Effects of Temperature on the FT NIR Raman Spectra of Fish Skin Collagen
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Raman Spectra of FS and BAT Collagen Measured at Room Temperature
3.2. Temperature Effect on the FS Collagen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- de Melo Oliveira, V.; Assis, C.R.D.; Costa, B.D.A.M.; de Araujo Neri, R.C.; Monte, F.T.D.; da Costa Vasconcelos, H.M.S.; Franca, R.C.P.; Santos, J.F.; Bezerra, R.d.S.; Porto, A.L.F. Physical, biochemical, densitometric and spectroscopic techniques for characterization collagen from alternative sources: A review based on the sustainable valorization of aquatic by-products. J. Mol. Struct. 2021, 1224, 129023. [Google Scholar] [CrossRef]
- Manon-Jensen, T.; Kjeld, N.G.; Karsdal, M.A. Collagen-mediated hemostasis. J. Thromb. Haemost. 2016, 14, 438–448. [Google Scholar] [CrossRef]
- Kubisz, L.; Hojan-Jezierska, D.; Szewczyk, M.; Majewska, A.; Kawałkiewicz, W.; Pankowski, E.; Janus, M.; Cwajda-Białasik, J.; Mościcka, P.; Jawień, A. In vivo electrical impedance measurement in human skin assessment. Pure Appl. Chem. 2019, 91, 1481–1491. [Google Scholar] [CrossRef]
- Park, K.H.; Kwon, J.B.; Park, J.H.; Shin, J.C.; Han, S.H.; Lee, J.W. Collagen dressing in the trement of diabetic foot ulcer: A prospective, randomized, placebo-controlled, single-center study. Diabetes Res. Clin. Pract. 2020, 156, 107861. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. 2019, 99, 1509–1522. [Google Scholar] [CrossRef]
- Chong, C.; Wang, Y.; Fathi, A.; Parungao, R.; Maitz, P.K.; Li, Z. Skin wound repair: Results of a pre-clinical study to evaluate electropsun collagen–elastin–PCL scaffolds as dermal substitutes. Burns 2019, 45, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Lukáč, P.; Hartinger, J.M.; Mlček, M.; Popková, M.; Suchý, T.; Šupová, M.; Zavora, L.; Adamkova, V.; Benakova, H.; Slanar, O. A novel gentamicin-releasing wound dressing prepared from freshwater fish Cyprinus carpio collagen cross-linked with carbodiimide. J. Bioact. Compat. Polym. 2019, 34, 246–262. [Google Scholar] [CrossRef]
