Effect of Urea Addition on Anatase Phase Enrichment and Nitrogen Doping of TiO2 for Photocatalytic Abatement of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Pure and Nitrogen-Doped TiO2 Samples
2.3. Materials Characterizations
2.4. Photocatalytic Performance Evaluation
3. Results and Discussion
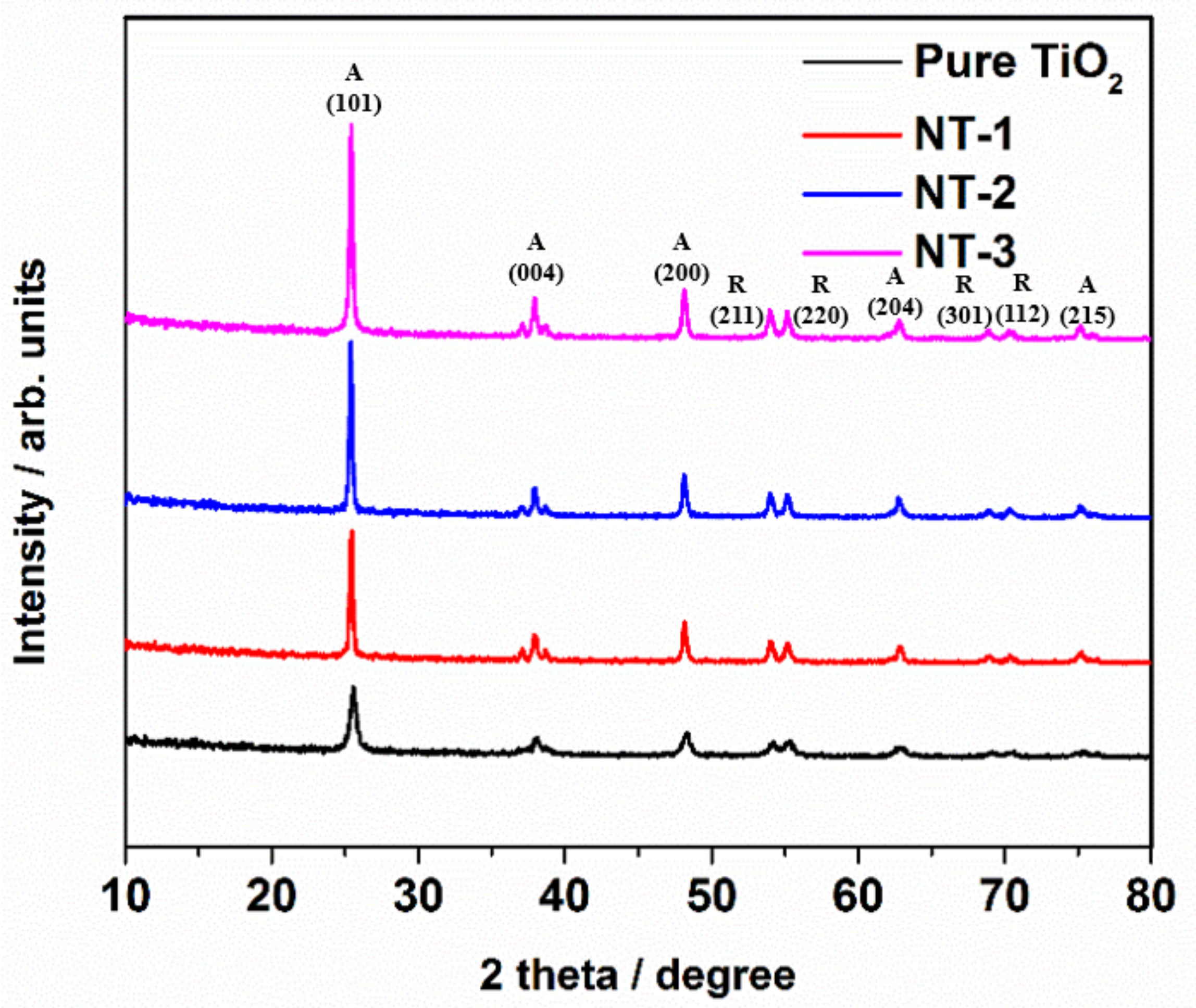
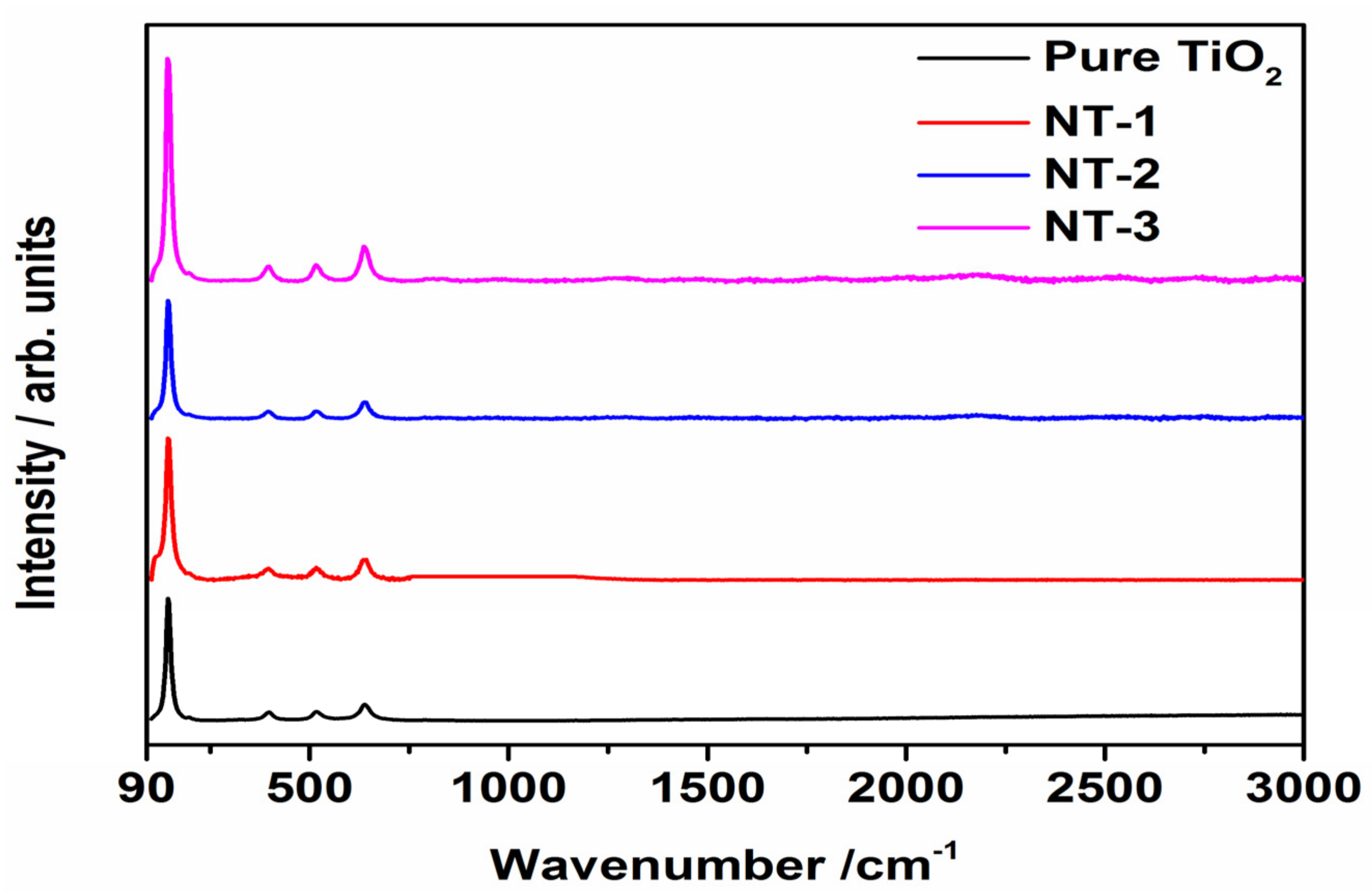
3.1. Crystallinity
3.2. Morphological Analysis
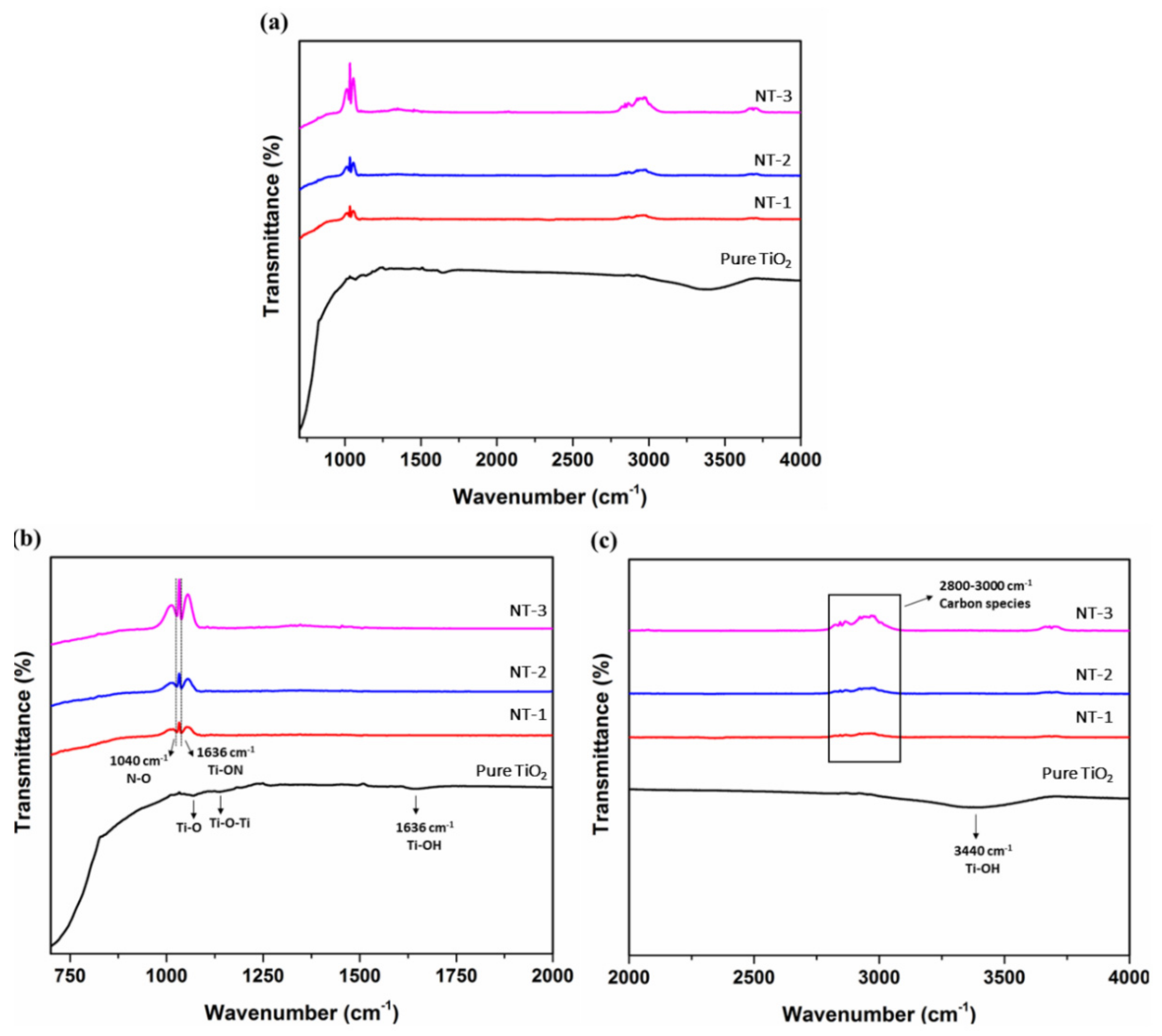
3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
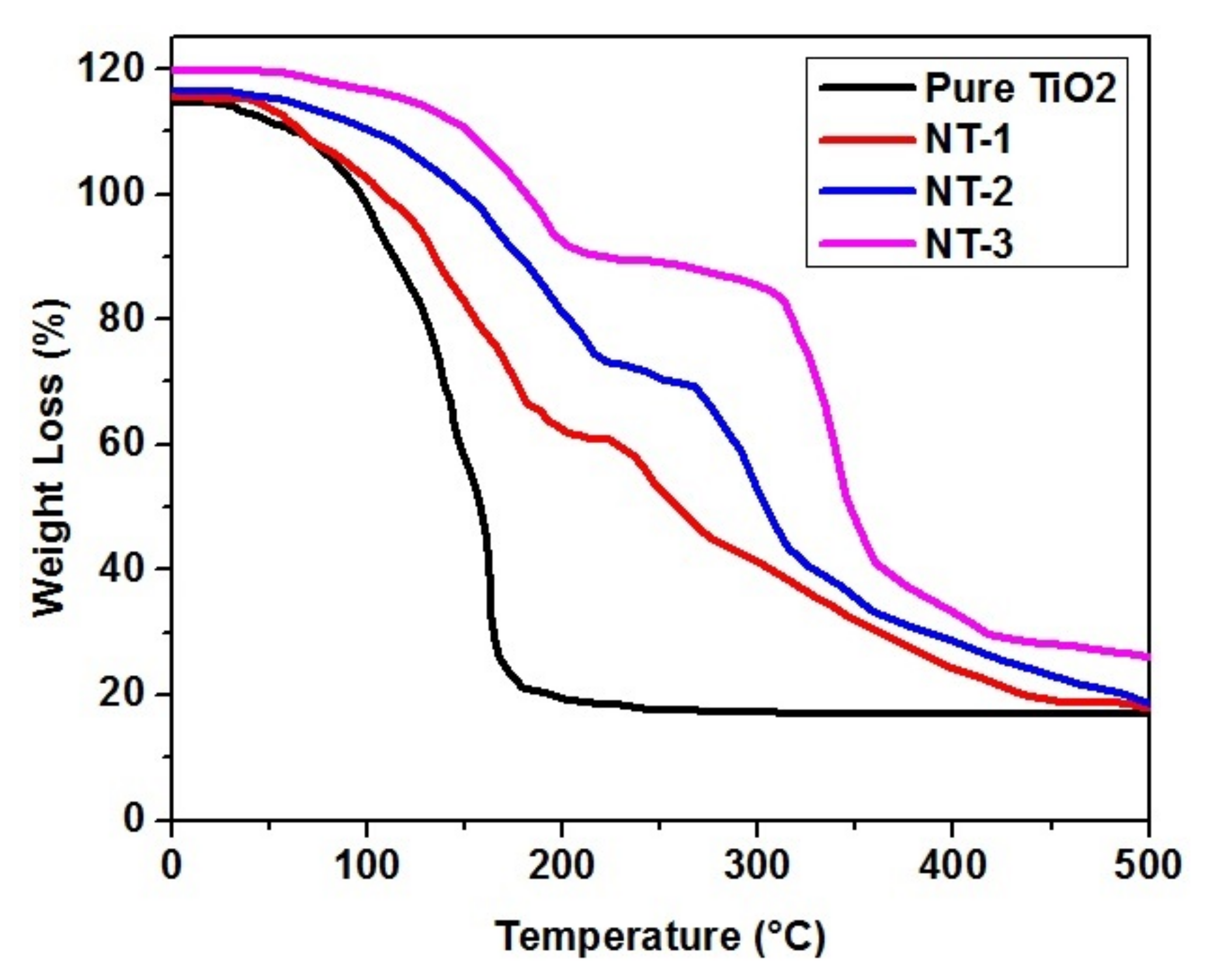
3.4. Thermogravimetrical Analysis (TGA)
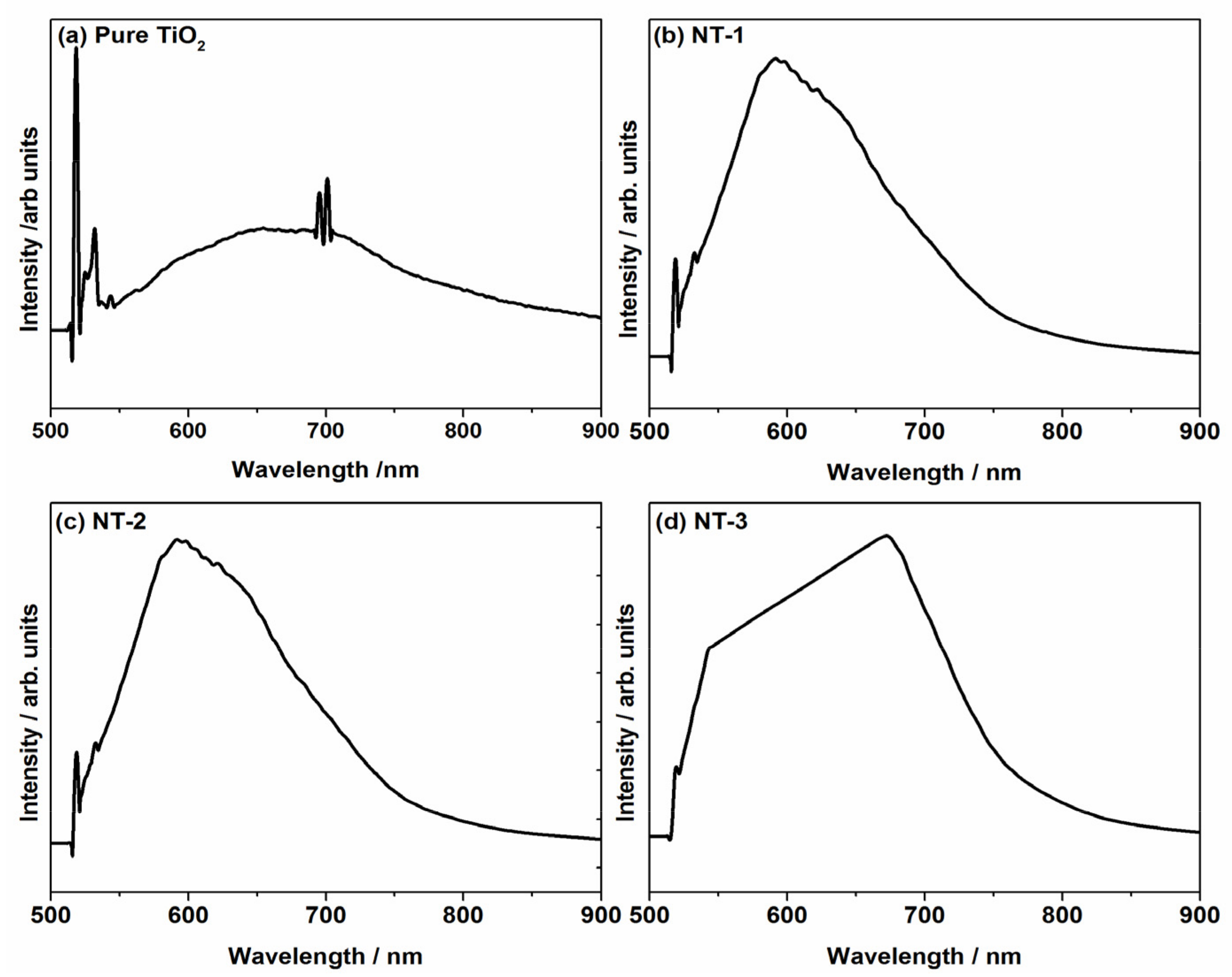
3.5. Optical Properties Investigation
3.6. Surface Area and Porosity Analysis
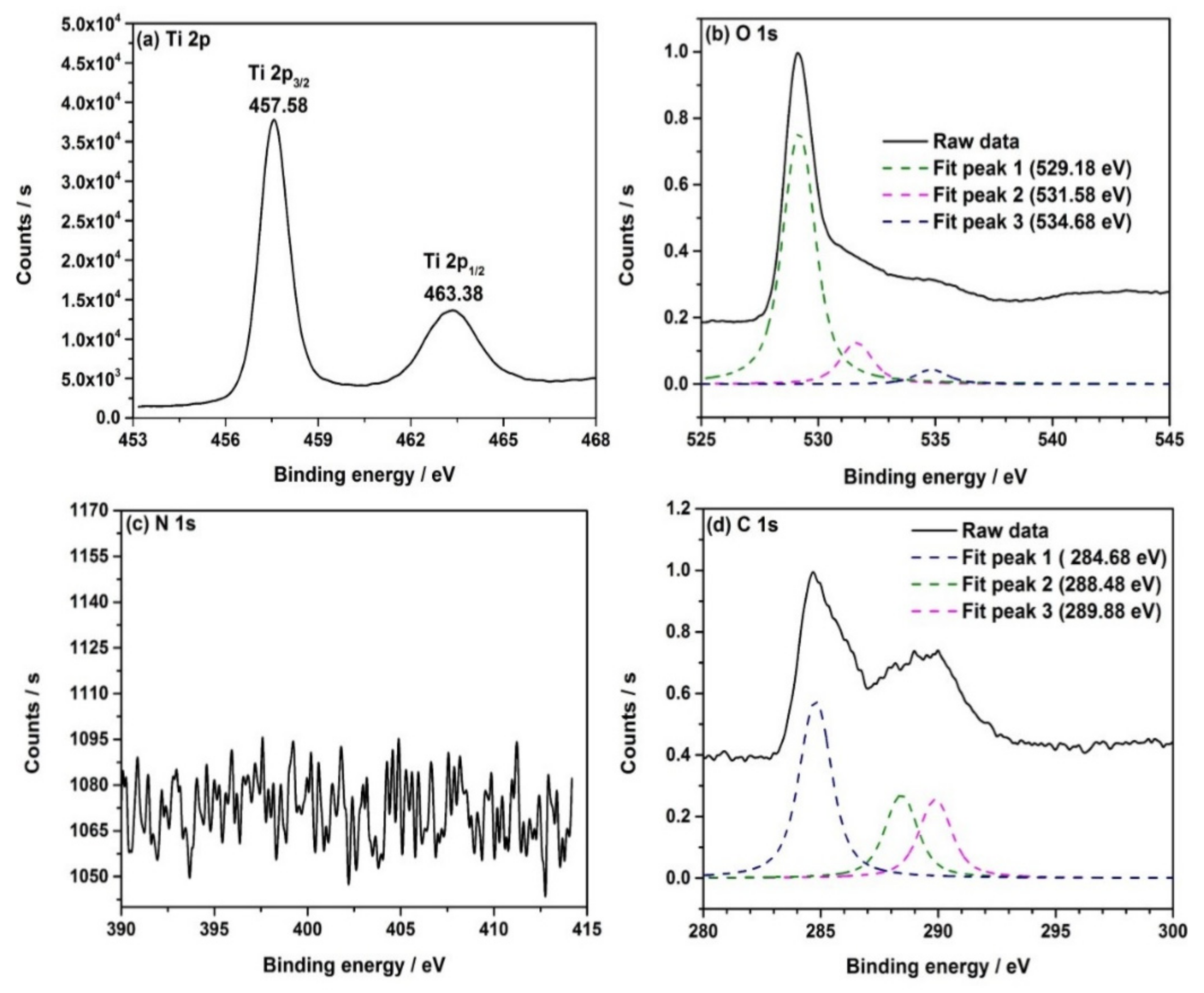
3.7. X-ray Photoelectron Spectroscopy (XPS)
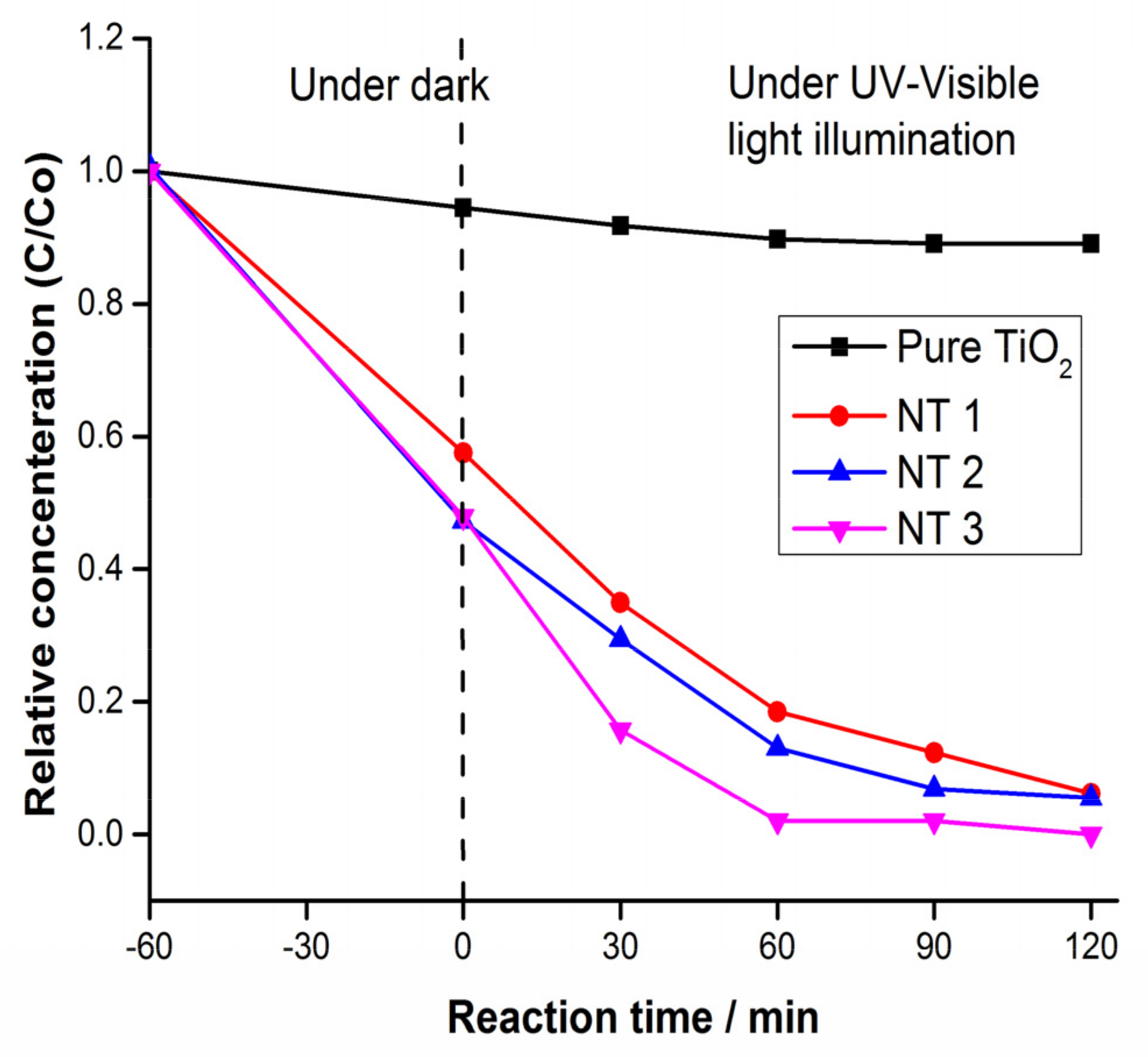
3.8. Photocatalytic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudakkar, S.R.; Zaman, K.; Khan, M.M.; Ahmad, M. Energy for economic growth, industrialization, environment and natural resources: Living with just enough. Renew. Sustain. Energy Rev. 2013, 25, 580–595. [Google Scholar] [CrossRef]
- Sihvonen, M.; Pihlainen, S.; Lai, T.Y.; Salo, T.; Hyytiäinen, K. Crop production, water pollution, or climate change mitigation—Which drives socially optimal fertilization management most? Agric. Syst. 2021, 186, 102985. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, J.; Ge, J. Dynamic game in agriculture and industry cross-sectoral water pollution governance in developing countries. Agric. Water Manag. 2021, 243, 106417. [Google Scholar] [CrossRef]
- Le Marechal, A.M.; Krianec, B.; Vajnhandl, S.; Volmajer, J. Textile Finishing Industry as an Important Source of Organic Pollutants. In Organic Pollutants Ten Years after the Stockholm Convention-Environmental and Analytical Update; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Bhaskar, Y.V.; Karthikeyan, J. Biological decolourisation of simulated azo dye in aqueous phase by algae Spirogyra species. Int. J. Environ. Pollut. 2004, 21, 211–222. [Google Scholar] [CrossRef]
