Application of Combined In Situ Chemical Reduction and Enhanced Bioremediation to Accelerate TCE Treatment in Groundwater
Abstract
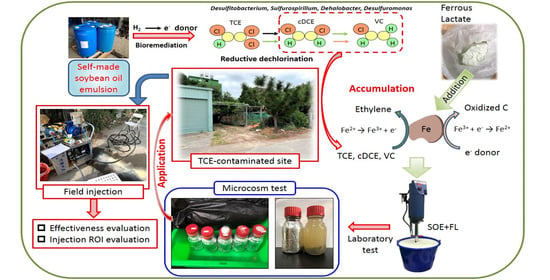
:1. Introduction
2. Materials and Methods
2.1. Materials and Analytical Methods
2.2. Microcosm Test
2.3. In Situ Biological Field Test
3. Results and Discussion
3.1. Microcosm Testing Results
3.2. In Situ Biological Field Test Results
3.2.1. TOC Concentration Variation
3.2.2. In Situ Water Quality Parameter Variation
3.2.3. TCE and Derivatives’ Concentration Variations
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morrison, R.D. Application of forensic techniques for age dating and source identification in environmental litigation. Environ. Forensics 2000, 1, 131–153. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. World Cancer Report 2014; Stewart, B.W., Wild, C.P., Eds.; IARC: Lyon Cedex 08, France, 2014; p. 152. ISBN 978-92-832-0443-5. [Google Scholar]
- Purdue, M.P.; Stewart, P.A.; Friesen, M.C.; Colt, J.S.; Locke, S.J.; Hein, M.J.; Waters, M.A.; Graubard, B.I.; Davis, F.; Ruterbusch, J.; et al. Occupational exposure to chlorinated solvents and kidney cancer: A case-control study. Occup. Environ. Med. 2017, 74, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; McCarty, P.L. Comparison between donor substrates for biologically enhanced tetrachloroethene DNAPL dissolution. Environ. Sci. Technol. 2002, 36, 3400–3404. [Google Scholar] [CrossRef]
- Hunter, W.J. Bioremediation of chlorate or perchlorate contaminated water using permeable barriers containing vegetable oil. Curr. Microbiol. 2002, 45, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.J. Injection of innocuous oils to create reactive barriers for bioremediation: Laboratory studies. J. Contam. Hydrol. 2005, 80, 31–48. [Google Scholar] [CrossRef]
- Hunter, W.J.; Shaner, D.L. Nitrogen limited biobarriers remove atrazine from contaminated water: Laboratory studies. J. Contam. Hydrol. 2009, 103, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.T.; Borden, R.C.; Zawtocki, C.; May, I.; Casey, C. Long term TCE source area remediation using short term emulsified oil substrate (EOS®) recirculation. In Proceedings of the 21st Annual International Conference on Soils, Sediments, and Water, University of Massachusetts, Amherst, MA, USA, 17 October 2005; pp. 17–20. [Google Scholar]
- Borden, R.C. Effective distribution of emulsified edible oil for enhanced anaerobic bioremediation. J. Contam. Hydrol. 2007, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Marks, C. Comparison of EHC®, EOS®, and Solid Potassium Permanganate Pilot Studies for Reducing Residual TCE Contaminant Mass; URS Corporation: San Francisco, CA, USA, 2012. [Google Scholar]
- Aulenta, F.; Fuoco, M.; Canosa, A.; Papini, M.P.; Majone, M. Use of poly-β-hydroxy-butyrate as a slow-release electron donor for the microbial reductive dechlorination of TCE. Water Sci. Technol. 2008, 57, 921–925. [Google Scholar] [CrossRef]
- Fuller, M.E.; Schaefer, C.E.; Steffan, R.J. Evaluation of a peat moss plus soybean oil (PMSO) technology for reducing explosive residue transport to groundwater at military training ranges under field conditions. Chemosphere 2009, 77, 1076–1083. [Google Scholar] [CrossRef]
- Hunter, W.J.; Shaner, D.L. Studies on removing sulfachloropyridazine from groundwater with microbial bioreactors. Curr. Microbiol. 2011, 62, 1560–1564. [Google Scholar] [CrossRef]