- Cwajda-Białasik, J.; Mościcka, P.; Szewczyk, M.T.; Hojan-Jezierska, D.; Kawałkiewicz, W.; Majewska, A.; Janus-Kubiak, M.; Kubisz, L.; Jawień, A. Venous leg ulcers treated with fish collagen gelin a 12-week randomized single-centre study. Adv. Dermatol. Allergol. 2021, 38, 1–9. [Google Scholar] [CrossRef]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.G.; Walles, H.; Groeber-Becker, F. Cross-linked collagen hydrogel matrix resisting contraction to facilitate full-thickness skin equivalents. ACS Appl. Mater. Interfaces 2017, 9, 204. [Google Scholar] [CrossRef]
- Miri, A.K.; Muja, N.; Kamranpour, N.O.; Lepry, W.C.; Boccaccini, A.R.; Clarke, S.A.; Nazhat, S.N. Ectopic bone formation in rapidly fabricated acellular injectable dense collagen-bioglass hybrid scaffolds via gel aspiration-ejection. Biomaterials 2016, 85, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A.; Skrzyński, S.; Śmiechowski, K.; Kołodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2017, 28, 4–9. [Google Scholar] [CrossRef]
- Chen, L.T.S.; Su, W.; Weng, W.; Osako, K.; Tanaka, M. Physicochemical properties and film-forming ability of fish skin collagen extracted from different freshwater species. Process. Biochem. 2015, 50, 148–155. [Google Scholar]
- Bardakova, K.N.; Grebenik, E.A.; Minaev, N.V.; Churbanov, S.N.; Moldagazyeva, Z.; Krupinov, G.E.; Kostjuk, S.V.; Timasheva, P.S. Tailoring the collagen film structural properties via direct laser crosslinking of star-shaped polylactide for robust scaffold formation. Mater. Sci. Eng. 2020, 107, 110300. [Google Scholar] [CrossRef] [PubMed]
- Szumała, P.; Jungnickel, C.; Kozłowska-Tylingo, K.; Jacyna, B.; Cal, K. Transdermal transport of collagen and hyaluronic acid using water in oil microemulsion. Int. J. Pharm. 2019, 572, 118738. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Lin, Y.S.; Brodsky, B. Collagen interactions: Drug design and delivery. Adv. drug Deliv. Rev. 2016, 97, 69–84. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Busra, M.F.M.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Idrus, R.B.H. Collagen type I: A versatile biomaterial. Nov. Biomater. Regen. Med. 2018, 1077, 389–414. [Google Scholar]