- Balapure, K.; Bhatt, N.; Madamwar, D. Mineralization of reactive azo dyes present in simulated textile waste water using down flow microaerophilic fixed film bioreactor. Bioresour. Technol. 2015, 175, 1–7. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef] [Green Version]
- Šíma, J.; Hasal, P. Photocatalytic degradation of textile dyes in aTiO2/UV system. Chem. Eng. Trans. 2013, 32, 79–84. [Google Scholar] [CrossRef]
- Shehzad, N.; Zafar, M.; Ashfaq, M.; Razzaq, A.; Akhter, P.; Ahmad, N.; Hafeez, A.; Azam, K.; Hussain, M.; Kim, W.Y. Development of AgFeO2/rGO/TiO2 Ternary Composite Photocatalysts for Enhanced Photocatalytic Dye Decolorization. Crystals 2020, 10, 923. [Google Scholar] [CrossRef]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Ur Rehman, M.S.; Faisal, A.; Rehman, F. Integrating Adsorption and Photocatalysis: A Cost Effective Strategy for Textile Wastewater Treatment Using Hybrid Biochar-TiO2 Composite; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 390, ISBN 9242111001007. [Google Scholar]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
- Razzaq, A.; In, S.-I. TiO2 Based Nanostructures for Photocatalytic CO2 Conversion to Valuable Chemicals. Micromachines 2019, 10, 326. [Google Scholar] [CrossRef] [Green Version]
- Parayil, S.; Razzaq, A.; Park, S.M.; Kim, H.R.; Grimes, C.A.; In, S. Il Photocatalytic conversion of CO2 to hydrocarbon fuel using carbon and nitrogen co-doped sodium titanate nanotubes. Appl. Catal. A Gen. 2015, 498, 205–213. [Google Scholar] [CrossRef]
- Razzaq, A.; Sinhamahapatra, A.; Kang, T.; Grimes, C.A.; Yu, J.; In, S. Applied Catalysis B: Environmental Efficient solar light photoreduction of CO2 to hydrocarbon fuels via magnesiothermally reduced TiO2 photocatalyst. Appl. Catal. B Environ. 2017, 215, 28–35. [Google Scholar] [CrossRef]
