017, 134, 146–153. [Google Scholar] [CrossRef]
- Hess, G.P.; McConn, J.; Ku, E.; McConkey, G. Studies of the activity of chymotrypsin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1970, 257, 89–104. [Google Scholar] [CrossRef]
- Fuchs, B.; Schiller, J.; Süß, R.; Nimptsch, A.; Schürenberg, M.; Suckau, D. Capabilities and disadvantages of combined matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and high-performance thin-layer chromatography (HPTLC): Analysis of egg yolk lipids. J. Planar Chromatogr. Mod. TLC 2009, 22, 35–42. [Google Scholar] [CrossRef]
- Teuber, K.; Schiller, J.; Fuchs, B.; Karas, M.; Jaskolla, T.W. Significant sensitivity improvements by matrix optimization: A MALDI-TOF mass spectrometric study of lipids from hen egg yolk. Chem. Phys. Lipids 2010, 163, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Kang, S.-J.; Evans, J.E.; Cresswell, P. Natural Lipid Ligands Associated with Human CD1d Targeted to Different Subcellular Compartments. J. Immunol. 2009, 182, 4784–4791. [Google Scholar] [CrossRef] [Green Version]
- Harvey, D.J. Matrix-assisted Laser Desorption/Ionization Mass Spectrometry of Phospholipids. J. Mass Spectrom. 1995, 30, 1333–1346. [Google Scholar] [CrossRef]
- Calvano, C.D.; van der Werf, I.D.; Sabbatini, L.; Palmisano, F. On plate graphite supported sample processing for simultaneous lipid and protein identification by matrix assisted laser desorption ionization mass spectrometry. Talanta 2015, 137, 161–166. [Google Scholar] [CrossRef]
- Meilunas, R.J.; Bentsen, J.G.; Steinberg, A. Analysis of aged paint binders by ftir spectroscopy. Stud. Conserv. 1990, 35, 33–51. [Google Scholar] [CrossRef]
- Švarcová, S.; Kočí, E.; Plocek, J.; Zhankina, A.; Hradilová, J.; Bezdička, P. Saponification in egg yolk-based tempera paintings with lead-tin yellow type I. J. Cult. Herit. 2019, 38, 8–19. [Google Scholar] [CrossRef]
- Sotiropoulou, S.; Sciutto, G.; Tenorio, A.L.; Mazurek, J.; Bonaduce, I.; Prati, S.; Mazzeo, R.; Schilling, M.; Colombini, M.P. Advanced analytical investigation on degradation markers in wall paintings. Microchem. J. 2018, 139, 278–294. [Google Scholar] [CrossRef] [Green Version]
- Vahur, S.; Teearu, A.; Peets, P.; Joosu, L.; Leito, I. ATR-FT-IR spectral collection of conservation materials in the extended region of 4000-80 cm–1. Anal. Bioanal. Chem. 2016, 408, 3373–3379. [Google Scholar] [CrossRef] [PubMed]
- Database of ATR-FT-IR Spectra of Various Materials. Available online: https://spectra.chem.ut.ee/paint/pigments/italian-gold-ochre-light/ (accessed on 26 July 2021).
- Getty Conservation Institute. IMP00034, Ochre, Avana w/calcite, gypsum, Pigment Yellow 43 (PY43), CI77492” Ed. Beth, A. Price, Boris Pretzel and Susanne Quillen Lomax. Infrared and Raman Users Group Spectral Database. Infrared and Raman Users Group. 1992. Available online: http://www.irug.org/ (accessed on 26 July 2021).