- Blidi, E.; del Omari, N.; Balahbib, A.; Ghchime, R.; del Menyiy, N.; Ibrahimi, A.; Kaddour, K.B.; Bouyahya, A.; Chokairi, O.; Barkiyou, M. Extraction Methods, Characterization and Biomedical Applications of Collagen: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13587–13613. [Google Scholar] [CrossRef]
- Salvatorea, L.; Gallob, N.; Natalia, M.L.; Campaa, L.; Lunettib, P.; Madaghieleb, M.; Blasib, F.S.; Corallob, A.; Capobiancoc, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. 2020, 113, 110963. [Google Scholar] [CrossRef]
- Rahman, A. Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications. Mar. Drugs 2018, 16, 118. [Google Scholar] [CrossRef] [Green Version]
- Jayaraman, A.; Price, C.; Sullivan, M.O.; Kiick, K.L. Collagen-Peptide-Based Drug Delivery Strategies. Technol. Innov. 2020, 21, 1–20. [Google Scholar] [CrossRef]
- Przybylski, J.E.; Siemaszko-Przybylska, K. Patent 190737 Patent Office, Poland. 2002. [Google Scholar]
- Sun, J.; Zhang, J.; Zhao, D.; Xue, C.; Liu, Z.; Mao, X. Characterization of turbot (scophthalmus maximus) skin and the extracted acid-soluble collagen. J. Ocean Univ. China 2019, 18, 687–692. [Google Scholar] [CrossRef]
- Rodziewicz-Motowidło, S.; Śladewska, A.; Mulkiewicz, E.; Kołodziejczyk, A.; Aleksandrowicz, A.; Miszkiewicz, J.; Stepnowski, P. Isolation and characterization of a thermally stable collagen preparation from the outer skin of the silver carp Hypophthalmichthys molitrix. Aquaculture 2008, 285, 130–134. [Google Scholar] [CrossRef]
- Janus, M.; Pankowski, E.; Hojan-Jezierska, D.; Kubisz, L. Study of fish skin collagen by viscosimetric method. In Proceedings of the 24th Annual World Forum on Advanced Materials, Poznań, Poland, 9–13 May 2016; pp. 76–77. [Google Scholar]
