Phytochemicals and Their Possible Mechanisms in Managing COVID-19 and Diabetes
Abstract
:1. Introduction
2. Diabetes
Respiratory Infections in Diabetes
3. COVID-19
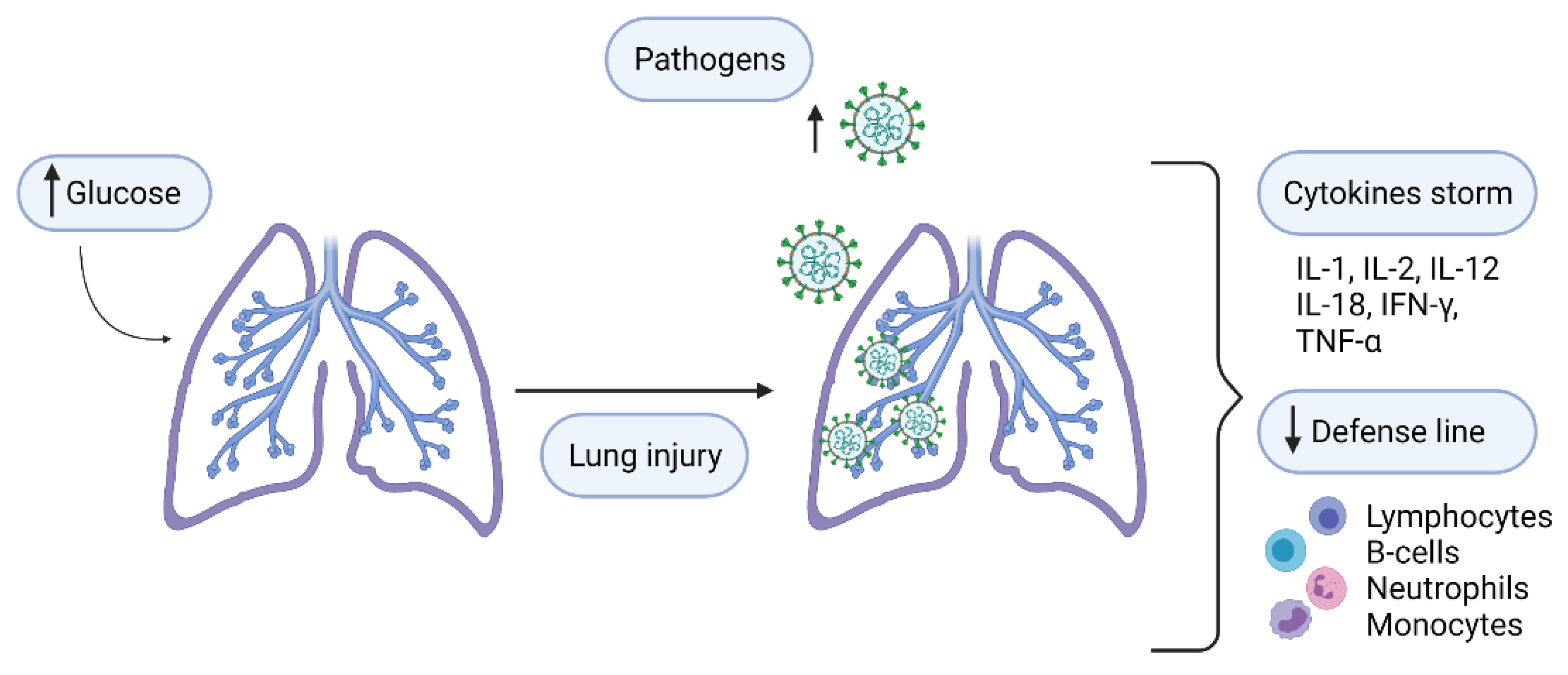
Diabetes and COVID-19
