Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dry Matter Content
2.3. Pretreatment of Turmeric
2.3.1. Thermal Pretreatment (TP)
2.3.2. Ultrasound Pretreatment (UP)
2.3.3. Pulsed Electric Field Pretreatment (PEF)
2.3.4. Enzyme Pretreatment (EP)
2.4. Rapid Extraction of Curcuminoids
2.5. Quantification of Curcuminoids
2.6. Determination of Cell Disintegration Index (Zp)
2.7. Particle Size
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Thermal Pretreatment on Total Curcuminoids Recovery of Fresh Turmeric
3.2. Effect of Ultrasound Pretreatment on Total Curcuminoids Recovery of Fresh Turmeric
3.3. Effect of Enzyme Pretreatment on Total Curcuminoids Recovery of Fresh Turmeric
3.4. Effect of PEF Pretreatment on Total Curcuminoids Recovery of Fresh Turmeric
3.5. Effect of Pretreatment Time on BDMC, DMC, CUR Recovery of Fresh Turmeric
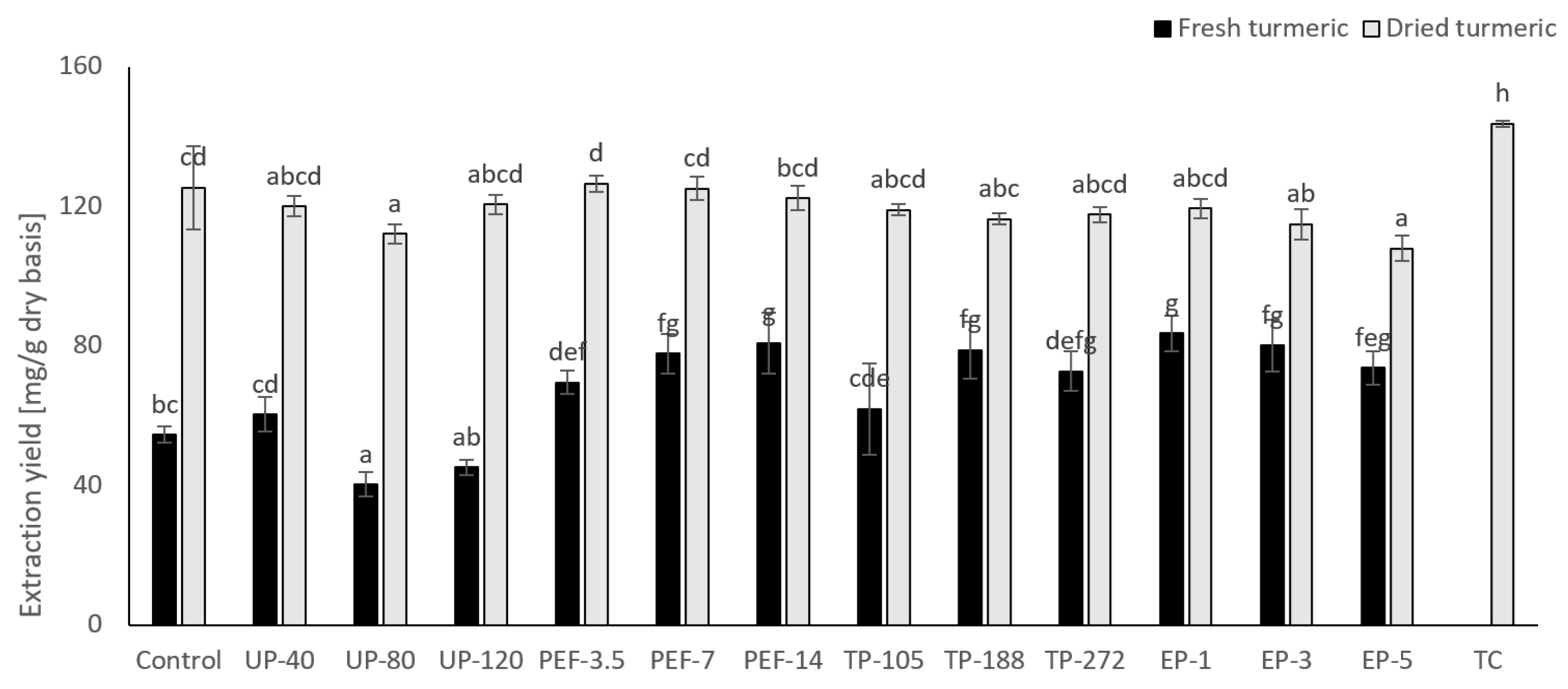
3.6. Effect of Different Pretreatment on Total Curcuminoids Recovery of Dried Turmeric
3.7. Comparison of Total Specific Energy Input of the Pretreatments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirsath, S.; Sable, S.; Gaikwad, S.; Sonawane, S.; Saini, D.; Gogate, P. Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrason. Sonochem. 2017, 38, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.I.; Uliana, M.R.; Costa, S.M.; Magro, M.; Vianello, F.; Ming, L.C.; Lima, G.P.P. Exclusion of solar UV radiation increases the yield of curcuminoid in Curcuma longa L. Ind. Crop. Prod. 2016, 89, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, P.; Babu, K.N.; Sivaraman, K. Turmeric: The Genus Curcuma; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction of biomolecules from seaweeds. In Seaweed Sustainability; Elsevier: Amsterdam, The Netherlands, 2015; pp. 243–269. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Ahmad, S.; Ali, M.; Ansari, S.H.; Ahmed, F. Phytoconstituents from the rhizomes of Curcuma aromatica Salisb. J. Saudi Chem. Soc. 2011, 15, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Govindarajan, V.; Stahl, W.H. Turmeric—Chemistry, technology, and quality. Crit. Rev. Food Sci. Nutr. 1980, 12, 199–301. [Google Scholar] [CrossRef]
- Sonawane, S.; Nirmal, S.; Patil, A.; Pattan, S. Development and validation of HPTLC method to detect curcumin and gallic acid in polyherbal formulation. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2664–2673. [Google Scholar] [CrossRef]
- Wakte, P.S.; Sachin, B.; Patil, A.; Mohato, D.; Band, T.; Shinde, D. Optimization of microwave, ultra-sonic and supercritical carbon dioxide assisted extraction techniques for curcumin from Curcuma longa. Sep. Purif. Technol. 2011, 79, 50–55. [Google Scholar] [CrossRef]
- Dandekar, D.V.; Gaikar, V. Microwave assisted extraction of curcuminoids from Curcuma longa. Sep. Sci. Technol. 2002, 37, 2669–2690. [Google Scholar] [CrossRef]