- Yunoki, S.; Suzuki, T.; Takai, M. Stabilization of low denaturation temperature collagen from fish by physical cross-linking methods. J. Biosci. Bioeng. 2003, 96, 575–577. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Bella, J.; Eaton, M.; Brodsky, B.; Berman, H.M. Crystal and molecular structure of a collagen like peptide at 1.9 Å resolution. Science 1994, 266, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Bella, J.; Berman, H.M. Crystallographic evidence for Cα–H··· O= C hydrogen bonds in a collagen triple helix. J. Mol. Biol. 1996, 264, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Xu, X.; Iguchi, M.; Noguchi, K. Revision of collagen structure. Biopolymers 2005, 84, 181–191. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagen-structure, function, and biosynthesis. Adv. Drug Del. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Bonifacio, A.; Sergo, V. Effects of sample orientation in Raman microspectroscopy of collagen fibers and their impact on the interpretation of the amide III band. Vib. Spectr. 2010, 53, 214–217. [Google Scholar] [CrossRef]
- Vogel, A.; Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef] [Green Version]
- Improta, R.; Benzi, C.; Barone, V. Understanding the role of stereelectronic effects in determining collagen stability. I. A quantum mechanical study of proline, hydroxyproline, and fluoroproline dipeptide analogues in aqueous solution. J. Am. Chem. Soc. 2001, 123, 12568–12577. [Google Scholar] [CrossRef] [PubMed]
- Carcamo, J.J.; Aliaga, A.E.; Clavijo, R.E.; Garrido, C.; Gomez-Jeria, J.S.; Campos-Vallette, M.M. Proline and hydroxyproline deposited on silver nanoparticles. A Raman, SERS and theoretical study. J. Raman Spectr. 2011, 439, 750–755. [Google Scholar] [CrossRef]
- Overman, S.A.; Thomas, G.J., Jr. Amide modes of the α-Helix:Raman Spectroscopy of Filamentous Virus fd containind peptide 13C and 2H labels in coat protein subunits. Biochemistry 1998, 37, 5654–5665. [Google Scholar] [CrossRef] [PubMed]