- Razzaq, A.; Grimes, C.A.; In, S.-I. Facile fabrication of a noble metal-free photocatalyst: TiO2 nanotube arrays covered with reduced graphene oxide. Carbon N. Y. 2016, 98, 537–544. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, T.N.; Pillai, C.S.; Seery, K.M.; Falaras, P.; Kontos, A.G.; Dunlop, S.M.P.; Hamilton, W.J.J.; Byrne, A.J.; O’Shea, K.; et al. A review on the visible light active titanium dioixde photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Razzaq, A.; Kim, Y.K.; Kim, S.; In, S.-I. Synthesis and characterization of p latinum modified TiO2-embedded carbon nanofibers for solar hydrogen generation. RSC Adv. 2014, 4, 51286–51293. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible-light irradiation. Appl. Catal. B Environ. 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Park, S.-M.; Razzaq, A.; Park, Y.H.; Sorcar, S.; Park, Y.; Grimes, C.A.; In, S.-I. Hybrid CuxO-TiO2 Heterostructured Composites for Photocatalytic CO2 Reduction into Methane Using Solar Irradiation: Sunlight into Fuel. ACS Omega 2016, 1, 868–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.R.; Razzaq, A.; Grimes, C.A.; In, S.-I. Heterojunction p-n-p Cu2O/S-TiO2/CuO: Synthesis and application to photocatalytic conversion of CO2 to methane. J. CO2 Util. 2017, 20, 91–96. [Google Scholar] [CrossRef]
- Kim, K.; Razzaq, A.; Sorcar, S.; Park, Y.; Grimes, C.A.; In, S.-I. Hybrid mesoporous Cu2ZnSnS4 (CZTS)-TiO2 photocatalyst for efficient photocatalytic conversion of CO2 into CH4 under solar irradiation. RSC Adv. 2016, 6, 38964–38971. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; In, S.-I. Development of graphene based photocatalysts for CO2 reduction to C1 chemicals: A brief overview. Catal. Today 2019, 335, 39–54. [Google Scholar] [CrossRef]
