The Study of Corrosion Behaviors of Carbon Steel Weldments and Their Inhibition in Simulated Pore Solution Using Multi-Electrode Array Technique
Abstract
:1. Introduction
2. Experimental Methods
2.1. The Preparation of the Electrodes and the Corrosion Coupon
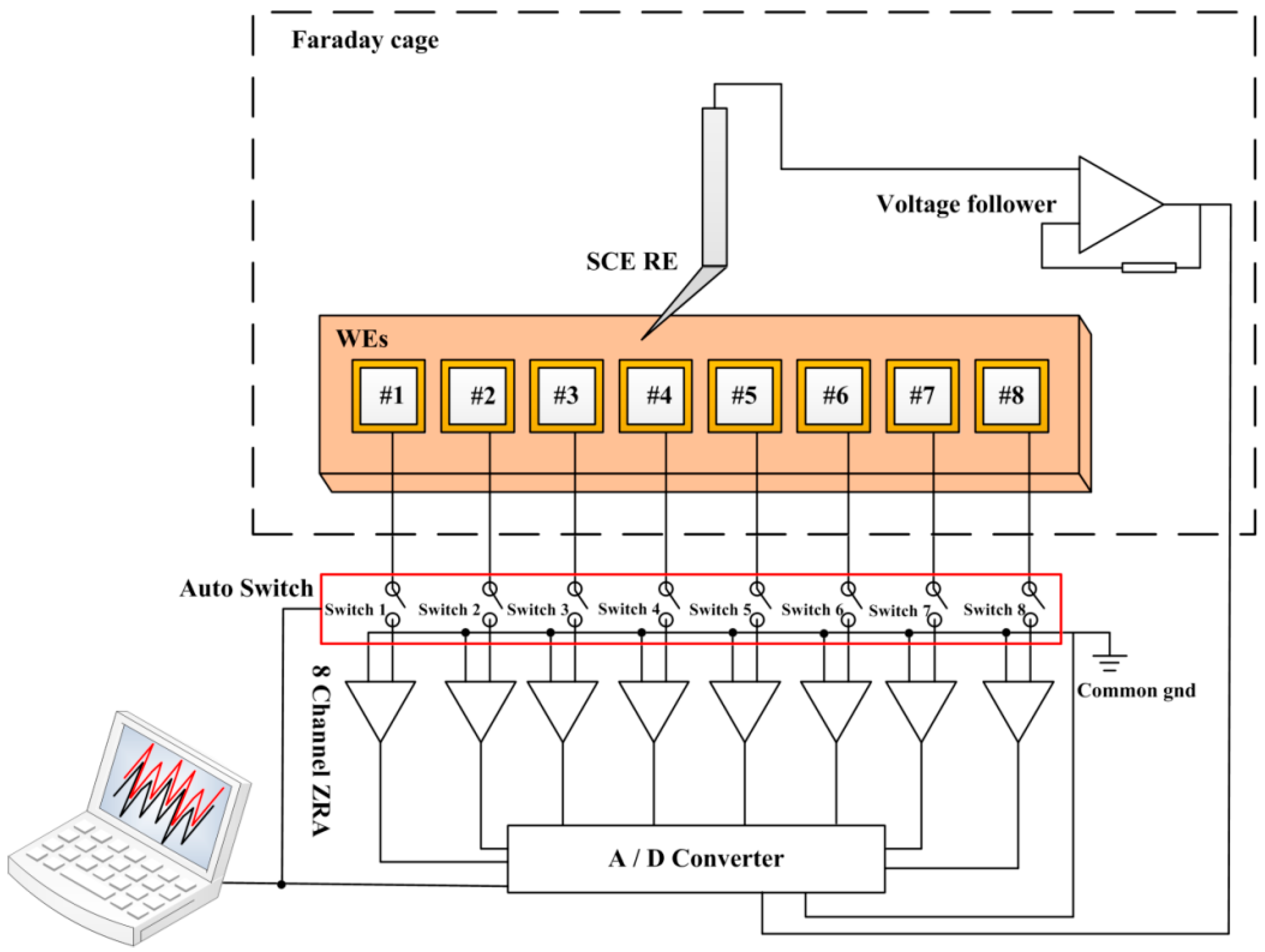
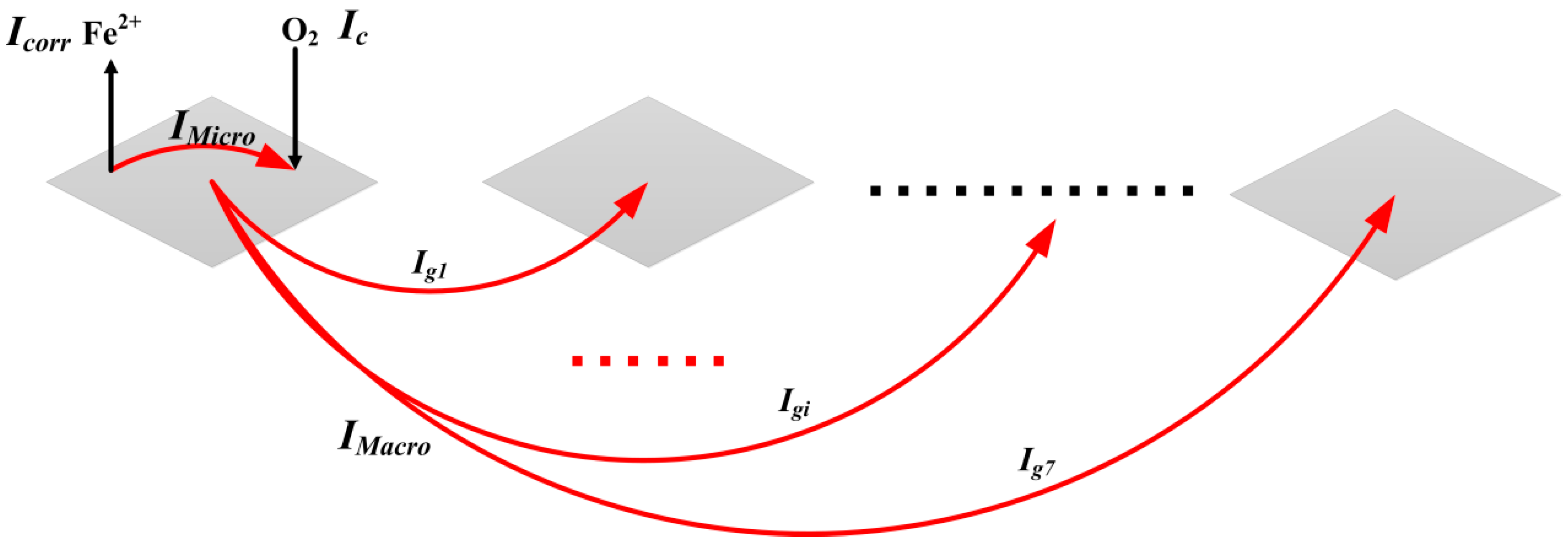