- Gullekson, K.; Lucas, L.; Hewitt, K.; Kreplak, L. Surface sensitive Raman spectroscopy of collagen I fibrils. Biophys. J. 2011, 100, 1837–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, C.L.; Bretscher, L.E.; Guzei, I.A.; Raines, R.T. Effect of 3-hydroxyproline residues on collagen stability. J. Am. Chem. Soc. 2003, 125, 5422–5427. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Sionkowska, A.; Kaczmarek, H.; Lazare, S.; Tokarev, V.; Belin, C. Spectroscopic study of a KrF excimer laser treated surface of the thin collagen films. J. Photochem. Photobiol. A Chem. 2007, 188, 192–199. [Google Scholar] [CrossRef]
- Renugopalakrishnan, V.; Carreira, L.A.; Collette, T.W.; Dobbs, J.C.; Chandraksasan, G.; Lord, R.C. Non-uniform triple helical structure in chick skin type I collagen on thermal denaturation: Raman spectroscopic study. Z. Naturforsch. 1998, 53, 383–388. [Google Scholar] [CrossRef]
- Careche, M.; Herrero, A.M.; Rodriguez-Casado, A.; del Mazo, M.L.; Carmona, P. Structural changes of hake (Merluccius merluccius L.) fillets: Effects of freezing and frozen storage. J. Agric. Food Chem. 1998, 47, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Herreiro, A.M.; Carmona, P.; Careche, M. Raman spectroscopic study of structural changes in hake (Merluccius merluccius L.) muscle proteins during frozen storage. J. Agric. Food Chem. 2004, 52, 2147–2153. [Google Scholar] [CrossRef] [Green Version]
- Herrero, A.M. Raman spectroscopy for monitoring protein structure in muscle food system. Crit. Rev. Food Sci. Nutr. 2008, 48, 512–523. [Google Scholar] [CrossRef]
- Kumar, R.; Sripriya, R.; Kumar, M.S.; Sehgal, P.K. Physical characterization of succinylated type I collagen by Raman spectra and MALDI-TOF/MS and in vitro evaluation for biomedical application. J. Mol. Struct. 2011, 994, 117–124. [Google Scholar] [CrossRef]
- Carcamo, J.J.; Aliaga, A.E.; Clavijo, R.E.; Branes, M.R.; Campos-Vallette, M.M. Raman study of the shockwave effect on collagens. Spectrochim. Acta Part. A. 2012, 86, 360–365. [Google Scholar] [CrossRef]