- Chambers, J.; Wilkinson, P.B.; Wealthall, G.P.; Loke, M.H.; Dearden, R.A.; Wilson, R.; White, D.; Ogilvy, R.D. Hydrogeophysical imaging of deposit heterogeneity and groundwater chemistry changes during DNAPL source zone bioremediation. J. Contam. Hydrol. 2010, 118, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Boni, M.R.; Sbaffoni, S. The potential of compost-based biobarriers for Cr (VI) removal from contaminated groundwater: Column test. J. Hazard. Mater. 2009, 166, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.J.; Shaner, D.L. Biological remediation of groundwater containing both nitrate and atrazine. Curr. Microbiol. 2010, 60, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Leombruni, A.; Mueller, M.; Seech, A.; Leigh, D. Field application of a reagent for in site chemical reduction and enhanced reductive dichlorination treatment of an aquifer contaminated with tetrachloroethylene (PCE), trichloroethylene, 1,1-dichloroethylene, dichloropropane and 1,1,2,2-tetrachloroethane (R-130). Environ. Eng. Manag. J. 2020, 19, 1791–1796. [Google Scholar]
- Philips, J.; Haest, P.J.; Springael, D.; Smolders, E. Inhibition of geobacter dechlorinators at elevated trichloroethene concentrations is explained by a reduced activity rather than by an enhanced cell decay. Environ. Sci. Technol. 2013, 47, 1510–1517. [Google Scholar]
- Sleep, B.E.; Seepersad, D.J.; Mo, K.; Heidorn, C.M.; Hrapovic, L.; Morrill, P.L.; McMaster, M.L.; Hood, E.D.; LeBron, C.; Lollar, B.S.; et al. Biological enhancement of tetrachloroethene dissolution and associated microbial community changes. Environ. Sci. Technol. 2006, 40, 3623–3633. [Google Scholar] [CrossRef]
- Matheson, L.J.; Tratnyek, P.G. Reductive dehalogenation of chlorinated methanes by iron metal. J. Environ. Sci. Technol. 1994, 28, 2045–2053. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W.; Krug, T.A.; Watling, M.T.; O’Hara, S.K.; Quinn, J.W.; Ruiz, N.E. A two and half-year-performance evaluation of a field test on treatment of source zone tetrachloroethene and its chlorinated daughter products using emulsified zero valent iron nanoparticles. Water Res. 2012, 46, 5071–5084. [Google Scholar] [CrossRef]
- Li, X.Q.; Brown, D.G.; Zhang, W.X. Stabilization of biosolids with nanoscale zero-valent iron (nZVI). J. Nanopart. Res. 2007, 9, 233–243. [Google Scholar] [CrossRef]
- Herrero, J.; Puigserver, D.; Nijenhuis, I.; Kuntze, K.; Carmona, J.M. Combined use of ISCR and biostimulation techniques in incomplete processes of reductive dehalogenation of chlorinated solvents. Sci. Total Environ. 2019, 648, 819–829. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W.; Krug, T.A.; Watling, M.T.; O’Hara, S.K.; Quinn, J.W.; Ruiz, N.E. Travel distance and transformation of injected emulsified zerovalent iron nanoparticles in the subsurface during two and half years. Water Res. 2013, 47, 4095–4106. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Analysis (NIEA); Taiwan EPA. T, NIEA W785.57B. 2020. Available online: https://www.epa.gov.tw/niea/3617C6A64E37A35A (accessed on 28 July 2021).
- National Institute of Environmental Analysis (NIEA); Taiwan EPA. Determination of Total Organic Carbon in Water-Peroxypyrosulfate Heating Oxidation/Infrared Measurement Method, NIEA W532.52C. 2009. Available online: https://www.epa.gov.tw/niea/99FCF9EFD3CB163C (accessed on 28 July 2021).
- National Institute of Environmental Analysis (NIEA); Taiwan EPA. Determination of Nitrate Nitrogen and Nitrite Nitrogen in Water-Cadmium Reduction Flow Analysis Method, NIEA W436.52C. 2015. Available online: https://www.epa.gov.tw/niea/C8FB816F3E6542A2 (accessed on 28 July 2021).
- National Institute of Environmental Analysis (NIEA); Taiwan EPA. Determination of Inorganic Anions in Drinking Water by Ion Chromatography, NIEA W415.54B. 2018. Available online: https://www.epa.gov.tw/niea/2C82301AFEA0B53A (accessed on 28 July 2021).