2.2. The Coupled Multi-Electrode Array Sensor System
2.3. Experimental Procedures
3. Experimental Results and Discussions
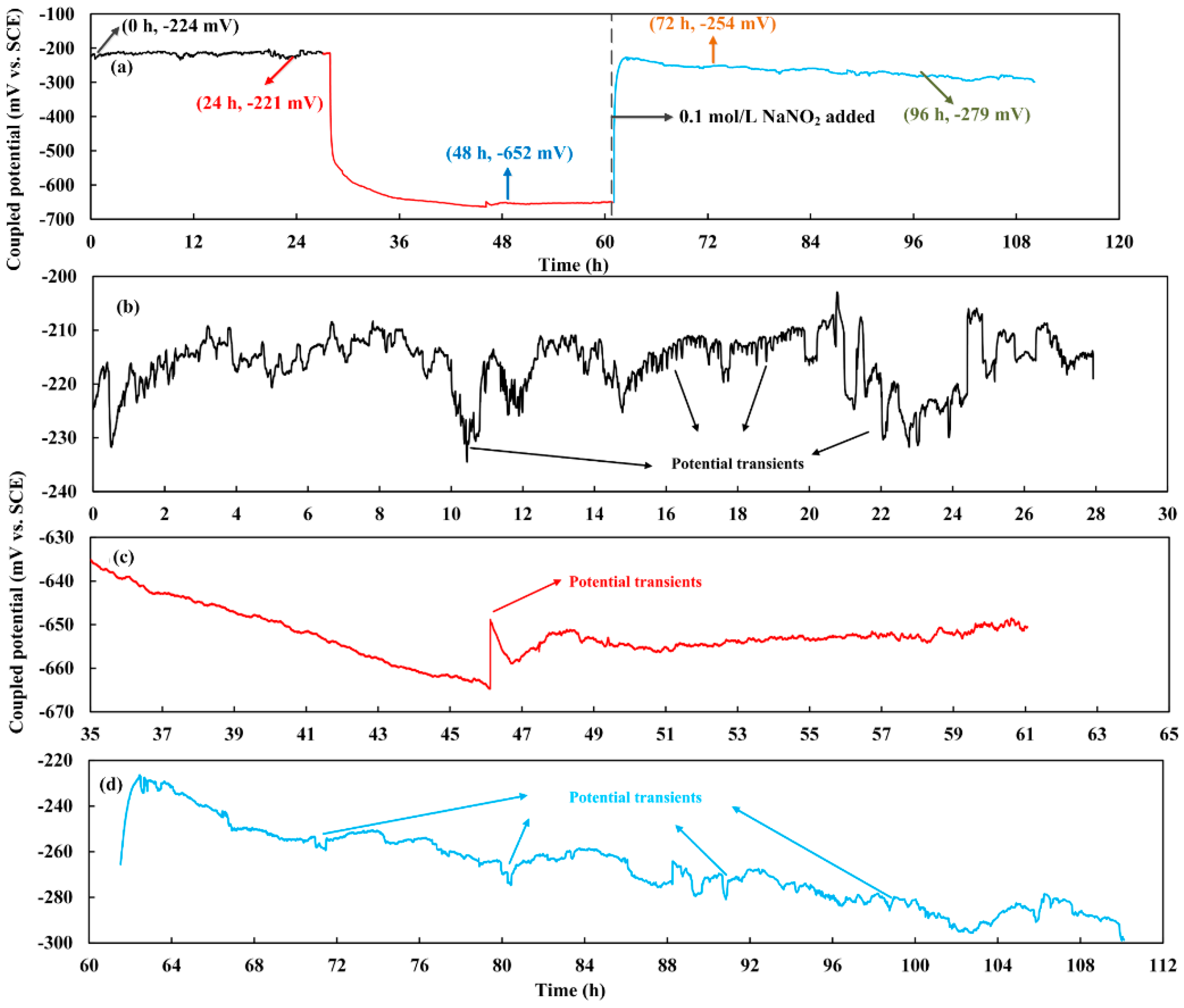
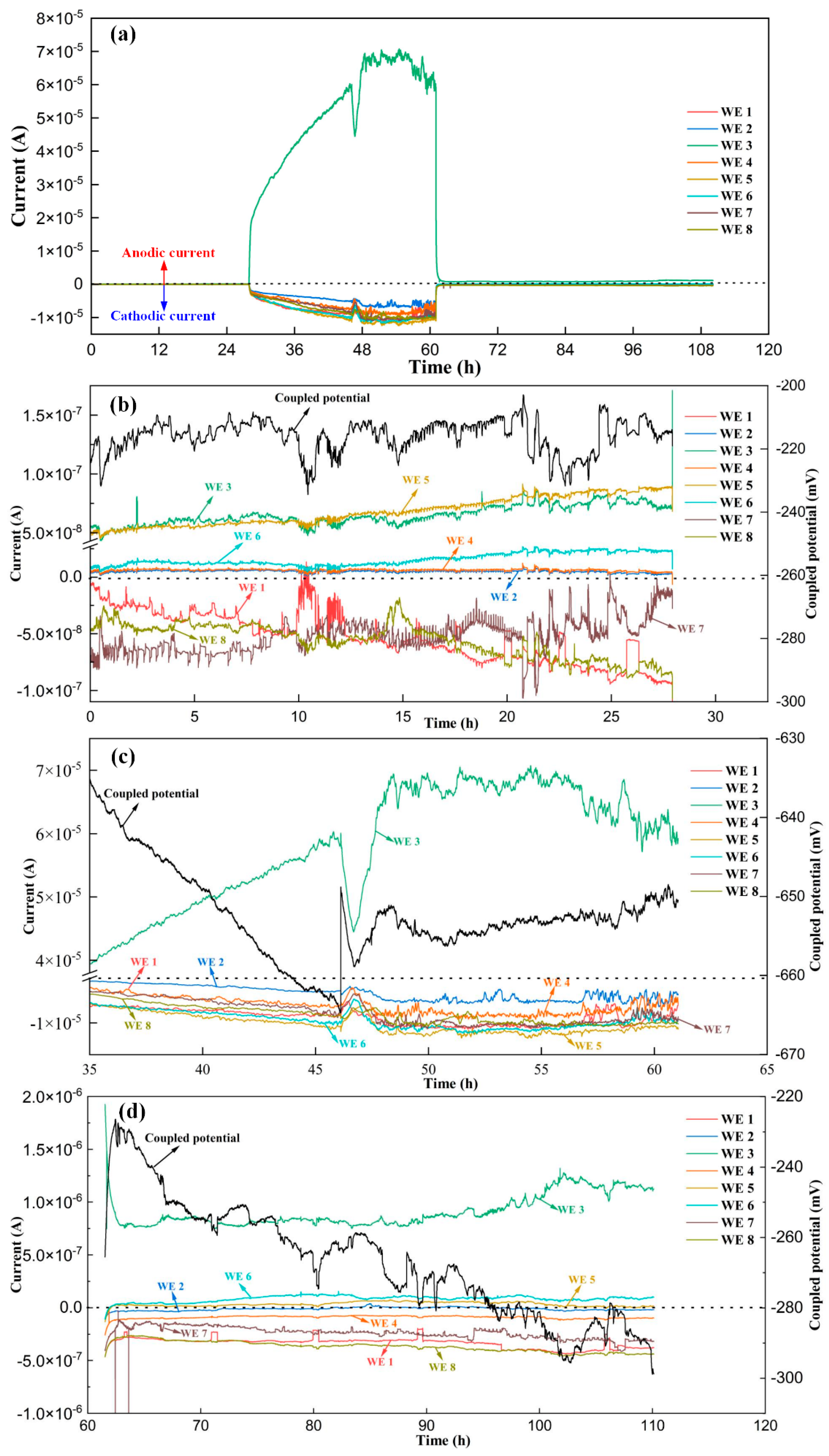
3.1. Measurement Results of the Coupled Potential and the OCP
3.2. Measurement Results of the Coupled Potential and the OCP
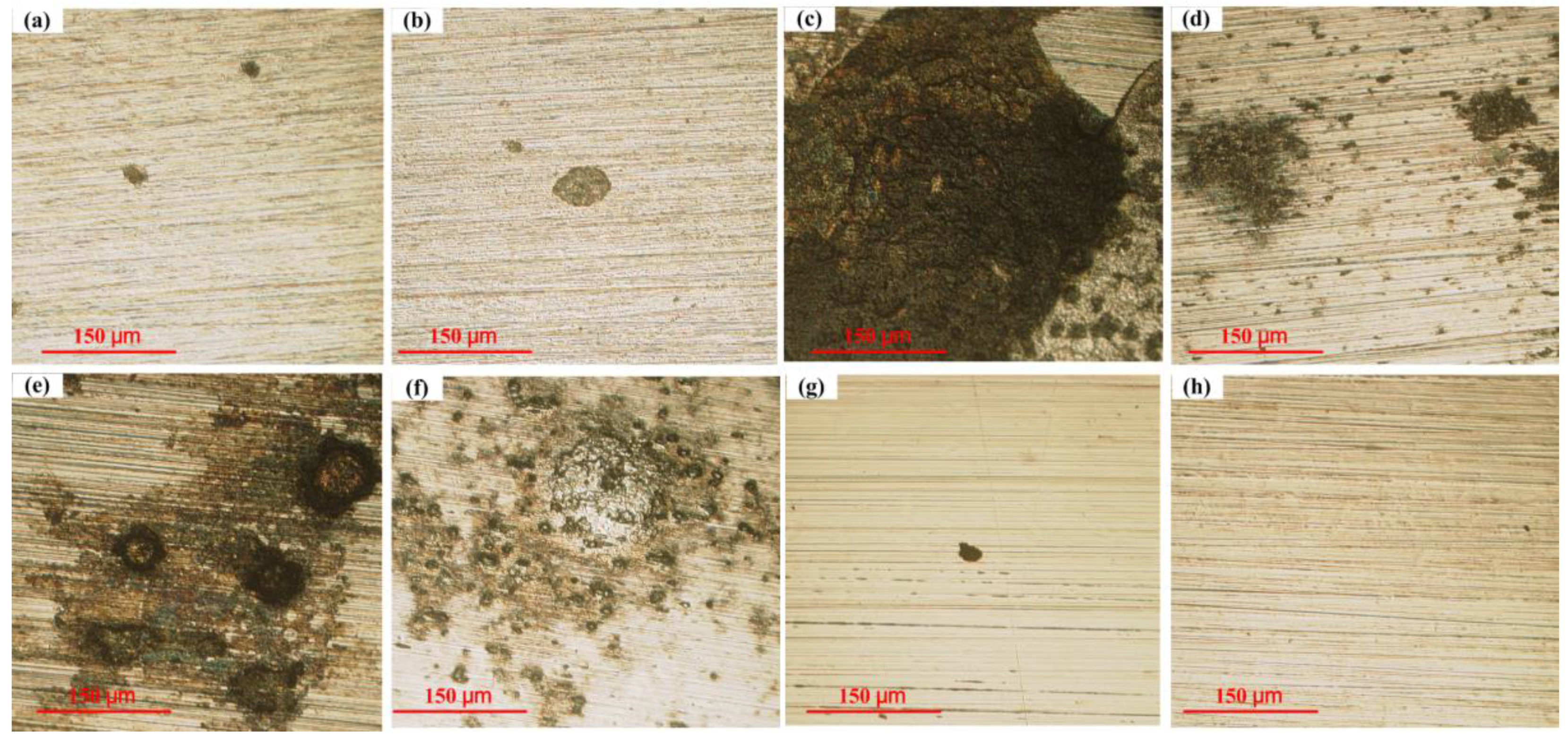
3.3. The Morphologies of the Working Electrodes and the Corrosion Coupon
3.4. The Measurement Error Analysis of the Multi-Electrode Sensor System
4. Conclusions
- (1)
- A complete welded structure can be well simulated by using the coupled multi-electrode array sensor system. The OCP of the electrodes, the coupled potential, and the galvanic currents among the electrodes can be online monitored. The maximum penetration rate of the weldment can be measured when serious localized corrosion happens. The metastable pitting corrosion and stable pitting corrosion process can also be revealed by the sensor system. This sensor system can be used as an effective method to monitor corrosion of weldments in marine concrete structures.