- Czerniecka-Kubicka, A.; Neilsen, G.; Dickson, M.S.; Woodfield, B.F.; Janus-Kubiak, M.; Kubisz, L.; Zarzyka, I.; Zielecki, W.; Skotnicki, M.; Hojan-Jezierska, D.; et al. Vibrational heat capacity of silver carp collagen. Int. J. Biol. Macromol. 2020, 163, 833–841. [Google Scholar]
- Kubisz, L.; Hojan-Jezierska, D. Application of in vivo electrical impedance measurement in human skin assessment. In Proceedings of the 1st International Conference on Chemistry for Beauty and Health, BEAUTY—TORUŃ, Torun, Poland, 13–16 June 2018. [Google Scholar]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 1–74. [Google Scholar] [CrossRef] [Green Version]
- Sionkowska, A.; Lewandowska, K.; Adamiak, K. The Influence of UV Light on Rheological Properties of Collagen Extracted from Silver Carp Skin. Materials 2020, 13, 4453. [Google Scholar] [CrossRef]
- Gauza-Włodarczyk, M.; Kubisz, L.; Włodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Wiliams, R.W.; Dunker, A.K. Determination of the secondary structure of proteins from the Amide I band of the laser Raman spectrum. J. Mol. Biol. 1981, 152, 783–813. [Google Scholar] [CrossRef]
- Wiliams, R.W. Estimation of protein secondary structure from the laser Raman amide I spectrum. J. Mol. Biol. 1983, 166, 581–603. [Google Scholar] [CrossRef]
- Alix, A.J.P.; Pedanou, G.; Berjot, M. Fast determination of the quantitative secondary structure of proteins by using some parameters of the Raman amide I band. J. Mol. Struct. 1988, 174, 159–164. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Farwell, D.W.; Holder, J.M.; Lawson, E.E. Fourier-tranform Raman spectroscopy of ivory: II.Spectroscopic analysis and assignments. J. Mol. Str. 1997, 435, 49–58. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. Identification of β-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef]
- Ignatiewa, N.; Zakharkina, O.; Leroy, G.; Sobol, E.; Vorobiewa, N.; Mordon, S. Molecular processes and structural alterations in laser reshaping of cartilage. Laser Phys. Lett. 2007, 4, 749–753. [Google Scholar] [CrossRef]