4. Alternative Treatments with Phytochemicals (Curcumin, Silymarin, and Sulphorafane)
4.1. Curcumin
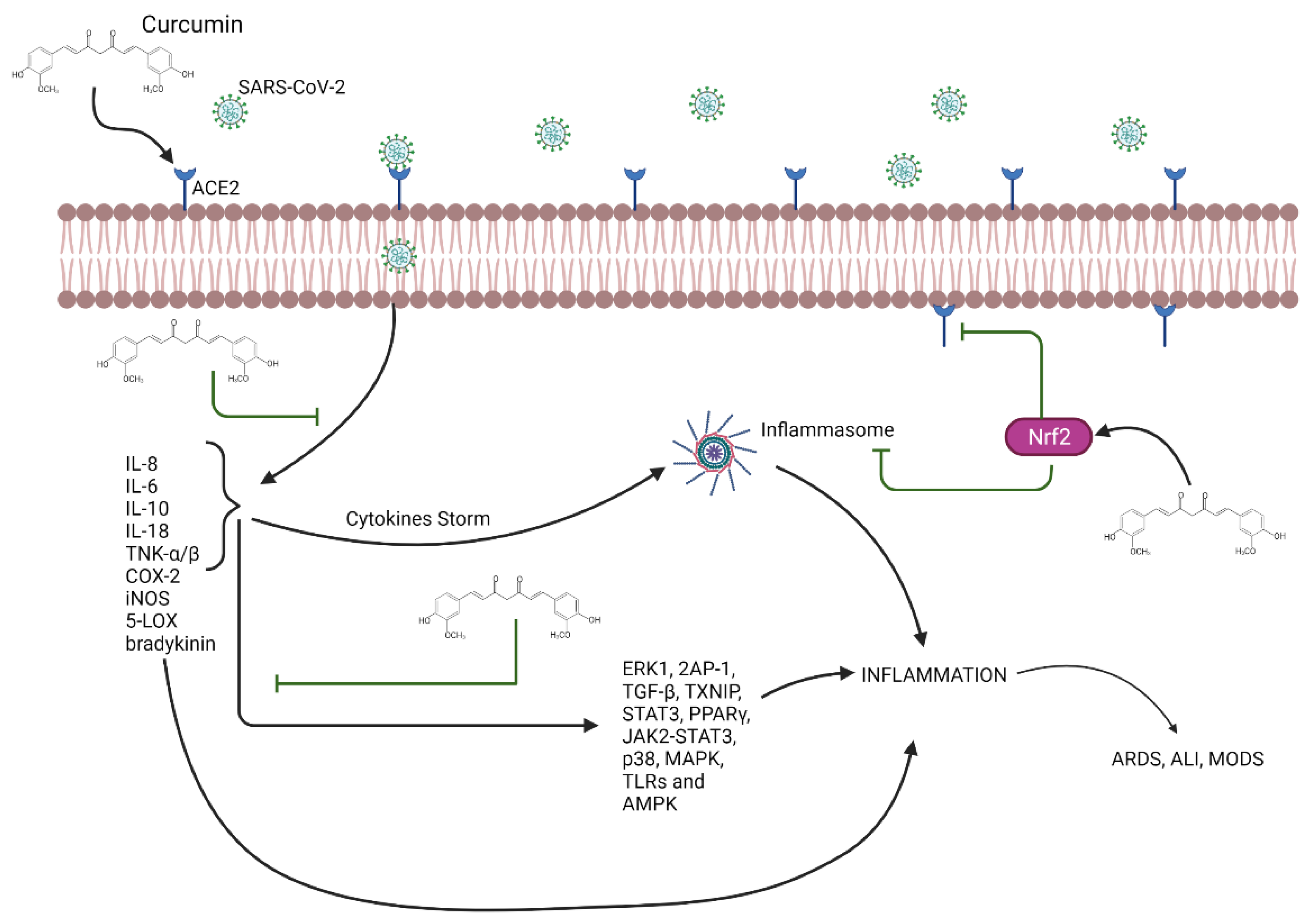
Curcumin and Its Effect on COVID-19 and Diabetes
4.2. Silymarin
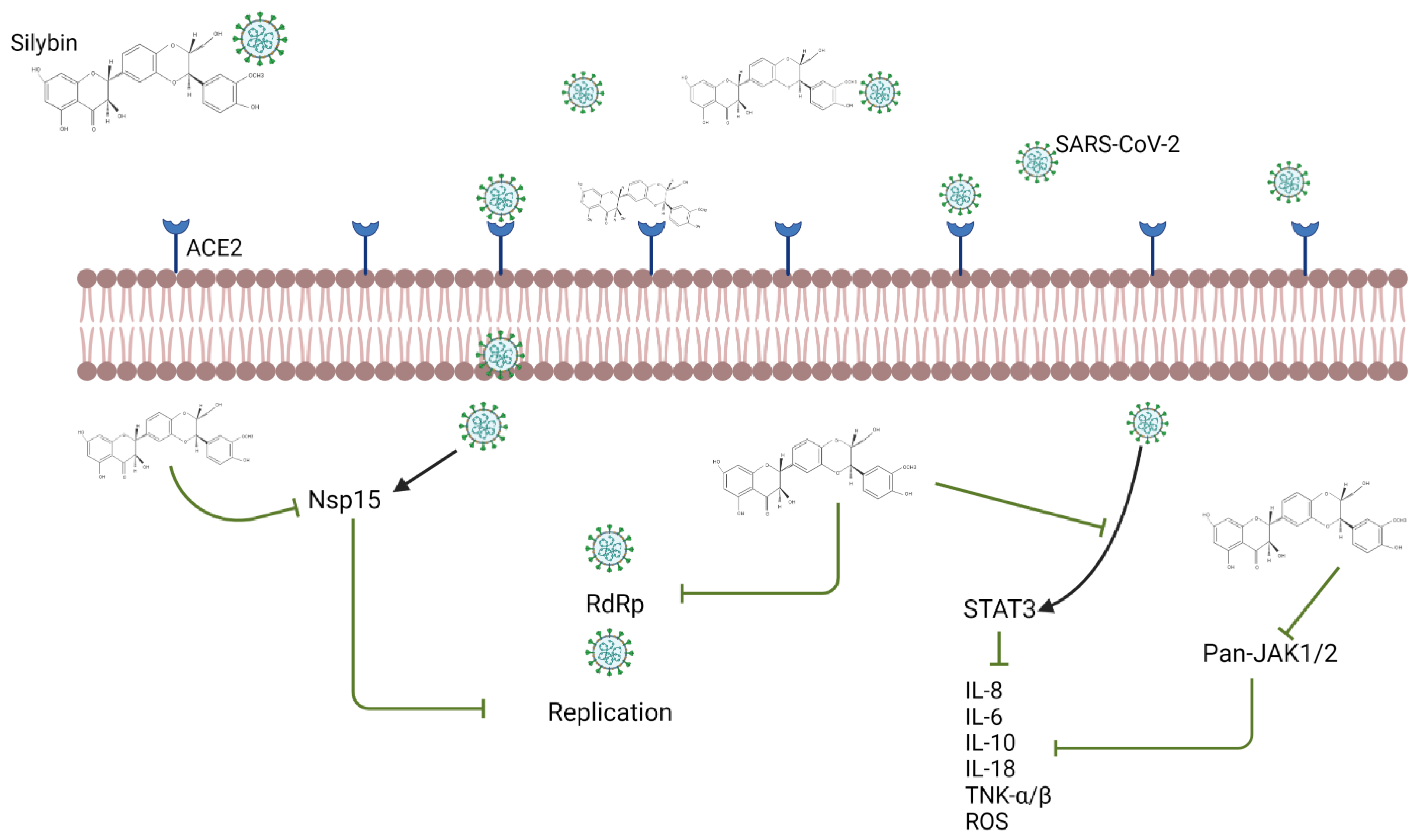
Silymarin and Its Effect on COVID-19 and Diabetes
4.3. Sulforaphane
Sulforaphane and Its Effect on COVID-19 and Diabetes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. COVID-19 Weekly Epidemiological Update, 30 March 2021. Available online: https://apps.who.int/iris/handle/10665/340513 (accessed on 30 March 2021).