- Mandal, V.; Dewanjee, S.; Sahu, R.; Mandal, S.C. Design and optimization of ultrasound assisted extraction of curcumin as an effective alternative for conventional solid liquid extraction of natural products. Nat. Prod. Commun. 2009, 4, 1934578X0900400121. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.O.; Pereira, R.N.; Tonon, R.V.; Cabral, L.M.C.; Santiago, M.C.P.; Vicente, A.A.; Teixeira, J.A.C.; Matta, V.M.; Freitas, S.P. Antioxidant compounds recovery from juçara residue by thermal assisted extraction. Plant Foods Hum. Nutr. 2018, 73, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Braga, M.E.; Leal, P.F.; Carvalho, J.E.; Meireles, M.A.A. Comparison of yield, composition, and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J. Agric. Food Chem. 2003, 51, 6604–6611. [Google Scholar] [CrossRef]
- Raj, A.S.; Chakraborty, S.; Rao, P.S. Thermal assisted high-pressure processing of Indian gooseberry (Embilica officinalis L.) juice–Impact on colour and nutritional attributes. LWT 2019, 99, 119–127. [Google Scholar] [CrossRef]
- Mane, S.; Bremner, D.H.; Tziboula-Clarke, A.; Lemos, M.A. Effect of ultrasound on the extraction of total anthocyanins from Purple Majesty potato. Ultrason. Sonochem. 2015, 27, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ngadi, M.O.; Ma, Y. Optimisation of pulsed ultrasonic and microwave-assisted extraction for curcuminoids by response surface methodology and kinetic study. Food Chem. 2014, 165, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Zhang, F.; Wang, Z. Study on Ultrasonic Extraction of Gastrodin from Gastrodia elata Bl. Sep. Sci. Technol. 2010, 45, 832–838. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction of curcumin by sample–solvent dual heating mechanism using Taguchi L9 orthogonal design. J. Pharm. Biomed. Anal. 2008, 46, 322–327. [Google Scholar] [CrossRef]
- Rouhani, S.; Alizadeh, N.; Salimi, S.; Haji-Ghasemi, T. Ultrasonic Assisted Extraction of Natural Pigments from Rhizomes of Curcuma Longa, L. Prog. Color. Colorants Coat. 2009, 2, 103–113. [Google Scholar]
- Kurmudle, N.; Kagliwal, L.D.; Bankar, S.B.; Singhal, R.S. Enzyme-assisted extraction for enhanced yields of turmeric oleoresin and its constituents. Food Biosci. 2013, 3, 36–41. [Google Scholar] [CrossRef]
- Kurmudle, N.N.; Bankar, S.B.; Bajaj, I.B.; Bule, M.V.; Singhal, R.S. Enzyme-assisted three phase partitioning: A novel approach for extraction of turmeric oleoresin. Process. Biochem. 2011, 46, 423–426. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Enzyme-assisted ionic liquid extraction of bioactive compound from turmeric (Curcuma longa L.): Isolation, purification and analysis of curcumin. Ind. Crop. Prod. 2017, 95, 686–694. [Google Scholar] [CrossRef]
- Harsha, M.R.; Chandra Prakash, S.V.; Dharmesh, S.M. Modified pectic polysaccharide from turmeric (Curcuma longa): A potent dietary component against gastric ulcer. Carbohydr. Polym. 2016, 138, 143–155. [Google Scholar] [CrossRef]