- (2)
- The passive film on the WM area and the HAZ closings to the WM area are fragile and it is easier for them to be destroyed by the aggressive ions in the marine environment. These areas will confront high preferential corrosion risks and they always act as anodes when coupled with the BM area. Once stable pits were formed on the WM area or the HAZ, a localized anodic area will form and leads to a high penetration rate. The high galvanic current among the WM zone, HAZ, and BM zone will also result in the acceleration of the localized corrosion. The highest localized corrosion rate of the weldment in the carbonated pore solution containing 0.2 mol/L Cl− can reach a value higher than 0.8 mm/a, which will result in the fast failure of the marine structure.
- (3)
- The inhibitor NaNO2 has an obvious inhibition effect on the corrosion of weldments when the ratio of NO2− and Cl− is 0.5. The maximum anodic current has a nearly 80 times decrease after the inhibitor was added. The OCP of the localized corrosion area also recovered with the introduction of the inhibitor. It indicates that NO2− has a strong repair function for the broken passive film on the WM zone and HAZ in carbonated pore solution.
- (4)
- The localized corrosion rate and the corrosion depths of the electrodes will be underestimated only based on the anodic current and anodic electric charges obtained by the sensor system. The electrodes with cathodic currents cannot be directly considered as the pure cathodic area. Both negative galvanic current and microcurrent may exist on these electrodes. For the pure anodic electrode, the main measurement error of the maximum localized corrosion rate is caused by its internal micro-current. When the coupled potential is close to the OCP of the pure anodic electrode, the measurement of the corrosion current at the OCP can provide a further evaluation of the maximum penetration rate.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, M.K.; Subramaniana, K.B.; Saha, S. Behaviour of prefabricated steel-concrete composite slabs with a novel interlocking system -Numerical Analysis. Eng. Struct. 2021, 245, 112905. [Google Scholar] [CrossRef]
- Haryanto, Y.; Wariyatno, N.G.; Hu, H.T. Investigation on Structural Behavior of Bamboo Reinforced Concrete Slabs under Concentrated Load. Sains Malays. 2021, 50, 227–238. [Google Scholar] [CrossRef]
- Xie, J.H.; Wei, M.W.; Huang, P.Y. Fatigue behavior of the basalt fiber-reinforced polymer/concrete interface under wet-dry cycling in a marine environment. Constr. Build. Mater. 2019, 228, 117065. [Google Scholar] [CrossRef]
- Gholipour, G.; Zhang, C.; Mousavi, A.A. Nonlinear failure analysis of bridge pier subjected to vessel impact combined with blast loads. Ocean. Eng. 2021, 234, 109209. [Google Scholar] [CrossRef]
- Liu, Y.; Asselin, E. Communication—The Galvanic Effect on the Under-Deposit Corrosion of Titanium in Chloride Solutions. J. Electrochem. Soc. 2021, 168, 071512. [Google Scholar] [CrossRef]
- Sohail, M.G.; Laurens, S.; Deby, F.; Balayssac, J.P.; Al Nuaimi, N. Electrochemical corrosion parameters for active and passive reinforcing steel in carbonated and sound concrete. Mater. Corros. 2021. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, C.G.; Na, W.B.; Kim, J.K. Chemical degradation characteristics of reinforced concrete reefs in South Korea. Ocean. Eng. 2008, 35, 738–748. [Google Scholar] [CrossRef]