- Rampazzi, L.; Corti, C. Are commercial pigments reliable references for the analysis of paintings? Int. J. Conserv. Sci. 2019, 10, 217–220. [Google Scholar]
- Guglielmi, V.; Fermo, P.; Andreoli, M.; Comite, V. A multi-analytical survey for the identification of the red and yellow pigments of coloured sherds discovered in the Monte d’Oro area (Rome). In Proceedings of the 2020 IMEKO TC-4 International Conference on Metrology for Archaeology and Cultural Heritage, Trento, Italy, 22–24 October 2020; pp. 548–553. [Google Scholar] [CrossRef]
- Saraiva, S.A.; Cabral, E.C.; Eberlin, M.N.; Catharino, R.R. Amazonian vegetable oils and fats: Fast typification and quality control via triacylglycerol (TAG) profiles from dry matrix-assisted laser desorption/lonization time-of-flight (MALDI-TOF) mass spectrometry fingerprinting. J. Agric. Food Chem. 2009, 57, 4030–4034. [Google Scholar] [CrossRef]
- Vereshchagin, A.G.; Novitskaya, G.V. The composition of linseed oil. J. Am. Oil Chem. Soc. 1965, 42, 970–974. [Google Scholar] [CrossRef]
- Tirat, S.; Degano, I.; Echard, J.P.; Lattuati-Derieux, A.; Lluveras-Tenorio, A.; Marie, A.; Serfaty, S.; Le Huerou, J.Y. Historical linseed oil/colophony varnishes formulations: Study of their molecular composition with micro-chemical chromatographic techniques. Microchem. J. 2016, 126, 200–213. [Google Scholar] [CrossRef]


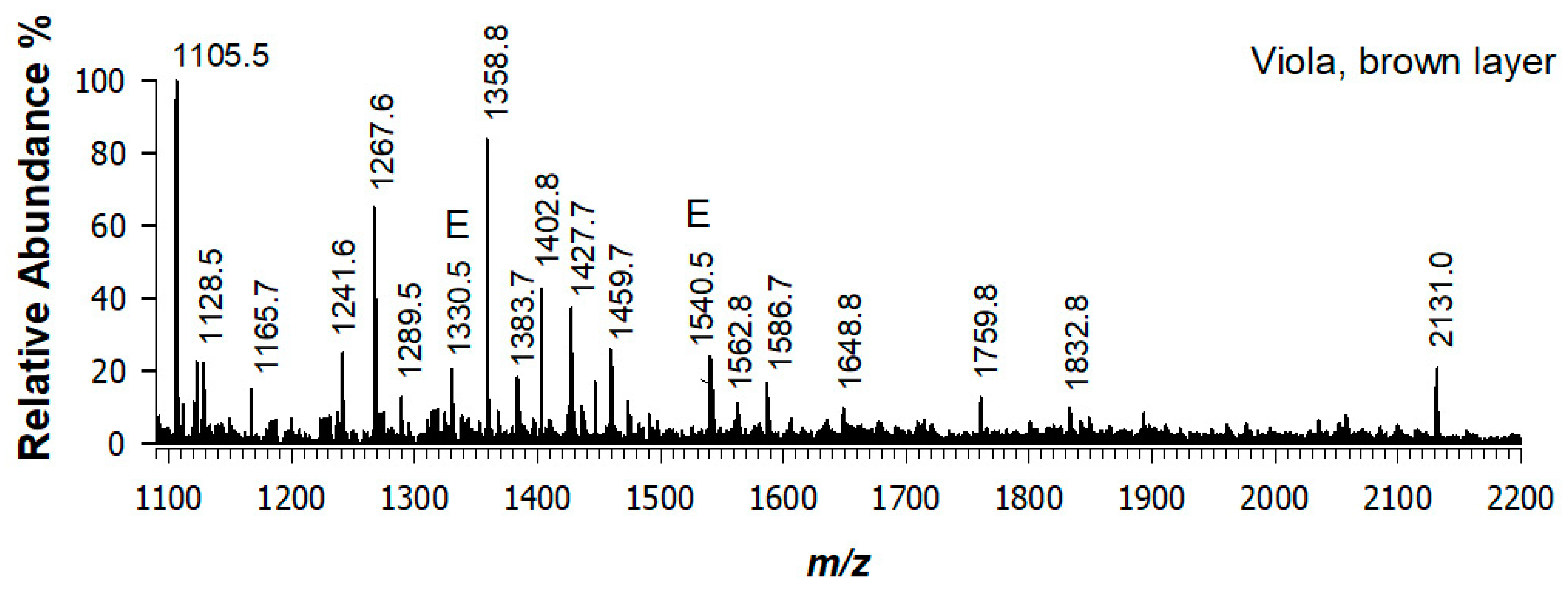


| Experimental m/z | Theoretical m/z | Sequence | Identified Protein |
|---|---|---|---|
| 1105.53 | 1105.57 | (R)GVQGPPGPAGPR(G) + 1 ox | Collagen alpha-1(I) |
| 1128.50 | 1128.55 1128.55 | (R)GLPGTPGTDGPK(G) + 2 ox (R)GLAGPPGMPGAR(G) + 3 ox | Collagen alpha-1(II) Collagen alpha-1(III) |
| 1241.59 | 1241.60 | (R)GSPGGPGAAGFPGGR(G) | Collagen alpha-1(III) |
| 1267.62 | 1267.68 | (R)GIPGPVGAAGATGAR(G) + 1 ox | Collagen alpha-2(I) |
| 1289.53 | 1289.59 | (R)GSPGGPGAAGFPGGR(G) + 3 ox | Collagen alpha-1(III) |
| 1358.75 | 1358.67 | (R)ADDANVVRDRDL(E) | Collagen alpha-1(I) |
| 1427.73 | 1427.70 1427.73 1427.79 | (R)GSAGPPGATGFPGAAGR(V) (R)GIPGEFGLPGPAGAR(G) + 2 ox (K)ALLIQGSNDVEIR(A) | Collagen alpha-1(I) Collagen alpha-2(I) Collagen alpha-1(II) |
| 1435.69 | 1435.68 1435.69 | (R)GEPGPAGLPGPPGER(G) + 3 ox (R)GPPGPPGTNGVPGQR(G) + 3 ox | Collagen alpha-1(I) Collagen alpha-1(III) |
| 1459.70 | 1459.69 | (R)GSAGPPGATGFPGAAGR(V) + 2 ox | Collagen alpha-1(I) |
| 1473.68 | 1473.67 | (R)GDGGPPGATGFPGAAGR(T) + 2 ox | Collagen alpha-2(I) |
| 1560.67 | 1560.73 | (K)STGISVPGPMGPSGPR(G) + 4 ox | Collagen alpha-1(I) |
| 1562.80 | 1562.79 1562.83 | (R)GDKGETGEQGDRGIK(G) + 1 ox (K)GAAGLPGVAGAPGLPGPR(G) + 3 ox | Collagen alpha-1(I) Collagen alpha-2(I) |
| 1586.74 | 1586.74 1586.70 | (K)GNSGEPGAPGSKGDTGAK(G) (R)GETGPAGPSGAPGPAGSR(G) + 4 ox | Collagen alpha-1(I) Collagen alpha-1(III) |