- Janko, M.; Davydovskaya, P.; Bauer, M.; Zink, A.; Stark, R.W. Anisotropic Raman scattering in collagen bundles. Optics Lett. 2010, 35, 2765–2767. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Sekar, S.; Chandrasekaran, N.; Mukherjee, A.; Sastry, T.P. Vibrational spectroscopic investigation on interaction of sago starch capped silver nanoparticles with collagen: A comparative physicochemical study using FT-IR and FT-Raman techniques. RSC Adv. 2015, 5, 15763–15771. [Google Scholar] [CrossRef]
- Knudsen, L.; Johansson, C.K.; Philipsen, P.A.; Gniadecka, M.; Wulf, H.C. Natural vibrations and reproducibility of in vivo near-infrared Fourier transform Raman spectroscopy of normal human skin. J. Raman Spectrosc. 2002, 33, 574–579. [Google Scholar] [CrossRef]
- Fernandes, R.M.T.; Neto, R.G.C.; Paschoal, C.W.A.; Rohling, J.H.; Bezerra, C.W.B. Collagen films from swim bladders: Preparation method and properties. Colloids Surf. B Biointerfaces 2008, 62, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Safandowska, M.; Pietrucha, K. Effect of fish collagen modification on its thermal and rheological properties. Int. J. Biol. Macromol. 2013, 53, 32–37. [Google Scholar] [CrossRef]
- Pietrucha, K.; Safandowska, M. Dialdehyde cellulose-crosslinked collagen and its physicochemical properties. Process. Biochem. 2015, 50, 2105–2111. [Google Scholar] [CrossRef]



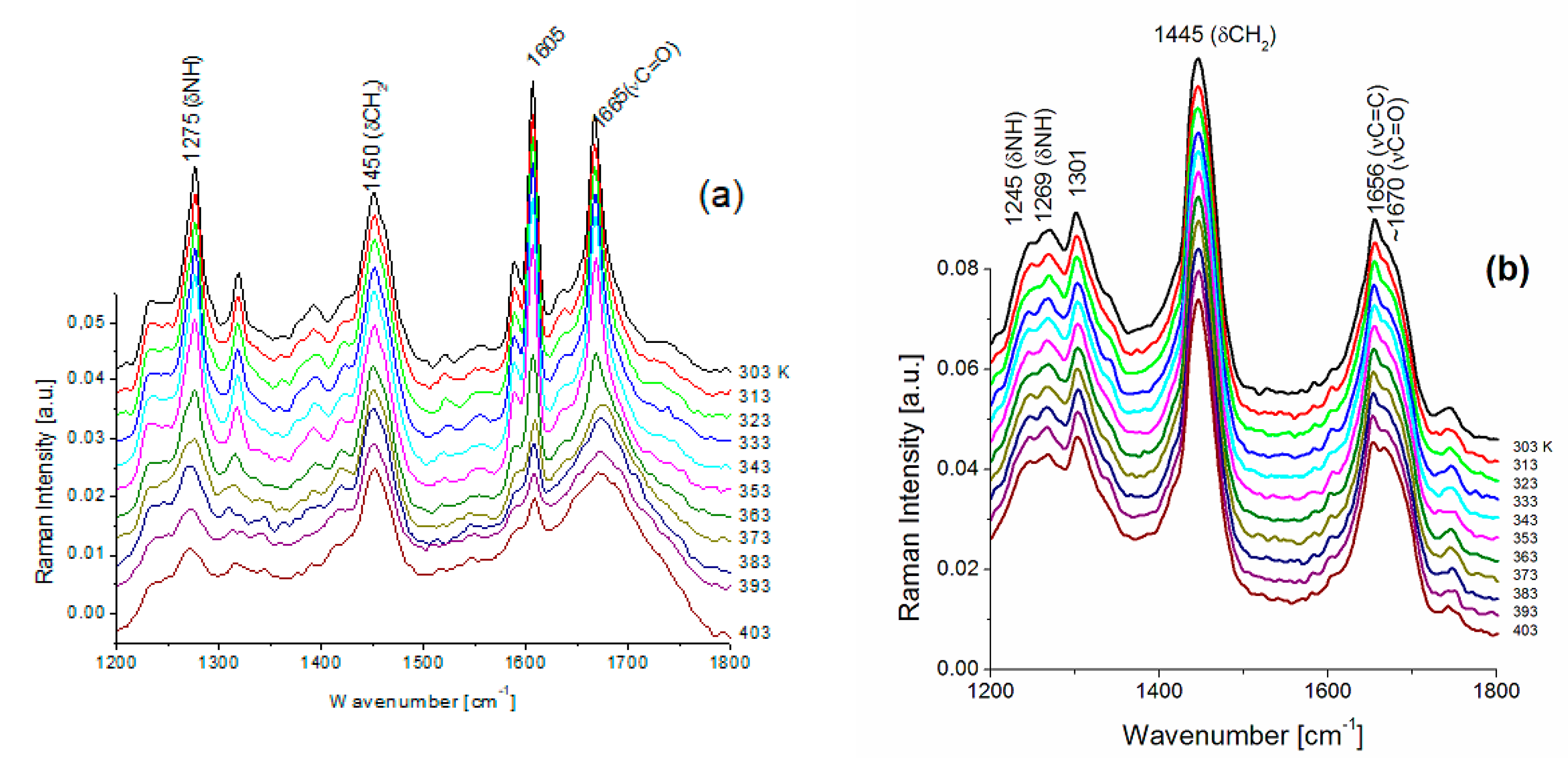
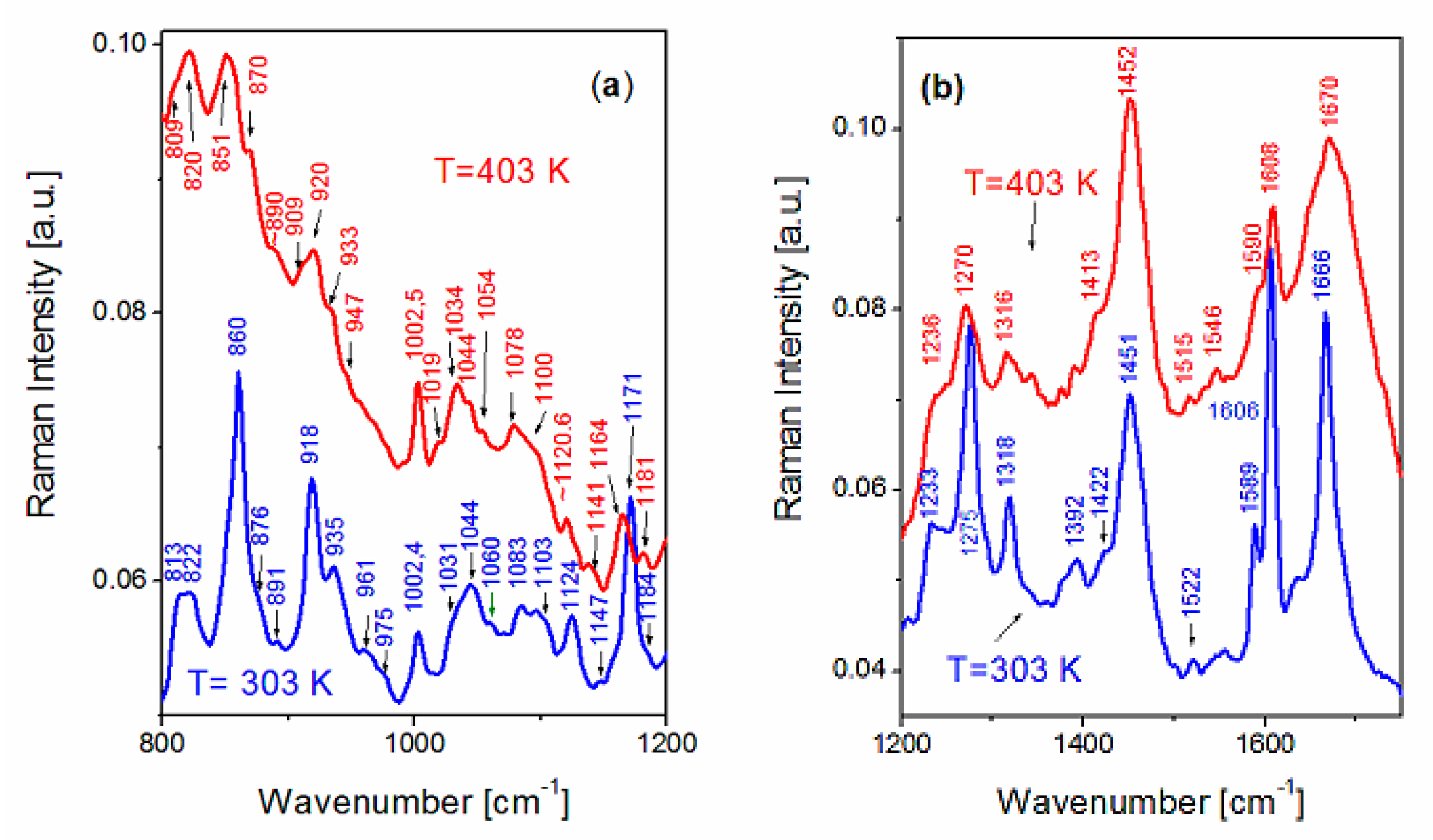
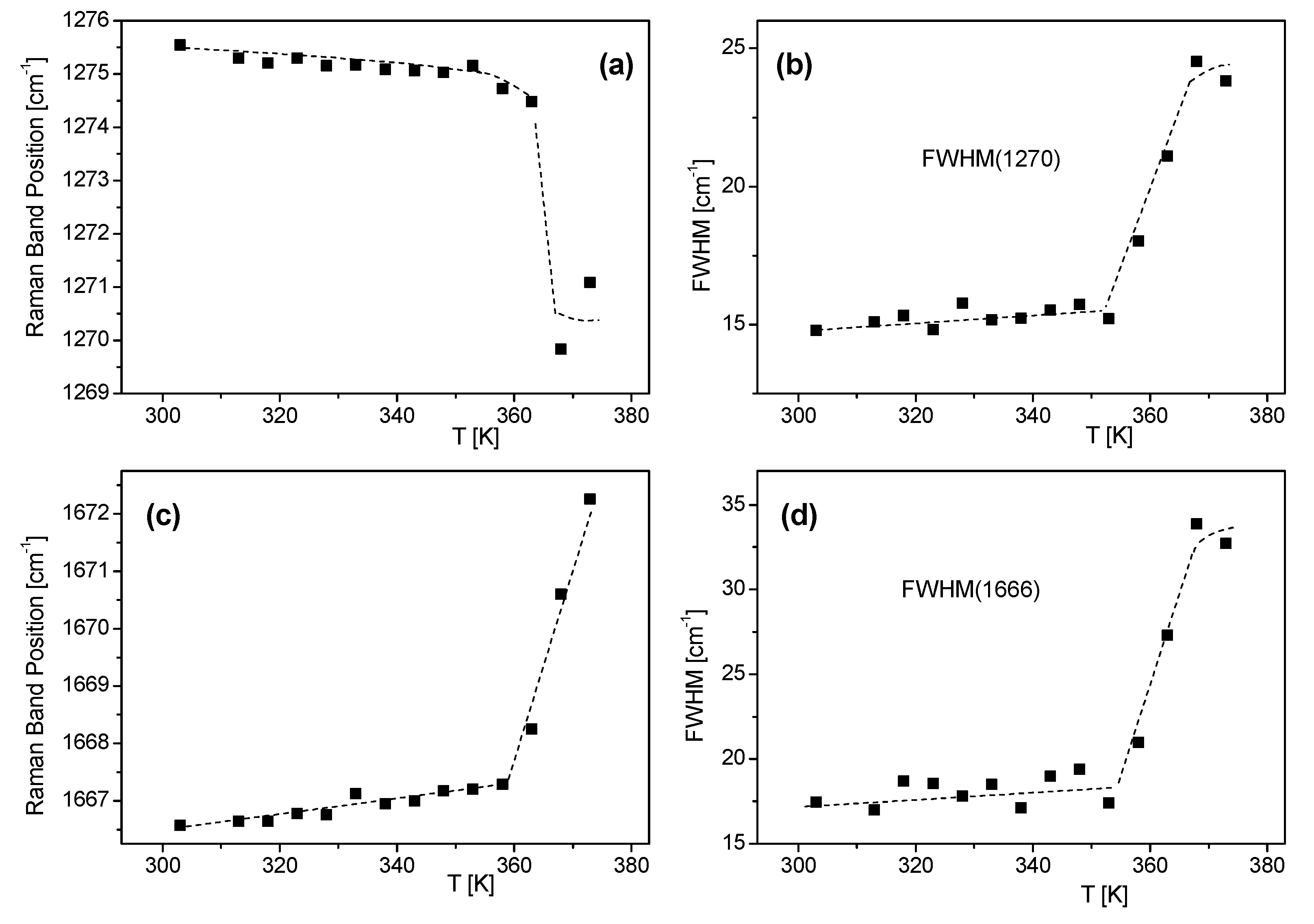
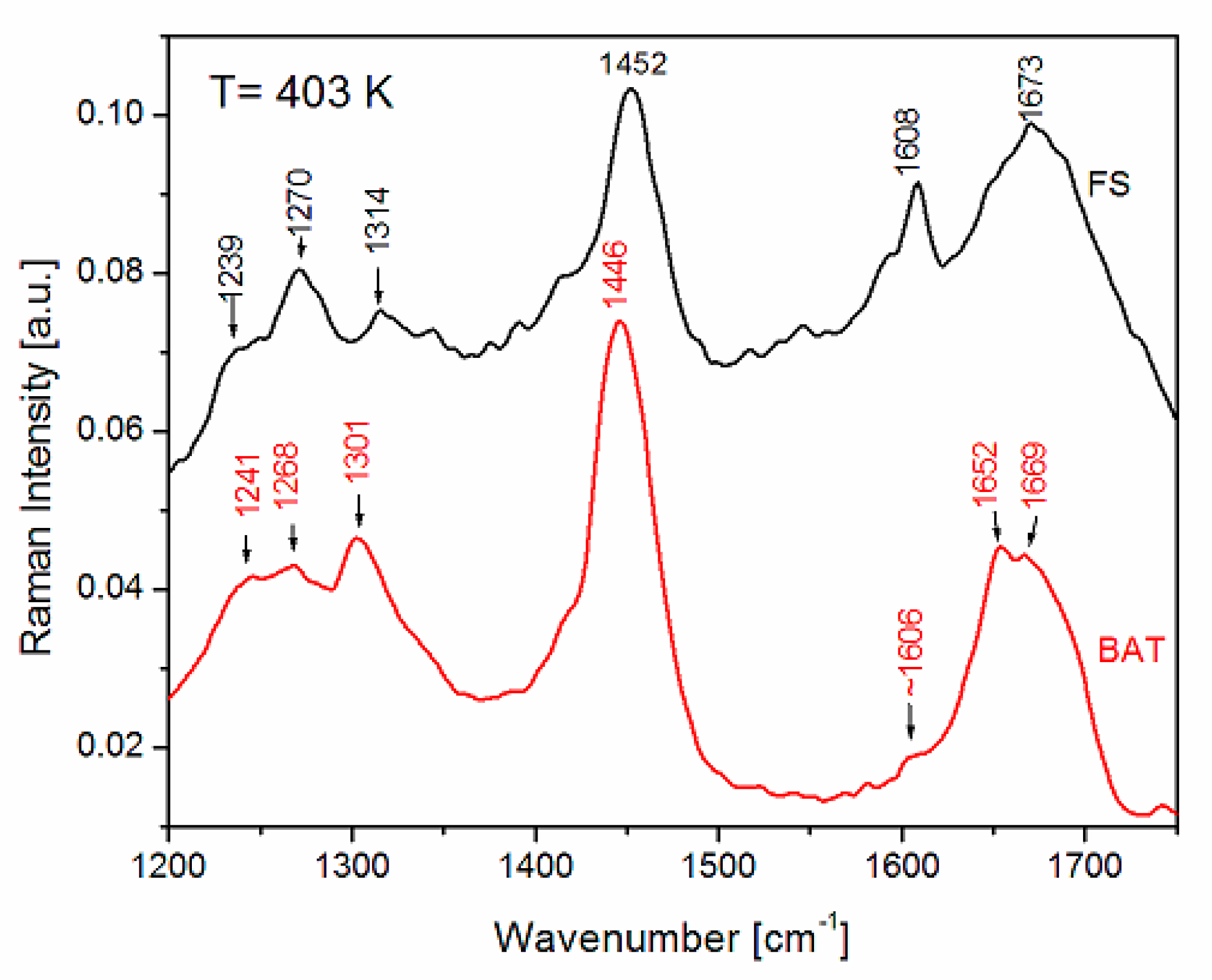
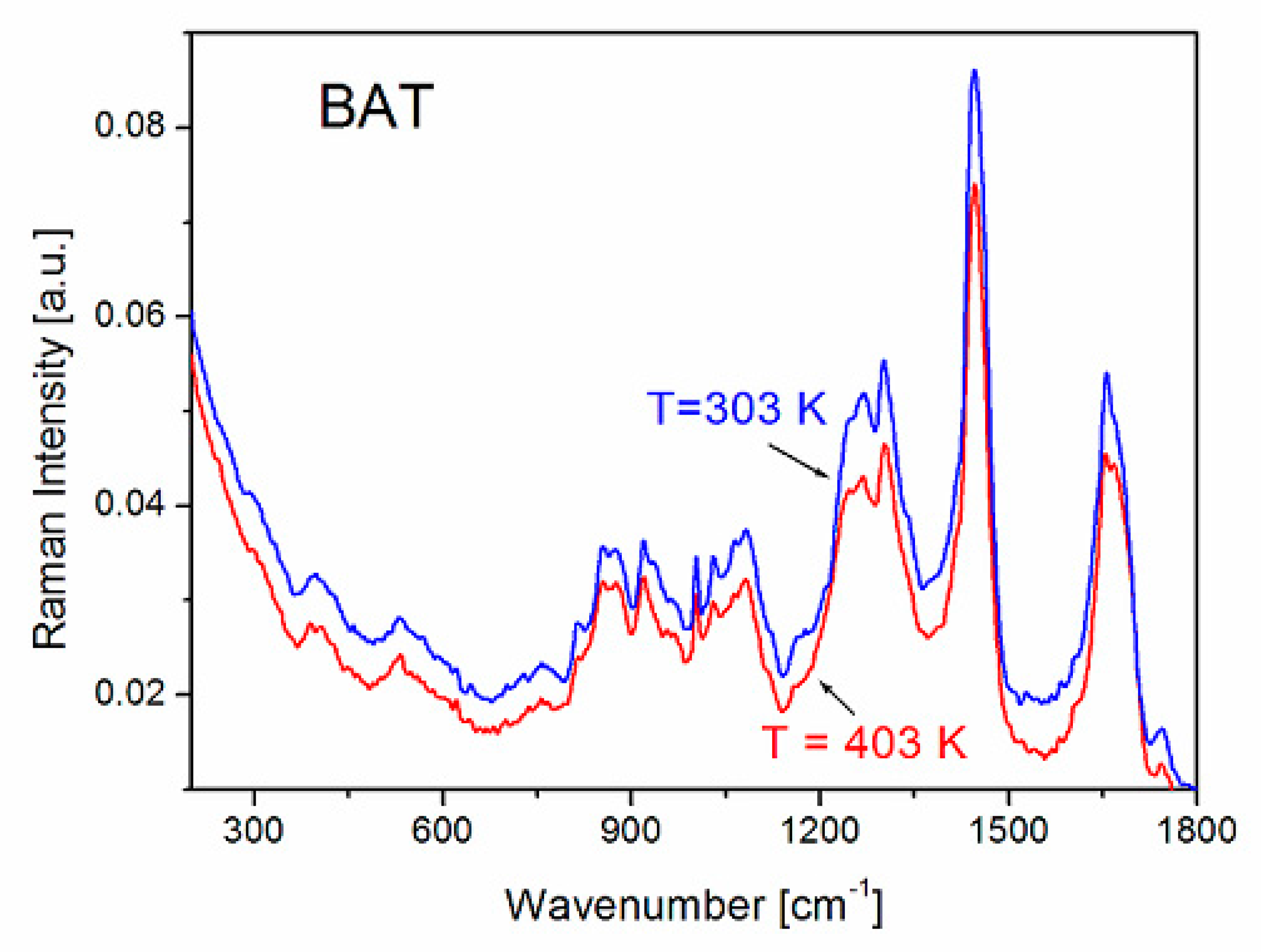
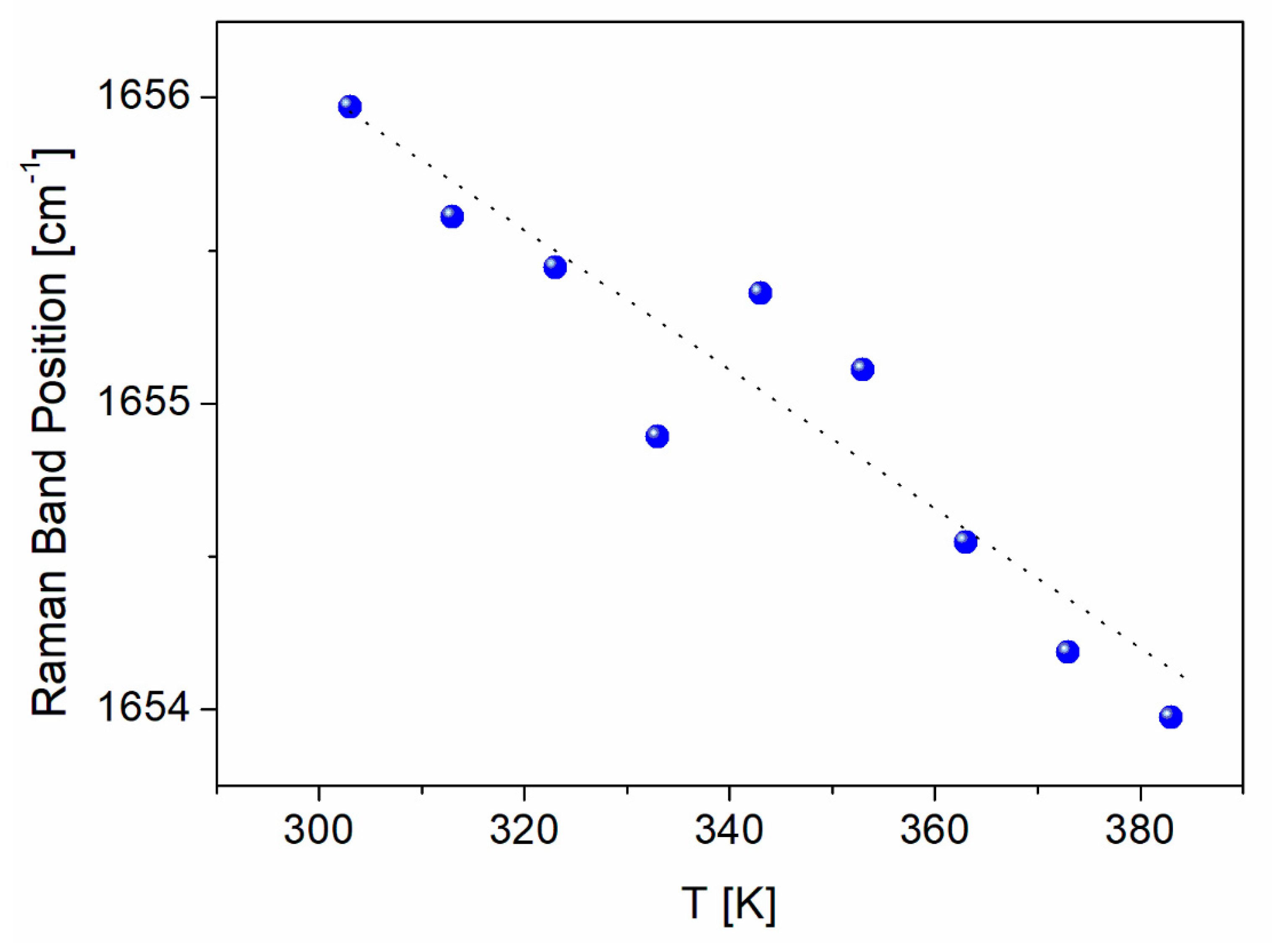
| FS Band Position/cm−1 | BAT Band Position/cm−1 | Assignment | Assignment |
|---|---|---|---|
| 282 w | 292 vw | 3-helix | |
| 314 w | 307 vw | 3-helix | |
| 388 vw | 396 w | ||
| 535 w | 530 w | δ(CCN) | |
| 639 w | 644 vw | δ,ω(COO−) | |
| 649 w | |||
| 760 w | 760 w | ν(CCO) | |
| 813 w | 815 w | ν(COC), ν(CC) | |
| 822 w | |||
| 850 wsh | 854 m | ν(COC), ν(CC) | Pro |
| 861 m | |||
| 870 wsh | 874 m | ν(CC) | Hyp |