- Zubair, M.; Razzaq, A.; Grimes, C.A.; In, S.-I. Cu2ZnSnS4 (CZTS)-ZnO: A noble metal-free hybrid Z-scheme photocatalyst for enhanced solar-spectrum photocatalytic conversion of CO2 to CH4. J. CO2 Util. 2017, 20, 301–311. [Google Scholar] [CrossRef]
- Zubair, M.; Kim, H.R.; Razzaq, A.; Grimes, C.A.; In, S.-I. Solar spectrum photocatalytic conversion of CO2 to CH4 utilizing TiO2 nanotube arrays embedded graphene quantum dots. J. CO2 Util. 2018, 26, 70–79. [Google Scholar] [CrossRef]
- Wongso, V.; Chen, C.J.; Razzaq, A.; Kamal, N.A.; Sambudi, N.S. Hybrid kaolin/TiO2 composite: Effect of urea addition towards an efficient photocatalyst for dye abatement under visible light irradiation. Appl. Clay Sci. 2019, 180, 105158. [Google Scholar] [CrossRef]
- Ismael, M. A review and recent advances in solar-to-hydrogen energy conversion based on photocatalytic water splitting over doped-TiO2 nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic degradation of pharmaceutical and pesticide compounds (PPCs) using doped TiO2 nanomaterials: A review. Water-Energy Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Sannino, D.; Ciambelli, P. Nanostructured N-doped TiO2 coated on glass spheres for the photocatalytic removal of organic dyes under UV or visible light irradiation. Appl. Catal. B Environ. 2015, 170–171, 153–161. [Google Scholar] [CrossRef]
- Powell, M.J.; Dunnill, C.W.; Parkin, I.P. N-doped TiO2 visible light photocatalyst films via a sol-gel route using TMEDA as the nitrogen source. J. Photochem. Photobiol. A Chem. 2014, 281, 27–34. [Google Scholar] [CrossRef]
- Saravanan, R.; Aviles, J.; Gracia, F.; Mosquera, E.; Gupta, V.K. Crystallinity and lowering band gap induced visible light photocatalytic activity of TiO2/CS (Chitosan) nanocomposites. Int. J. Biol. Macromol. 2018, 109, 1239–1245. [Google Scholar] [CrossRef]
