Removal of Cesium from Radioactive Waste Liquids Using Geomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Adsorbent
2.2. Characterization of the Adsorbent
2.3. Adsorption Experiments
2.4. Adsorption Isotherm
2.5. Kinetic Studies
3. Results and Discussion
3.1. Adsorption Experiment
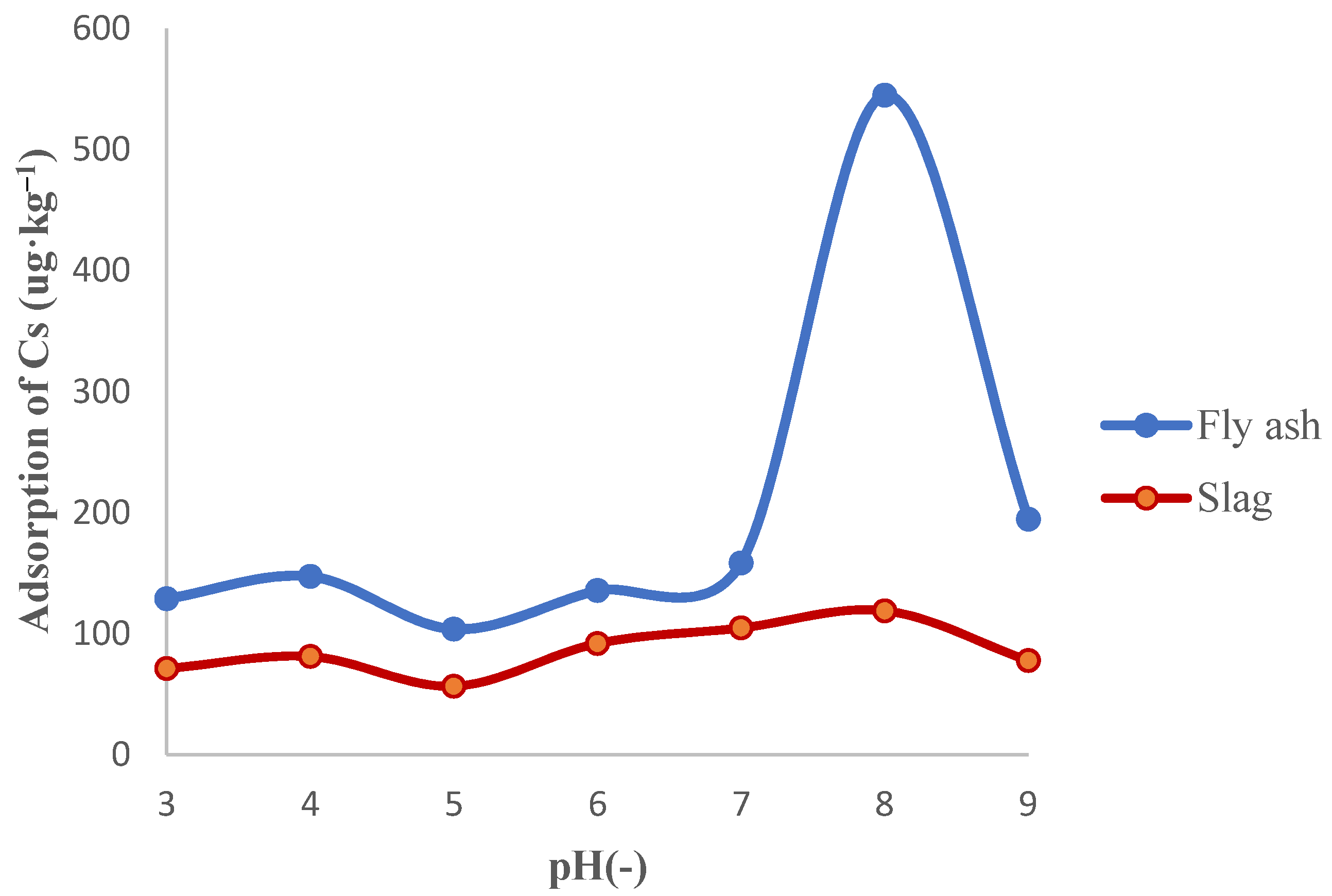
3.1.1. Effect of pH on the Adsorption of Cs
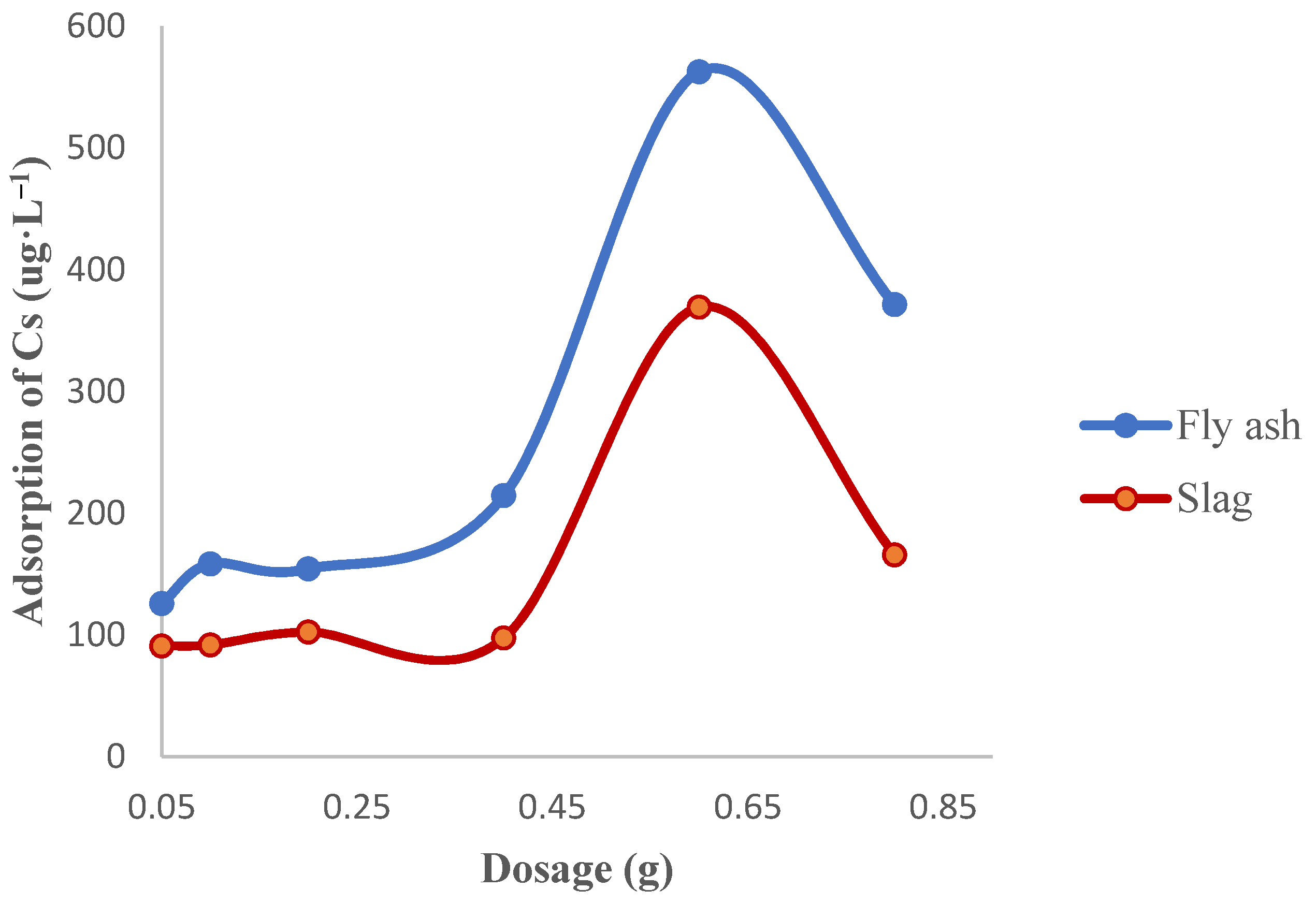
3.1.2. Effect of the Adsorbent Dosage on the Adsorption of Cs