- Ritalahti, K.M.; Amos, B.K.; Sung, Y.; Wu, Q.; Koenigsberg, S.S.; Löffler, F.E. Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains. Appl. Environ. Microbiol. 2006, 72, 2765–2774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiwan Environmental Protection Administration. Groundwater Pollution Control Standards. In Soil and Groundwater Pollution Remediation Act; Taiwan Environmental Protection Administration: Taipei, Taiwan, 2000. [Google Scholar]
- Tarnawski, S.E.; Rossi, P.; Brennerova, M.V.; Stavelova, M.; Holliger, C. Validation of an integrative methodology to assess and monitor reductive dechlorination of chlorinated ethenes in contaminated aquifers. Front. Environ. Sci. 2016, 4, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sheu, Y.T. Application of a Long-Lasting Colloidal Substrate with pH and Hydrogen Sulfide Control Capabilities for TCE-Contaminated Groundwater Remediation. Ph.D. Dissertation, Institute of Environmental Engineering, National Sun Yat-Sen University, Kaohsiung, Taiwan, 2015. [Google Scholar]









| Well | Sampling Day | pH | DO (mg/L) | ORP (mV) | Nitrate (mg/L) | Nitrite (mg/L) | Fe (Ⅱ)(mg/L) | Sulfate (mg/L) | Sulfide (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| OW2 | 0 | 6.41 | 1.94 | 328 | 7.92 | ND | 0.09 | 45.7 | ND |
| 29 | 6.42 | 2.32 | 316 | 8.99 | 0.002 | 0.06 | 43.7 | ND | |
| 61 | 6.41 | 0.52 | 72 | 9.49 | ND | 0.5 | 39.8 | ND | |
| 119 | 6.44 | 1.83 | 85 | 8.62 | 0.005 | 0.18 | 44.1 | <0.04 | |
| 140 | 6.26 | 1.11 | -2 | 0.84 | ND | 3.6 | 50.3 | 0.22 | |
| CW | 0 | 6.78 | 1.6 | -5 | 0.02 | ND | 0.08 | 122 | ND |
| 29 | 6.67 | 1.73 | 23 | 0.03 | ND | 0.35 | 128 | ND | |
| 61 | 6.57 | 0.65 | -8 | 0.02 | ND | 1.05 | 130 | 0.02 | |
| 92 | 6.26 | 0.07 | 103 | 0.05 | N.D. | 0.02 | 122 | 0.03 | |
| 119 | 6.45 | 1.38 | 202 | 0.08 | 0.003 | 0.35 | 127 | <0.04 | |
| 140 | 6.16 | 1.8 | 64 | 0.04 | ND | 1.98 | 127 | ND |
| Control Item | Control Standards (mg/L) | |
|---|---|---|
| Class I Groundwater | Class II Groundwater | |
| TCE | 0.005 | 0.05 |
| cis-1,2-DCE | 0.07 | 0.7 |
| trans-1,2-DCE | 0.1 | 1.0 |
| VC | 0.002 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.-H.; Hsiao, C.-M.; Lin, C.-E.; Leu, J. Application of Combined In Situ Chemical Reduction and Enhanced Bioremediation to Accelerate TCE Treatment in Groundwater. Appl. Sci. 2021, 11, 8374. https://doi.org/10.3390/app11188374
Liu M-H, Hsiao C-M, Lin C-E, Leu J. Application of Combined In Situ Chemical Reduction and Enhanced Bioremediation to Accelerate TCE Treatment in Groundwater. Applied Sciences. 2021; 11(18):8374. https://doi.org/10.3390/app11188374
Chicago/Turabian StyleLiu, Min-Hsin, Chung-Ming Hsiao, Chih-En Lin, and Jim Leu. 2021. "Application of Combined In Situ Chemical Reduction and Enhanced Bioremediation to Accelerate TCE Treatment in Groundwater" Applied Sciences 11, no. 18: 8374. https://doi.org/10.3390/app11188374
APA StyleLiu, M.-H., Hsiao, C.-M., Lin, C.-E., & Leu, J. (2021). Application of Combined In Situ Chemical Reduction and Enhanced Bioremediation to Accelerate TCE Treatment in Groundwater. Applied Sciences, 11(18), 8374. https://doi.org/10.3390/app11188374