- Liu, T.; Chen, W.; Liu, X.; Zhu, J.; Lu, L. Well-dispersed ultrafine nitrogen-doped TiO2 with polyvinylpyrrolidone (PVP) acted as N-source and stabilizer for water splitting. J. Energy Chem. 2016, 25, 1–9. [Google Scholar] [CrossRef]
- Liu, W.X.; Jiang, P.; Shao, W.N.; Zhang, J.; Cao, W. Bin A novel approach for the synthesis of visible-light-active nanocrystalline N-doped TiO2 photocatalytic hydrosol. Solid State Sci. 2014, 33, 45–48. [Google Scholar] [CrossRef]
- Gohari-Bajestani, Z.; Akhlaghi, O.; Yürüm, Y.; Yürüm, A. Synthesis of anatase TiO2 with exposed (001) facets grown on N-doped reduced graphene oxide for enhanced hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 6096–6103. [Google Scholar] [CrossRef]
- Ferrari-Lima, A.M.; Marques, R.G.; Gimenes, M.L.; Fernandes-Machado, N.R.C. Synthesis, characterisation and photocatalytic activity of N-doped TiO2-Nb2O5 mixed oxides. Catal. Today 2015, 254, 119–128. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, Z.; Liu, W.; Xue, Y.; Yin, S. Solvothermal synthesis of different phase N-TiO2 and their kinetics, isotherm and thermodynamic studies on the adsorption of methyl orange. J. Colloid Interface Sci. 2016, 470, 229–236. [Google Scholar] [CrossRef]
- Bakar, S.A.; Ribeiro, C. Nitrogen-doped titanium dioxide: An overview of material design and dimensionality effect over modern applications. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 1–29. [Google Scholar] [CrossRef]
- Katoueizadeh, E.; Zebarjad, S.M.; Janghorban, K. Synthesis and enhanced visible-light activity of N-doped TiO2 nano-additives applied over cotton textiles. J. Mater. Res. Technol. 2018, 7, 204–211. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Koop-Santa, C.; López-Mena, E.R.; Orozco-Guareño, E.; García-Guaderrama, M. N-doped TiO2 nanoparticles obtained by a facile coprecipitation method at low temperature. Ceram. Int. 2018, 44, 5273–5283. [Google Scholar] [CrossRef]