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Lima-Martínez, M.M.; Carrera Boada, C.; Madera-Silva, M.D.; Marín, W.; Contreras, M. COVID-19 y diabetes mellitus: Una relación bidireccional. Clínica Investig. Arterioscler. 2020, 33, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, R.C.; Zimmet, P. Editorial: Diabetic kidney disease: Act now or pay later. Kidney Int. 2010, 77, 375–377. [Google Scholar] [CrossRef] [Green Version]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16, S27–S36. [Google Scholar]
- Saini, V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J. Diabetes 2010, 1, 68–75. [Google Scholar] [CrossRef]
- Hull, C.M.; Peakman, M.; Tree, T.I.M. Regulatory T cell dysfunction in type 1 diabetes: What’s broken and how can we fix it? Diabetologia 2017, 60, 1839–1850. [Google Scholar] [CrossRef] [Green Version]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Erener, S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef]
- Klekotka, R.B.; Mizgała, E.; Król, W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol. Alergol. Pol. 2015, 83, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Visca, D.; Pignatti, P.; Spanevello, A.; Lucini, E.; La Rocca, E. Relationship between diabetes and respiratory diseases—Clinical and therapeutic aspects. Pharm. Res. 2018, 137, 230–235. [Google Scholar] [CrossRef]
- Thomsen, R.W.; Mor, A. Diabetes and Risk of Community-Acquired Respiratory Tract Infections, Urinary Tract Infections, and Bacteremia. Open Infect Dis. J. 2012, 6, 27–39. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19). JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Bhattacharya, M.; Lee, S.S. SARS-CoV-2 causing pneumonia-associated respiratory disorder (COVID-19): Diagnostic and proposed therapeutic options. Eur. Rev. Med. Pharm. Sci. 2020, 24, 4016–4026. [Google Scholar]
- Chen, Y.; Klei, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 2021, 65, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Y.; Wang, Y.; Huang, Z.; Song, B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur. Radiol. 2020, 30, 4381–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Zhao, R.; Gao, L.-J.; Gao, X.-F.; Wang, D.-P.; Cao, J.-M. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front. Cell Infect Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; Van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Gubbi, S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020, 318, 736–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, M.A.; Mantzoros, C.; Sowers, J.R. Commentary: COVID-19 in patients with diabetes. Metabolism 2020, 107, 154217. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Rubino, F.; Khunti, K.; Mingrone, G.; Hopkins, D.; Birkenfeld, A.L.; Boehm, B.; Amiel, S.; Holt, R.I.G.; Skyler, J.S.; et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 546–550. [Google Scholar] [CrossRef]
- Das, S.K.R.A.; Birangal, S.R.; Nikam, A.N.; Pandey, A.; Mutalik, S.; Joseph, A. Role of comorbidities like diabetes on severe acute respiratory syndrome coronavirus-2: A review. Life Sci. 2020, 258, 118202. [Google Scholar] [CrossRef] [PubMed]
- Codo, A.C.; Davanzo, G.G.; Monteiro, L.d.B.; de Souza, G.F.; Muraro, S.P.; Virgilio-da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Lamas-Barreiro, J.M.; Alonso-Suárez, M.; Fernández-Martín, J.J.; Saavedra-Alonso, J.A. Supresión de angiotensina II en la infección por el virus SARS-CoV-2: Una propuesta terapéutica. Nefrología 2020, 40, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.W.; Holt, R.I.G. COVID-19 and diabetes. Diabet. Med. 2020, 37, 723–725. [Google Scholar] [CrossRef] [Green Version]
- Mesa Ramírez-Tortosa, M.C.; Aguilera, C.M.; Ramírez-Boscá, A.Y.; Gil, A. Efectos farmacológicos y nutricionales de los extractos de Curcuma longa L. y de los cucuminoides Pharmacological and nutritional effects of Curcuma longa L. extracts and curcuminoids. Ars. Pharm. 2000, 41, 307–321. [Google Scholar]
- Lestari, M.L.; Indrayanto, G. Curcumin. In Profiles of Drug Subst, Excip and Relat Methodol; Brittan, H.G., Ed.; Academic Press: London, UK, 2014; Volume 39, pp. 113–204. [Google Scholar]