- Bautista, A.; Paredes, E.C.; Alvarez, S.M.; Velasco, F. Welded, sandblasted, stainless steel corrugated bars in non-carbonated and carbonated mortars: A 9-year corrosion study. Corros. Sci. 2016, 102, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, R.I.; Sakka, Z.I. Strength and ductility of reinforced concrete slabs containing welded wire fabric and subjected to support settlement. Eng. Struct. 2010, 32, 1509–1521. [Google Scholar] [CrossRef]
- Ayyub, B.M.; Mutairi, N.A.; Chang, P. Structural strength of bidge decks reinforces with welded wire fabric. J. Struct. Eng. 1996, 122, 989–997. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Li, K. Experimental study on non-uniform corrosion of elbow-to-pipe weldment using multiple ring form electrical resistance sensor array. Measurement 2019, 138, 8–24. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Song, S. Probing the nonuniform corrosion of pipeline weldments under stepwise increasing solution temperature using a coupled multielement electrical resistance sensor. Mater. Corros. 2020, 71, 1386–1399. [Google Scholar] [CrossRef]
- Mohammadi, F.; Eliyan, F.F.; Alfantazi, A. Corrosion of simulated weld HAZ of API X-80 pipeline steel. Corros. Sci. 2012, 63, 323–333. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, L.J.; Xu, Y.Z.; Cao, Y.Z.; Song, S.D. A novel system for corrosion protection of reinforced steels in the underwater zone, Corrosion Engineering. Sci. Technol. 2016, 51, 566–572. [Google Scholar]
- James, A.; Bazarchi, E.; Chiniforush, A.A. Rebar corrosion detection, protection, and rehabilitation of reinforced concrete structures in coastal environments: A review. Constr. Build. Mater. 2019, 224, 1026–1039. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, L.; Feng, Z.; Frankel, G.S.; Lu, M.; Chang, W. Galvanic corrosion of a welded joint in 3Cr low alloy pipeline steel. Corros. Sci. 2016, 111, 391–403. [Google Scholar] [CrossRef]
- Scott, R.H.; Chikermane, S.; Vidakovic, M. Development of low cost packaged fibre optic sensors for use in reinforced concrete structures. Measurement 2019, 135, 617–624. [Google Scholar] [CrossRef]
- Bouzaffour, K.; Lescop, B.; Talbot, P. Development of an Embedded UHF-RFID Corrosion Sensor for Monitoring Corrosion of Steel in Concrete. IEEE Sens. J. 2021, 21, 12306–12312. [Google Scholar]
- Huang, Y.; Xu, Y.; Li, B.; Ying, L.; Yang, F.; Wang, X. Novel electrical resistance method to measure underdeposit corrosion and its inhibition in pipeline steels, Corrosion Engineering. Sci. Technol. 2016, 51, 211–222. [Google Scholar]
- Tan, Y.; Fwu, Y.; Bhardwaj, K. Electrochemical evaluation of under-deposit corrosion and its inhibition using the wire beam electrode method. Corros. Sci. 2011, 53, 1254–1261. [Google Scholar] [CrossRef]
- Karelina, M.Y.; Didmanidze, O.N. Methods of electrochemical research of corrosion resistance of metal treated with corrosion inhibitors, created on the basis of plant-based raw materials. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Digital Solutions for Automotive Industry, Roadway Maintenance and Traffic Control (DS ART 2020), Moscow, Russia, 14–16 December 2020; IOP Publishing: Bristol, UK, 2021; Volume 1159, p. 012086. [Google Scholar]
- Tan, Y. Sensing localised corrosion by means of electrochemical noise detection and analysis. Sens. Actuators B Chem. 2009, 139, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.H.; Shi, W.; Guo, X.P. Initiation and repassivation of pitting corrosion of carbon steel in carbonated concrete pore solution. Corros. Sci. 2011, 53, 1322–1330. [Google Scholar] [CrossRef]