- Lebovka, N.; Bazhal, M.; Vorobiev, E. Simulation and experimental investigation of food material breakage using pulsed electric field treatment. J. Food Eng. 2000, 44, 213–223. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Neumann, E.; Kakorin, S.; Toensing, K. Principles of membrane electroporation and transport of macromolecules. In Electrochemotherapy, Electrogenetherapy, and Transdermal Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–35. [Google Scholar]
- Maskooki, A.; Eshtiaghi, M.N. Impact of pulsed electric field on cell disintegration and mass transfer in sugar beet. Food Bioprod. Process. 2012, 90, 377–384. [Google Scholar] [CrossRef]
- Chemat, F.; Zill e, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Périno-Issartier, S.; Abert-Vian, M.; Chemat, F. Solvent free microwave-assisted extraction of antioxidants from sea buckthorn (Hippophae rhamnoides) food by-products. Food Bioprocess. Technol. 2011, 4, 1020–1028. [Google Scholar] [CrossRef]
- Knorr, D.; Angersbach, A. Impact of high-intensity electric field pulses on plant membrane permeabilization. Trends Food Sci. Technol. 1998, 9, 185–191. [Google Scholar] [CrossRef]
- Singh, G.; Arora, S.; Kumar, S. Effect of mechanical drying air conditions on quality of turmeric powder. J. Food Sci. Technol. 2010, 47, 347–350. [Google Scholar] [CrossRef] [Green Version]
- El Darra, N.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field, ultrasound, and thermal pretreatments for better phenolic extraction during red fermentation. Eur. Food Res. Technol. 2013, 236, 47–56. [Google Scholar] [CrossRef]
- Fauster, T.; Schlossnikl, D.; Rath, F.; Ostermeier, R.; Teufel, F.; Toepfl, S.; Jaeger, H. Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. J. Food Eng. 2018, 235, 16–22. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.; Obernberger, J.; Zehetner, E.; Kiesslich, A.; Volkert, M.; Jaeger, H. Impact of powder particle structure on the oxidation stability and color of encapsulated crystalline and emulsified carotenoids in carrot concentrate powders. J. Food Eng. 2019, 263, 398–408. [Google Scholar] [CrossRef]
- Kuttigounder, D.; Lingamallu, J.R.; Bhattacharya, S. Turmeric powder and starch: Selected physical, physicochemical, and microstructural properties. J. Food Sci. 2011, 76, C1284–C1291. [Google Scholar] [CrossRef]
- Fauster, T.; Philipp, C.; Hanz, K.; Scheibelberger, R.; Teufl, T.; Nauer, S.; Scheiblhofer, H.; Jaeger, H. Impact of a combined pulsed electric field (PEF) and enzymatic mash treatment on yield, fermentation behaviour and composition of white wine. Eur. Food Res. Technol. 2020, 246, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Lestari, M.L.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 39, pp. 113–204. [Google Scholar]
- Izadifar, Z.; Babyn, P.; Chapman, D. Mechanical and Biological Effects of Ultrasound: A Review of Present Knowledge. Ultrasound Med. Biol. 2017, 43, 1085–1104. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.; Wang, H.; Zhang, M.; Wang, Z. Improved extraction of oil from chickpea under ultrasound in a dynamic system. J. Food Eng. 2010, 98, 13–18. [Google Scholar] [CrossRef]