- Bharat, B.A.; Chitra, S.; Nikita, M.; Haruyo, I. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar]
- Krishnaswamy, K.; Raghuramulu, N. Bioactive phytochemicals with emphasis on dietary practices. Indian J. Med. Res. 1998, 108, 167–181. [Google Scholar]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Lelli, D.; Sahebkar, A.; Johnston, T.P.; Pedone, C. Curcumin use in pulmonary diseases: State of the art and future perspectives. Pharm. Res. 2017, 115, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, N.; He, H.; Tang, X. Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application. J. Control. Release 2019, 316, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Aller, L.L. What about bioavailability of oral curcumin? CMAJ 2019, 191, E427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaei-Malazy, O.; Abdollahi, M.; Larijani, B. Beneficial Effects of Anti-Oxidative Herbal Medicines in Diabetic Patients Infected with COVID-19: A Hypothesis. Diabetes Metab. Syndr. Obes. 2020, 13, 3113–3116. [Google Scholar] [CrossRef]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R.; Bahadoram, M.; Alghasi, A. COVID-19: A Case for Inhibiting NLRP3 Inflammasome, Suppression of Inflammation with Curcumin. Basic. Clin. Pharm. Toxicol. 2021, 128, 37–45. [Google Scholar] [CrossRef]
- Rahban, M.; Habibi-Rezaei, M.; Mazaheri, M.; Saso, L.; Moosavi-Movahedi, A.A. Anti-viral potential and modulation of nrf2 by curcumin: Pharmacological implications. Antioxidants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Liu, Z.; Ying, Y. The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and Its Potential Use in the Associated Severe Pneumonia. Front. Cell Dev. Biol. 2020, 8, 479. [Google Scholar] [CrossRef]
- Das, S.; Sarmah, S.; Lyndem, S.; Singha Roy, A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 2020, 39, 3347–3357. [Google Scholar] [CrossRef]
- Patel, A.; Rajendran, M.; Shah, A.; Patel, H.; Pakala, S.B.; Karyala, P. Virtual screening of curcumin and its analogs against the spike surface glycoprotein of SARS-CoV-2 and SARS-CoV. J. Biomol. Struct. Dyn. 2021, 5, 1–9. [Google Scholar]
- Kandeel, M.; Al-Nazawi, M. Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease. Life Sci. 2020, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Shanmugarajan, D.P.P.; Kumar, B.R.P.; Suresh, B. Curcumin to inhibit binding of spike glycoprotein to ACE2 receptors: Computational modelling, simulations, and ADMET studies to explore curcuminoids against novel SARS-CoV-2 targets. RSC Adv. 2020, 10, 31385–31399. [Google Scholar] [CrossRef]
- Zhang, D.W.; Fu, M.; Gao, S.H.; Liu, J.L. Curcumin and diabetes: A systematic review. Evidence-based Complementary and Alternative. Medicine 2013, 2013, 636053. [Google Scholar]
- Rivera-Mancía, S.; Trujillo, J.; Chaverri, J.P. Utility of curcumin for the treatment of diabetes mellitus: Evidence from preclinical and clinical studies. JNIM 2018, 14, 29–41. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roxo, D.F.; Arcaro, C.A.; Gutierres, V.O.; Costa, M.C.; Oliveira, J.O.; Lima, T.F.O.; Assis, R.P.; Lorenco, B.I.; Martins, B.A. Curcumin combined with metformin decreases glycemia and dyslipidemia, and increases paraoxonase activity in diabetic rats. Diabetol. Metab. Syndr. 2019, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin Extract for Prevention of Type 2 Diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Mendoza, N.; Morales-González, Á.; Morales-Martínez, M.; Soriano-Ursúa, M.A.; Delgado-Olivares, L.; Sandoval-Gallegos, E.M.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Madrigal-Santillán, E.; Morales-Gonzalez, J.A. Flavolignans from silymarin as Nrf2 bioactivators and their therapeutic applications. Biomedicines 2020, 8, 122. [Google Scholar] [CrossRef]
- Post-White, J.; Ladas, E.J.; Kelly, K.M. Advances in the use of milk thistle (Silybum marianum). Integr. Cancer Ther. 2007, 6, 104–109. [Google Scholar] [CrossRef]
- Radjabian, T.; Fallah Huseini, H. Anti-Hyperlipidemic and Anti-Atherosclerotic Activities of Silymarins from Cultivated and Wild Plants of Silybum marianum L. with Different Content of Flavonolignans. Iran. J. Pharmacol. Ther. 2010, 9, 63–67. [Google Scholar]