| 1648.79 | 1648.78 | (K)AGEDGHPGKPGRPGER(G) + 2 ox | Collagen alpha-2(I) |
| 1759.79 | 1759.82 | (K)GEPGPAGPQGAPGPAGEEGK(R) | Collagen alpha-1(II) |
| 1832.80 | 1832.81 | (R)GPPGPMGPPGLAGPPGESGR(E) + 3 ox | Collagen alpha-1(I) |
| 1922.94 | 1922.93 1922.86 | (R)GERGPPGESGAAGPTGPIGSR(G) + 1 ox (K)GDSGAPGERGPPGAGGPPGPR(G) + 5 ox | Collagen alpha-2(I) Collagen alpha-1(III) |
| 1975.90 | 1975.99 1975.95 1976.03 | (K)SGDRGETGPAGPAGPIGPVGAR(G) (K)GEPGAVGQPGPPGPSGEEGKR(G) + 1 ox (R)GPPGPQGARGFPGTPGLPGVK(G) + 2 ox | Collagen alpha-1(I) Collagen alpha-2(I) Collagen alpha-1(II) |
| 2131.01 | 2131.03 2131.11 2130.95 | (R)GVQGPPGPAGPRGANGAPGNDGAK(G) + 2 ox (R)GLPGVAGSVGEPGPLGIAGPPGAR(G) + 3 ox (R)GMPGPQGPRGDKGETGEAGER(G) + 3 ox | Collagen alpha-1(I) Collagen alpha-2(I) Collagen alpha-1(II) |
| Sampled Zone | Components | Analytical Techniques |
|---|---|---|
| Angel’s wing, blue layer | Prussian Blue (FeIII4[FeII(CN)6]3) Linseed oil Egg white | ATR-FTIR ATR-FTIR, MALDI-MS(/MS) MALDI-MS, RPLC-ESI-MS/MS |
| Angel’s viola, brown layer | Animal collagen | MALDI-MS |
| Angel’s blouse, blue layer | Prussian Blue (FeIII4[FeII(CN)6]3) Egg yolk | ATR-FTIR ATR-FTIR, MALDI-MS(/MS) |
| Lying lamb | Yellow (Italian gold) Ochre (α-FeOOH) Linseed oil | ATR-FTIR ATR-FTIR, MALDI-MS(/MS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigante, E.C.L.; Calvano, C.D.; Picca, R.A.; Armenise, S.; Cataldi, T.R.I.; Sabbatini, L. Multi-Technique Characterization of Pictorial Organic Binders on XV Century Polychrome Sculptures by Combining Micro- and Non-Invasive Sampling Approaches. Appl. Sci. 2021, 11, 8017. https://doi.org/10.3390/app11178017
Rigante ECL, Calvano CD, Picca RA, Armenise S, Cataldi TRI, Sabbatini L. Multi-Technique Characterization of Pictorial Organic Binders on XV Century Polychrome Sculptures by Combining Micro- and Non-Invasive Sampling Approaches. Applied Sciences. 2021; 11(17):8017. https://doi.org/10.3390/app11178017
Chicago/Turabian StyleRigante, Elena C. L., Cosima D. Calvano, Rosaria A. Picca, Simona Armenise, Tommaso R. I. Cataldi, and Luigia Sabbatini. 2021. "Multi-Technique Characterization of Pictorial Organic Binders on XV Century Polychrome Sculptures by Combining Micro- and Non-Invasive Sampling Approaches" Applied Sciences 11, no. 17: 8017. https://doi.org/10.3390/app11178017
APA StyleRigante, E. C. L., Calvano, C. D., Picca, R. A., Armenise, S., Cataldi, T. R. I., & Sabbatini, L. (2021). Multi-Technique Characterization of Pictorial Organic Binders on XV Century Polychrome Sculptures by Combining Micro- and Non-Invasive Sampling Approaches. Applied Sciences, 11(17), 8017. https://doi.org/10.3390/app11178017