- Albrbar, A.J.; Djokić, V.; Bjelajac, A.; Kovač, J.; Ćirković, J.; Mitrić, M.; Janaćković, D.; Petrović, R. Visible-light active mesoporous, nanocrystalline N,S-doped and co-doped titania photocatalysts synthesized by non-hydrolytic sol-gel route. Ceram. Int. 2016, 42, 16718–16728. [Google Scholar] [CrossRef]
- Bae, Y.S.; Yazayd’n, A.Ö.; Snurr, R.Q. Evaluation of the BET method for determining surface areas of MOFs and zeolites that contain Ultra-Micropores. Langmuir 2010, 26, 5475–5483. [Google Scholar] [CrossRef] [PubMed]
- Sorcar, S.; Razzaq, A.; Tian, H.; Grimes, C.A.; In, S.-I. Facile electrochemical synthesis of anatase nano-architectured titanium dioxide films with reversible superhydrophilic behavior. J. Ind. Eng. Chem. 2017, 46. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Wang, F.; Di, L.; Yang, S.; Zhu, S.; Yao, Y.; Ma, C.; Dai, B.; Yu, F. Enhanced photocatalytic degradation of organic dyes via defect-rich tio2 prepared by dielectric barrier discharge plasma. Nanomaterials 2019, 9, 720. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, L.; Huang, X.; Li, Q.; Li, G. New insights into fluorinated TiO2 (brookite, anatase and rutile) nanoparticles as efficient photocatalytic redox catalysts. RSC Adv. 2015, 5, 34302–34313. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol-gel synthesis of mesoporous anatase-brookite and anatase-brookite-rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef]
- Parayil, S.K.; Razzaq, A.; In, S.-I. Formation of titania-silica mixed oxides in solvent mixtures and their influences for the photocatalytic CO2 conversion to hydrocarbon. J. Nanosci. Nanotechnol. 2015, 15, 7285–7292. [Google Scholar] [CrossRef]
- Tran, V.A.; Truong, T.T.; Phan, T.A.P.; Nguyen, T.N.; Van Huynh, T.; Agresti, A.; Pescetelli, S.; Le, T.K.; Di Carlo, A.; Lund, T.; et al. Application of nitrogen-doped TiO2 nano-tubes in dye-sensitized solar cells. Appl. Surf. Sci. 2017, 399, 515–522. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z. Characterization and mechanism analysis of N doped TiO2 with visible light response and its enhanced visible activity. Appl. Surf. Sci. 2012, 258, 3244–3248. [Google Scholar] [CrossRef]