- Porwal, O.; Mohammed Ameen, M.S.; Anwer, E.T.; Uthirapathy, S.; Ahamad, J.; Tahsin, A. Silybum marianum (Milk Thistle): Review on Its chemistry, morphology, ethno medical uses, phytochemistry and pharmacological activities. J. Drug Deliv. Ther. 2019, 9, 199–206. [Google Scholar] [CrossRef]
- Silveira, D.; Prieto-Garcia, M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jama, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Front. Pharmacol. 2020, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sun, G. Sylimarin mitigates lung impairments in a rat model of acute respiratory distress syndrome. Inflammopharmacology 2018, 26, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [Green Version]
- Javed, S.; Kohli, K.; Ali, M. Patented bioavailability enhancement techniques of silymarin. Recent Pat. Drug Deliv. Formul. 2010, 4, 145–152. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, H. Anti-Parkinson Potential of Silymarin: Mechanistic Insight and Therapeutic Standing. Front. Pharm. 2018, 9, 422. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Zhang, D.; Zhang, J.; Yuan, J. Metabolism, Transport and Drug-Drug Interactions of Silymarin. Molecules 2019, 24, 3693. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Trinh, N.T.; Tran, H.N.; Tran, H.T.; Le, P.Q.; Ngo, D.N.; Tran-Van, H.; Van Vo, T.; Vong, L.B.; Nagasaki, Y. Improving silymarin oral bioavailability using silica-installed redox nanoparticle to suppress inflammatory bowel disease. J. Control. Release 2021, 331, 515–524. [Google Scholar] [CrossRef]
- Sornsuvit, C.; Hongwiset, D.; Yotsawimonwat, S.; Toonkum, M.; Thongsawat, S.; Taesotikul, W. The Bioavailability and Pharmacokinetics of Silymarin SMEDDS Formulation Study in Healthy Thai Volunteers. Evid.-Based Complement. Altern. Med. 2021, 24, 879–890. [Google Scholar]
- Gorla, U.S.; Rao, G.K.; Kulandaivelu, U.S.; Alavala, R.R.; Panda, S.P. Lead Finding from Selected Flavonoids with Antiviral (SARS-CoV-2) Potentials against COVID-19: An In-Silico Evaluation. Comb. Chem. High Throughput Screen. 2021, 24, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, J.; Singh, P.; Patel, R.A. Computational approach for the screening of potential antiviral compounds against SARS-CoV-2 protease: Ionic liquid vs. herbal and natural compounds. J. Mol. Liq. 2021, 326, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kashyap, P.; Chowdhury, S.; Kumar, S.; Panwar, A.; Kumar, A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine 2020, 85, 153317. [Google Scholar] [CrossRef]
- Bosch-Barrera, J.; Martin-Castillo, B.; Buxó, M.; Brunet, J.; Encinar, J.; Menendez, J.A.A. Silibinin and SARS-CoV-2: Dual Targeting of Host Cytokine Storm and Virus Replication Machinery for Clinical Management of COVID-19 Patients. J. Clin. Med. 2020, 9, 1770. [Google Scholar] [CrossRef]
- Pandit, M.; Latha, N. In Silico studies reveal potential antiviral activity of phytochemicals from medicinal plants for the treatment of COVID-19 infection. Res. Sq. 2020. [Google Scholar] [CrossRef] [Green Version]
- Stolf, A.M.; Cardoso, C.C.; Acco, A. Effects of Silymarin on Diabetes Mellitus Complications: A Review. Phytother. Res. 2017, 31, 366–374. [Google Scholar] [CrossRef]
- Manonmani, A.J.; Narmadha, M.P.; Anjana, A. Effect of silymarin in diabetes mellitus patients with liver diseases. J. Pharm. Pharm. 2011, 2, 287–289. [Google Scholar]
- Tuorkey, M.J.; El-Desouki, N.I.; Kamel, R.A. Cytoprotective Effect of Silymarin against Diabetes-Induced Cardiomyocyte Apoptosis in Diabetic Rats. Biomed. Env. Sci. 2015, 28, 36–43. [Google Scholar]
- Rafieian-Kopaie, M.; Nasri, H. Silymarin and diabetic nephropathy. J. Ren. Inj. Prev. 2012, 1, 3–5. [Google Scholar]
- Fallahzadeh, M.K.; Dormanesh, B.; Sagheb, M.M.; Roozbeh, J.; Vessal, G.; Pakfetrat, M.; Daneshbod, Y.; Kamali-Sarvestani, E.; Lankarani, K.B. Effect of Addition of Silymarin to Renin-Angiotensin System Inhibitors on Proteinuria in Type 2 Diabetic Patients With Overt Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Kidney Dis. 2012, 60, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential Protective Phytochemical against Neurodegenerative Diseases. Oxid. Med. Cell Longev. 2013, 2013, 415078. [Google Scholar] [CrossRef]