| 918 m | 918 m | ν(C-COO−) | α-helix |
| 935 m | 935 m | ν(CC) | α-helix |
| 962 w | 964 w | ||
| 975 w | 974 | ||
| 1003 w | 1002 m | ν(CC) aromatic ring | phenylalanine, tyrosine, tryptophane |
| 1029 wsh | 1030 m | ν(CCN) | phenylalanine |
| 1045 w | 1042 wsh | β-sheet | |
| 1060 sh | 1064 m | ω(CH2) | β-sheet |
| 1085 w | 1082 m | ν(CN) | |
| 1096 w | 1096 msh | ν(CN) | |
| 1124 | 1123 msh | ||
| 1171 m | δ(CH), ν(CCC) out-of-phase | tyrosine | |
| 1233 m | ν(CN), δ(CH), δ(NH) | ||
| 1245 ssh | δ(NH) | disordered (amide III) | |
| 1275 m | 1270 s | δ(NH) | α-helix (amide III) |
| 1301 s | |||
| 1318 w | 1318 sh | γ(CH2) | |
| 1340 wsh | 1340 msh | δ(CH2) | |
| 1391 vw | δ(CH2), ν(COO−) | ||
| 1449 s | 1445 s | δ(CH2) | |
| 1588 wsh | 1583 s | ν(CCH) aromatic ring | |
| 1607 s | 1604 | ν(CCH) aromatic ring | |
| 1633 wsh | ν(C=O) | 3-helix | |
| 1666 s | 1656 s | ν(C=O) | α-helix (amide I) |
| 1671 s | β-sheet (amide I) | ||
| 1680 | disordered (amide I) | ||
| 2861 ssh | 2853 ssh | νas(CH2) | |
| 2888 ssh | |||
| 2938 vs | 2934 vs | νs (CH2) | |
| 2982 ssh | |||
| 3071 w | ν(C=CH) |
| Amide I I(~1660)/I(~1450) | Amide III I(~1270)/I(~1450) | Amide I I(~1660)/I(~1450) | Amide III I(~1270)/I(~1450) | |
|---|---|---|---|---|
| Temperature | 303 K | 403 K | ||
| FS | 1.56 | 1.16 | 0.65 | 0.49 |
| BAT | 0.62 | 0.39 | 0.63 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Połomska, M.; Kubisz, L.; Wolak, J.; Hojan-Jezierska, D. Effects of Temperature on the FT NIR Raman Spectra of Fish Skin Collagen. Appl. Sci. 2021, 11, 8358. https://doi.org/10.3390/app11188358
Połomska M, Kubisz L, Wolak J, Hojan-Jezierska D. Effects of Temperature on the FT NIR Raman Spectra of Fish Skin Collagen. Applied Sciences. 2021; 11(18):8358. https://doi.org/10.3390/app11188358
Chicago/Turabian StylePołomska, Maria, Leszek Kubisz, Jacek Wolak, and Dorota Hojan-Jezierska. 2021. "Effects of Temperature on the FT NIR Raman Spectra of Fish Skin Collagen" Applied Sciences 11, no. 18: 8358. https://doi.org/10.3390/app11188358