- In, S.-I.; Vesborg, C.K.P.; Abrams, B.L.; Hou, Y.; Chorkendof, I. A comparative study of two techniques for determining photocatalytic activity of nitrogen doped TiO2 nanotubes under visible light irradiation: Photocatalytic reduction of dye and photocatalytic oxidation of organic molecules. J. Photchem. Photobiol. A Chem. 2011, 222, 258–262. [Google Scholar] [CrossRef]
- Larumbe, S.; Monge, M.; Gómez-Polo, C. Comparative study of (N, Fe) doped TiO2 photocatalysts. Appl. Surf. Sci. 2015, 327, 490–497. [Google Scholar] [CrossRef]
- Raciti, R.; Bahariqushchi, R.; Summonte, C.; Aydinli, A.; Terrasi, A.; Mirabella, S. Optical bandgap of semiconductor nanostructures: Methods for experimental data analysis. J. Appl. Phys. 2017, 121. [Google Scholar] [CrossRef]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S.; Joshi, H.M.; Patil, S.I.; Kale, B.B.; Ogale, S.B. N-doped TiO2 nanoparticle based visible light photocatalyst by modified peroxide sol-gel method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Chen, Z.; Cheng, H.M.; Lu, G.Q. (Max) The role of crystal phase in determining photocatalytic activity of nitrogen doped TiO2. J. Colloid Interface Sci. 2009, 329, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, L. Selective nonaqueous synthesis of C-Cl-codoped TiO2 with visible-light photocatalytic activity. J. Phys. Chem. C 2010, 114, 11534–11541. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Soares, G.B.; Bravin, B.; Vaz, C.M.P.; Ribeiro, C. Facile synthesis of N-doped TiO2 nanoparticles by a modified polymeric precursor method and its photocatalytic properties. Appl. Catal. B Environ. 2011, 106, 287–294. [Google Scholar] [CrossRef]