- Conzatti, A.; Fróes, F.C.T.d.S.; Perry, I.D.S.; de Souza, C.G. Evidencias Clínicas y moleculares del consumo de brócoli, glucorafanina y sulforafano en humanos. Nutr. Hosp. 2015, 31, 559–569. [Google Scholar]
- Biswas, S.; Rahman, I. Chapter 33 - Dietary Bioactive Functional Polyphenols in Chronic Lung Diseases. In Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 513–523. [Google Scholar]
- Fofaria, N.M.; Ranjan, A.; Kim, S.-H.; Srivastava, S.K. Mechanisms of the Anticancer Effects of Isothiocyanates. Enzymes 2015, 37, 111–137. [Google Scholar]
- Wang, Y.; Miao, X.; Sun, J.; Cai, L. Chapter 6 - Oxidative Stress in Diabetes: Molecular Basis for Diet Supplementation. In Mol. Nutr. Diabetes; Mauricio, D., Ed.; Academic Press: London, UK, 2016; pp. 65–72. [Google Scholar]
- Persico, A.M.; Ricciardello, A.; Cucinotta, F. The psychopharmacology of autism spectrum disorder and Rett syndrome. Handb. Clin. Neurol. 2019, 165, 391–414. [Google Scholar] [PubMed]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.; van den Berg, R.; Vaes, W.H. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–15509. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef]
- Zinovkin, R.A.; Grebenchikov, O.A. Transcription Factor Nrf2 as a Potential Therapeutic Target for Prevention of Cytokine Storm in COVID-19 Patients. Biochemistry 2020, 85, 833–837. [Google Scholar] [CrossRef]
- Cuadrado, A.; Pajares, M.; Benito, C.; Jiménez-Villegas, J.; Escoll, M.; Fernández-Ginés, R.; Garcia Yagüe, A.J.; Lastra, D.; Manda, G.; Rojo, A.I.; et al. Can Activation of NRF2 Be a Strategy against COVID-19? Trends Pharmacol. Sci. 2020, 41, 598–610. [Google Scholar] [CrossRef]
- Benarba, B.; Pandiella, A. Medicinal Plants as Sources of Active Molecules Against COVID-19. Front Pharm. 2020, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Diabetes Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.; Mann, G.E.; Chapple, S.J. Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome. Free Radic. Biol. Med. 2018, 122, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Bao, S.; Liu, T.; Wei, L.; Wang, D.; Ye, W.; Wang, N.; Song, S.; Li, J.; Chudhary, M. Sulforaphane delays diabetes-induced retinal photoreceptor cell degeneration. Cell Tissue Res. 2020, 382, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Bhowmik, B.; do Vale Moreira, N.C. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020, 162, 108142. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-Gallegos, E.M.; Ramírez-Moreno, E.; Vargas-Mendoza, N.; Arias-Rico, J.; Estrada-Luna, D.; Cuevas-Cancino, J.J.; Jiménez-Sánchez, R.C.; Flores-Chávez, O.R.; Baltazar-Téllez, R.M.; Morales-González, J.A. Phytochemicals and Their Possible Mechanisms in Managing COVID-19 and Diabetes. Appl. Sci. 2021, 11, 8163. https://doi.org/10.3390/app11178163
Sandoval-Gallegos EM, Ramírez-Moreno E, Vargas-Mendoza N, Arias-Rico J, Estrada-Luna D, Cuevas-Cancino JJ, Jiménez-Sánchez RC, Flores-Chávez OR, Baltazar-Téllez RM, Morales-González JA. Phytochemicals and Their Possible Mechanisms in Managing COVID-19 and Diabetes. Applied Sciences. 2021; 11(17):8163. https://doi.org/10.3390/app11178163
Chicago/Turabian StyleSandoval-Gallegos, Eli Mireya, Esther Ramírez-Moreno, Nancy Vargas-Mendoza, José Arias-Rico, Diego Estrada-Luna, José Javier Cuevas-Cancino, Reyna Cristina Jiménez-Sánchez, Olga Rocío Flores-Chávez, Rosa María Baltazar-Téllez, and José A. Morales-González. 2021. "Phytochemicals and Their Possible Mechanisms in Managing COVID-19 and Diabetes" Applied Sciences 11, no. 17: 8163. https://doi.org/10.3390/app11178163
APA StyleSandoval-Gallegos, E. M., Ramírez-Moreno, E., Vargas-Mendoza, N., Arias-Rico, J., Estrada-Luna, D., Cuevas-Cancino, J. J., Jiménez-Sánchez, R. C., Flores-Chávez, O. R., Baltazar-Téllez, R. M., & Morales-González, J. A. (2021). Phytochemicals and Their Possible Mechanisms in Managing COVID-19 and Diabetes. Applied Sciences, 11(17), 8163. https://doi.org/10.3390/app11178163