- Balachandran, S.; Kentish, S.E.; Mawson, R.; Ashokkumar, M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Doktycz, S.J.; Suslick, K.S. Interparticle collisions driven by ultrasound. Science 1990, 247, 1067–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muniglia, L.; Claisse, N.; Baudelet, P.-H.; Ricochon, G. Enzymatic Aqueous Extraction (EAE). In Alternative Solvents for Natural Products Extraction; Springer: Berlin/Heidelberg, Germany, 2014; pp. 167–204. [Google Scholar]
- Eshtiaghi, M.; Knorr, D. High electric field pulse pretreatment: Potential for sugar beet processing. J. Food Eng. 2002, 52, 265–272. [Google Scholar] [CrossRef]
- Lebovka, N.; Bazhal, M.; Vorobiev, E. Estimation of characteristic damage time of food materials in pulsed-electric fields. J. Food Eng. 2002, 54, 337–346. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Gordon, O.N.; Luis, P.B.; Ashley, R.E.; Osheroff, N.; Schneider, C. Oxidative transformation of demethoxy-and bisdemethoxycurcumin: Products, mechanism of formation, and poisoning of human topoisomerase IIα. Chem. Res. Toxicol. 2015, 28, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-assisted extraction of flavorings and colorants from plant materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, E.; Lebovka, N. Pulse electric field-assisted extraction. In Enhancing Extraction Processes in the Food Industry; CRC Press: Boca Raton, FL, USA, 2011; pp. 25–28. [Google Scholar]


| Pretreatment | Pretreatment Time | Specific Energy Input [kJ/kg] | Start Temperature [°C] | End Temperature [°C] |
|---|---|---|---|---|
| UP-40 | 1 min | 40 | 21.9 ± 0.2 | 26.1 ± 0.1 |
| UP-80 | 1 min | 80 | 22.5 ± 0.2 | 37.6 ± 0.2 |
| UP-120 | 1 min | 120 | 22.8 ± 0.1 | 44.3 ± 0.3 |
| PEF-3.5 | 0.54 ms | 3.5 | 22.8 ± 0.2 | 22.8 ± 0.2 |
| PEF-7 | 1.03 ms | 7 | 24.2 ± 0.3 | 25.8 ± 0.2 |
| PEF-14 | 2.05 ms | 14 | 24.1 ± 0.2 | 26.2 ± 0.2 |
| TP-105 | 5 min | 105 | 22.6 ± 0.1 | 34.5 ± 0.2 |
| TP-188 | 5 min | 188 | 23.5 ± 0.2 | 52.7 ± 0.2 |
| TP-272 | 5 min | 272 | 24.3 ± 0.2 | 67.4 ± 0.3 |
| EP-1 | 60 min | 105 1 | 20.2 ± 0.3 | 51.3 ± 0.3 |
| EP-3 | 180 min | 105 1 | 20.3 ± 0.3 | 51.3 ± 0.2 |
| EP-5 | 300 min | 105 1 | 20.2 ± 0.2 | 51.2 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le-Tan, H.; Fauster, T.; Vladic, J.; Gerhardt, T.; Haas, K.; Jaeger, H. Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa. Appl. Sci. 2021, 11, 8238. https://doi.org/10.3390/app11178238
Le-Tan H, Fauster T, Vladic J, Gerhardt T, Haas K, Jaeger H. Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa. Applied Sciences. 2021; 11(17):8238. https://doi.org/10.3390/app11178238
Chicago/Turabian StyleLe-Tan, Hoang, Thomas Fauster, Jelena Vladic, Tina Gerhardt, Klara Haas, and Henry Jaeger. 2021. "Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa" Applied Sciences 11, no. 17: 8238. https://doi.org/10.3390/app11178238