- Zhang, L.; Han, M.; Tan, O.K.; Tse, M.S.; Wang, Y.X.; Sze, C.C. Facile fabrication of Ag/C-TiO2 nanoparticles with enhanced visible light photocatalytic activity for disinfection of Escherichia coli and Enterococcus faecalis. J. Mater. Chem. B 2013, 1, 564–570. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Q.; Wang, Y. Semiconductor-based nanocomposites for photocatalytic H2 production and CO2 conversion. Phys. Chem. Chem. Phys. 2013, 15, 2632–2649. [Google Scholar] [CrossRef] [PubMed]




| Photocatalyst | BET Surface Area [m2/g] | Pore Size [nm] |
|---|---|---|
| Pure TiO2 | 42.98 | 0.6427 |
| NT-1 | 38.87 | 0.5947 |
| NT-2 | 30.18 | 0.5788 |
| NT-3 | 23.93 | 0.5776 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asif, M.; Zafar, M.; Akhter, P.; Hussain, M.; Umer, A.; Razzaq, A.; Kim, W.-Y. Effect of Urea Addition on Anatase Phase Enrichment and Nitrogen Doping of TiO2 for Photocatalytic Abatement of Methylene Blue. Appl. Sci. 2021, 11, 8264. https://doi.org/10.3390/app11178264
Asif M, Zafar M, Akhter P, Hussain M, Umer A, Razzaq A, Kim W-Y. Effect of Urea Addition on Anatase Phase Enrichment and Nitrogen Doping of TiO2 for Photocatalytic Abatement of Methylene Blue. Applied Sciences. 2021; 11(17):8264. https://doi.org/10.3390/app11178264
Chicago/Turabian StyleAsif, Maira, Muhammad Zafar, Parveen Akhter, Murid Hussain, Adeel Umer, Abdul Razzaq, and Woo-Young Kim. 2021. "Effect of Urea Addition on Anatase Phase Enrichment and Nitrogen Doping of TiO2 for Photocatalytic Abatement of Methylene Blue" Applied Sciences 11, no. 17: 8264. https://doi.org/10.3390/app11178264
APA StyleAsif, M., Zafar, M., Akhter, P., Hussain, M., Umer, A., Razzaq, A., & Kim, W.-Y. (2021). Effect of Urea Addition on Anatase Phase Enrichment and Nitrogen Doping of TiO2 for Photocatalytic Abatement of Methylene Blue. Applied Sciences, 11(17), 8264. https://doi.org/10.3390/app11178264