- Dong, Z.H.; Shi, W.; Zhang, G.A.; Guo, X.P. The role of inhibitors on the repassivation of pitting corrosion of carbon steel in synthetic carbonated concrete pore solution. Electrochim. Acta 2011, 56, 5890–5897. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Yang, L.J.; He, L.M.; Huang, Y.; Wang, X.N. The monitoring of galvanic corrosion behaviour caused by mineral deposit in pipeline working conditions using ring form electronic resistance sensor system. Corros. Eng. Sci. Technol. 2016, 51, 606–620. [Google Scholar] [CrossRef]
- Sun, X.; Sun, D.; Yang, L. Corrosion monitoring under cathodic protection conditions using multielectrode array sensors. In Techniques for Corrosion Monitoring, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Naganuma, A.; Fushimi, K.; Azumi, K.; Habazaki, H.; Konno, H. Application of the multichannel electrode method to monitoring of corrosion of steel in an artificial crevice. Corros. Sci. 2010, 52, 1179–1186. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.T.; Sun, X.D. Measurement of cumulative localized corrosion rate using coupled multielectrode array sensors. In Proceedings of the Corrosion Conference and Expo (CORROSION 2007), Nashville, TN, USA, 11 March 2007. [Google Scholar]
- Yang, L.T.; Chiang, K.T.; Yu, H.; Pabalan, R.T.; Dasgupta, B.; Ibarra, L. Threshold chloride levels for localized carbon steel corrosion in simulated concrete pore solutions using coupled multielectrode array sensor. Corrosion 2014, 70, 850–857. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Wang, J.; Peng, X.; Wang, Y.; Zhang, P. Study of corrosion behavior of carbon steel under seawater film using the wire beam electrode method. Corros. Sci. 2014, 80, 523–527. [Google Scholar] [CrossRef]
- Huo, Y.; Tan, M.Y.; Forsyth, M. Visualising dynamic passivation and localised corrosion processes occurring on buried steel surfaces under the effect of anodic transients. Electrochem. Commun. 2016, 66, 21–24. [Google Scholar] [CrossRef]
- Tan, Y. Experimental methods designed for measuring corrosion in highly resistive and inhomogeneous media. Corros. Sci. 2011, 53, 1145–1155. [Google Scholar] [CrossRef]
- Bastos, A.C.; Ferreira, M. Application of the scanning vibrating electrode technique to the characterization of modern coatings. In Handbook of Modern Coating Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–43. [Google Scholar]
- Yang, L.J.; Xu, Y.Z.; Zhu, Y.S. Evaluation of Interaction Effect of Sulfate and Chloride Ions on Reinforcements in Simulated Marine Environment Using Electrochemical Methods. Int. J. Electrochem. Sci. 2016, 11, 6943–6958. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, Y.; Xu, Y.Z. The Roles of Fluid Hydrodynamics, Mass Transfer, Rust Layer and Macro-Cell Current on Flow Accelerated Corrosion of Carbon Steel in Oxygen Containing Electrolyte. J. Electrochem. Soc. 2020, 167, 141510. [Google Scholar] [CrossRef]
- Wood, M.H.; Welbourn, R.J.L. An X-ray and Neutron Reflectometry Study of Iron Corrosion in Seawater. Langmuir 2018, 34, 5990–6002. [Google Scholar] [CrossRef]
- Köliö, A.; Honkanen, M.; Lahdensivu, J. Corrosion products of carbonation induced corrosion in existing reinforced concrete facades. Cem. Concr. Res. 2015, 78, 200–207. [Google Scholar] [CrossRef]
- Yohai, L.; Schreiner, W.; Vázquez, M. Phosphate ions as effective inhibitors for carbon steel in carbonated solutions contaminated with chloride ions. Electrochim. Acta 2016, 202, 231–242. [Google Scholar] [CrossRef]
- Zhu, Y.; Ding, J.; Zhang, J. Effects of Sodium Phosphate and Sodium Nitrite on the Pitting Corrosion Process of X70 Carbon Steel in Sodium Chloride Solution. Materials 2020, 13, 5392. [Google Scholar] [CrossRef] [PubMed]
- ASTM International. ASTM C876-91, Standard Test Method for Half-Cell Potentials of Uncoated Reinforcing Steel in Concrete; Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 1991. [Google Scholar]
- Xu, Y.Z.; Huang, Y.; Ying, L. Corrosion behavior of pipeline steel under deposit corrosion and the inhibition performance of organic phosphine inhibitor. Acta Metall. Sin. 2016, 52, 320–330. [Google Scholar]
- Gsa, B.; Vca, B.; Nbc, D. On the in-situ characterisation of metastable pitting using 316L stainless steel as a case study. Corros. Sci. 2020, 177, 109004. [Google Scholar]
- Glasser, F.P.; Sagoe-Crentsil, K.K. Steel in concrete: Part II Electron microscopy analysis. Mag. Concr. Res. 1989, 149, 213–220. [Google Scholar] [CrossRef]
- Andrade, C.; Garcés, P.; Martínez, I. Galvanic currents and corrosion rates of reinforcements measured in cells simulating different pitting areas caused by chloride attack in sodium hydroxide. Corros. Sci. 2008, 50, 2959–2964. [Google Scholar] [CrossRef]
- Legat, A. Monitoring of steel corrosion in concrete by electrode arrays and electrical resistance probes. Electrochim. Acta 2007, 52, 7590–7598. [Google Scholar] [CrossRef]


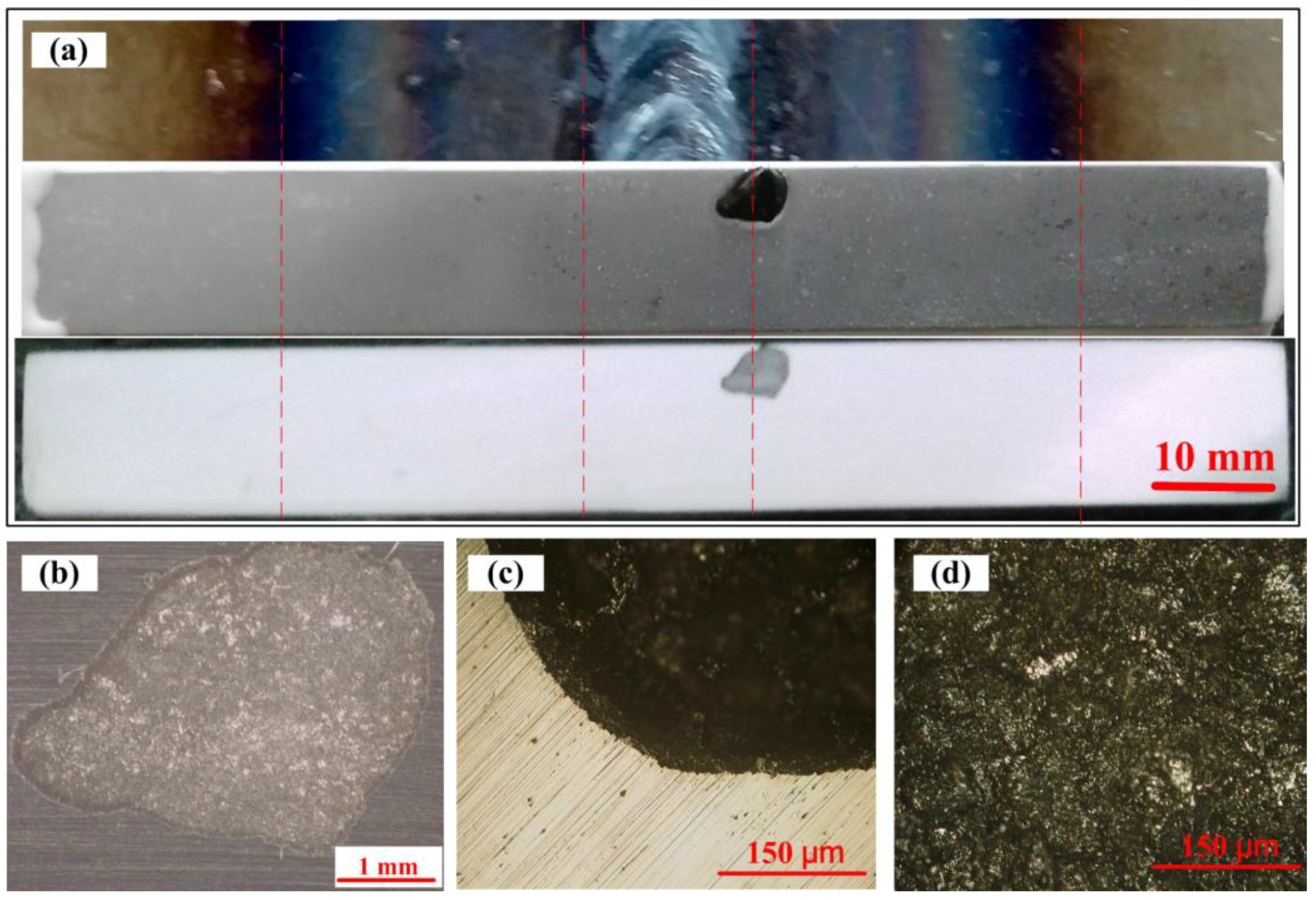

| C | Si | Mn | P | S | Cr | Ni | Fe | |
|---|---|---|---|---|---|---|---|---|
| Q235 | 0.22% | 0.30% | 0.40% | 0.04% | 0.04% | 0.01% | 0.01% | Bal. |
| J442 | 0.25% | 0.18% | 0.31% | 0.04% | 0.03% | <0.01% | <0.01% | Bal. |
| Time (h) | The OCP of the Electrodes (mV) | |||||||
|---|---|---|---|---|---|---|---|---|
| WE 1 | WE 2 | WE 3 | WE 4 | WE 5 | WE 6 | WE 7 | WE 8 | |
| 0 | −180 | −210 | −275 | −219 | −247 | −240 | −224 | −185 |
| 24 | −173 | −207 | −327 | −221 | −263 | −253 | −237 | −169 |
| 48 | −158 | −237 | −697 | −283 | −310 | −279 | −258 | −166 |
| 72 | −150 | −200 | −289 | −190 | −224 | −240 | −175 | −148 |
| 96 | −161 | −235 | −313 | −232 | −247 | −259 | −203 | −157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Ma, Z.; Zheng, Y.; Wang, X.; Huang, Y.; Wang, K.; Song, S.; Jin, W. The Study of Corrosion Behaviors of Carbon Steel Weldments and Their Inhibition in Simulated Pore Solution Using Multi-Electrode Array Technique. Appl. Sci. 2021, 11, 8278. https://doi.org/10.3390/app11188278
Yang L, Ma Z, Zheng Y, Wang X, Huang Y, Wang K, Song S, Jin W. The Study of Corrosion Behaviors of Carbon Steel Weldments and Their Inhibition in Simulated Pore Solution Using Multi-Electrode Array Technique. Applied Sciences. 2021; 11(18):8278. https://doi.org/10.3390/app11188278
Chicago/Turabian StyleYang, Lujia, Zhenping Ma, Yufeng Zheng, Xiaona Wang, Yi Huang, Kangchen Wang, Shenyou Song, and Wenliang Jin. 2021. "The Study of Corrosion Behaviors of Carbon Steel Weldments and Their Inhibition in Simulated Pore Solution Using Multi-Electrode Array Technique" Applied Sciences 11, no. 18: 8278. https://doi.org/10.3390/app11188278
APA StyleYang, L., Ma, Z., Zheng, Y., Wang, X., Huang, Y., Wang, K., Song, S., & Jin, W. (2021). The Study of Corrosion Behaviors of Carbon Steel Weldments and Their Inhibition in Simulated Pore Solution Using Multi-Electrode Array Technique. Applied Sciences, 11(18), 8278. https://doi.org/10.3390/app11188278