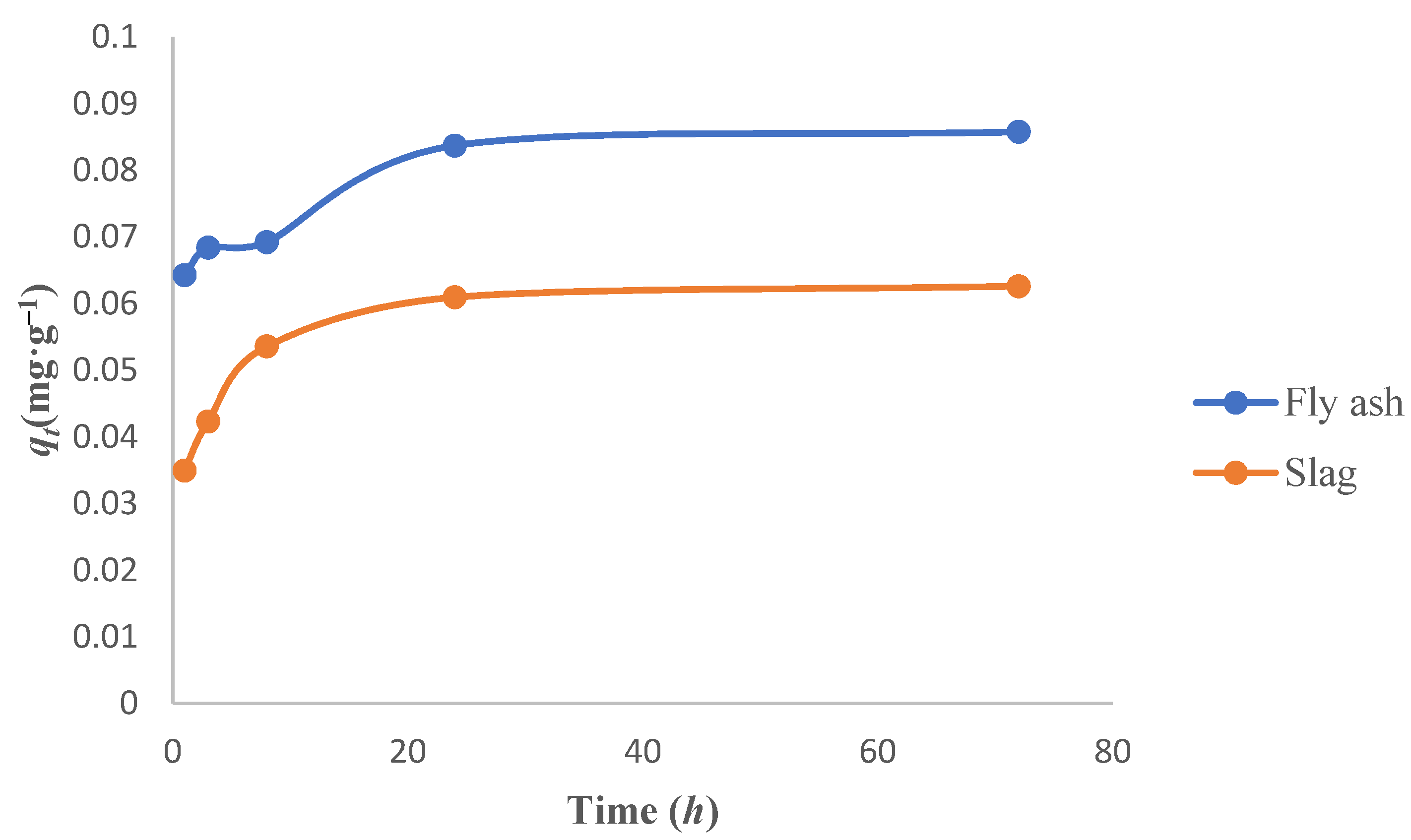
3.1.3. Effect of Contact Time on the Adsorption of Cs
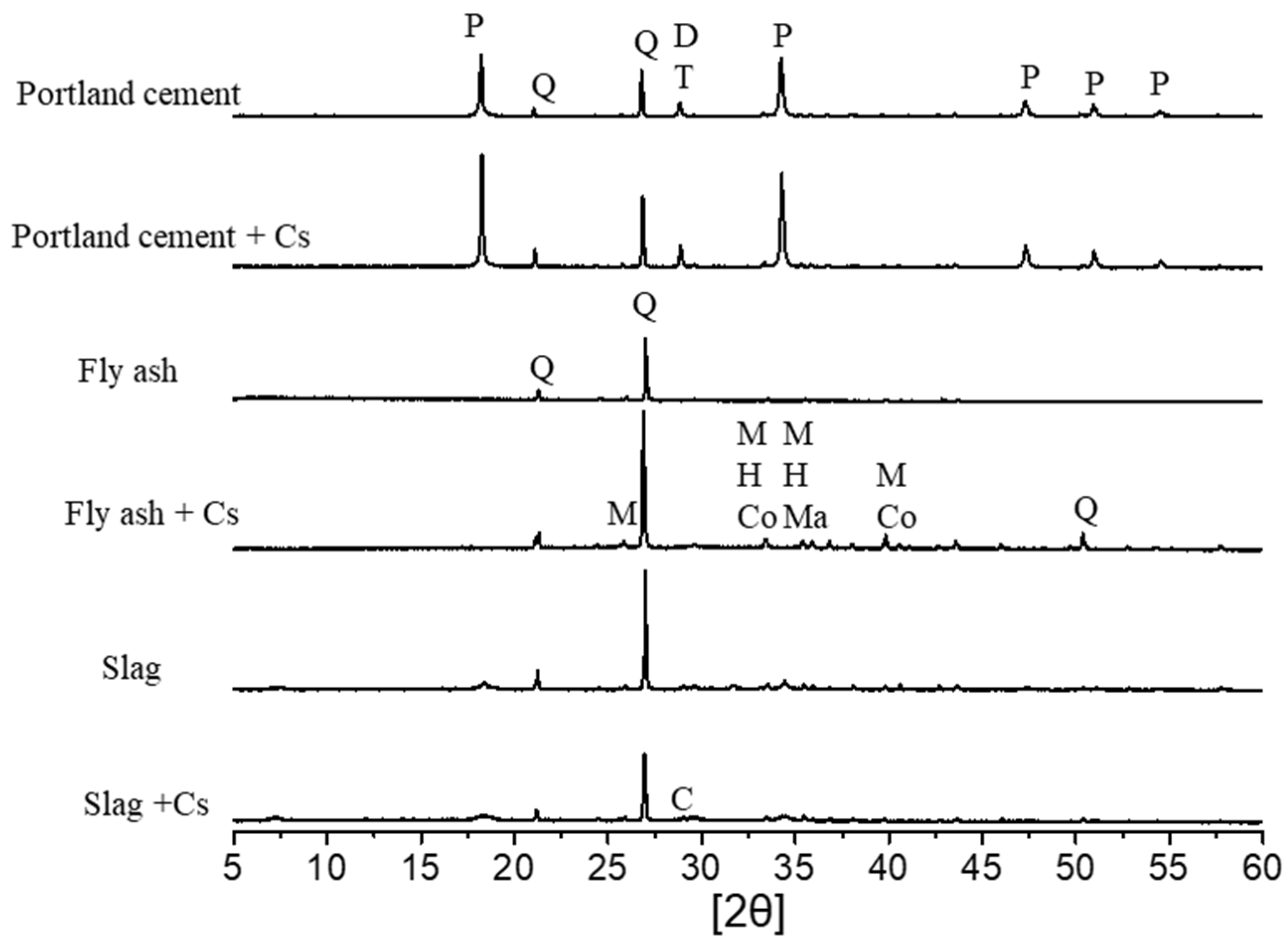
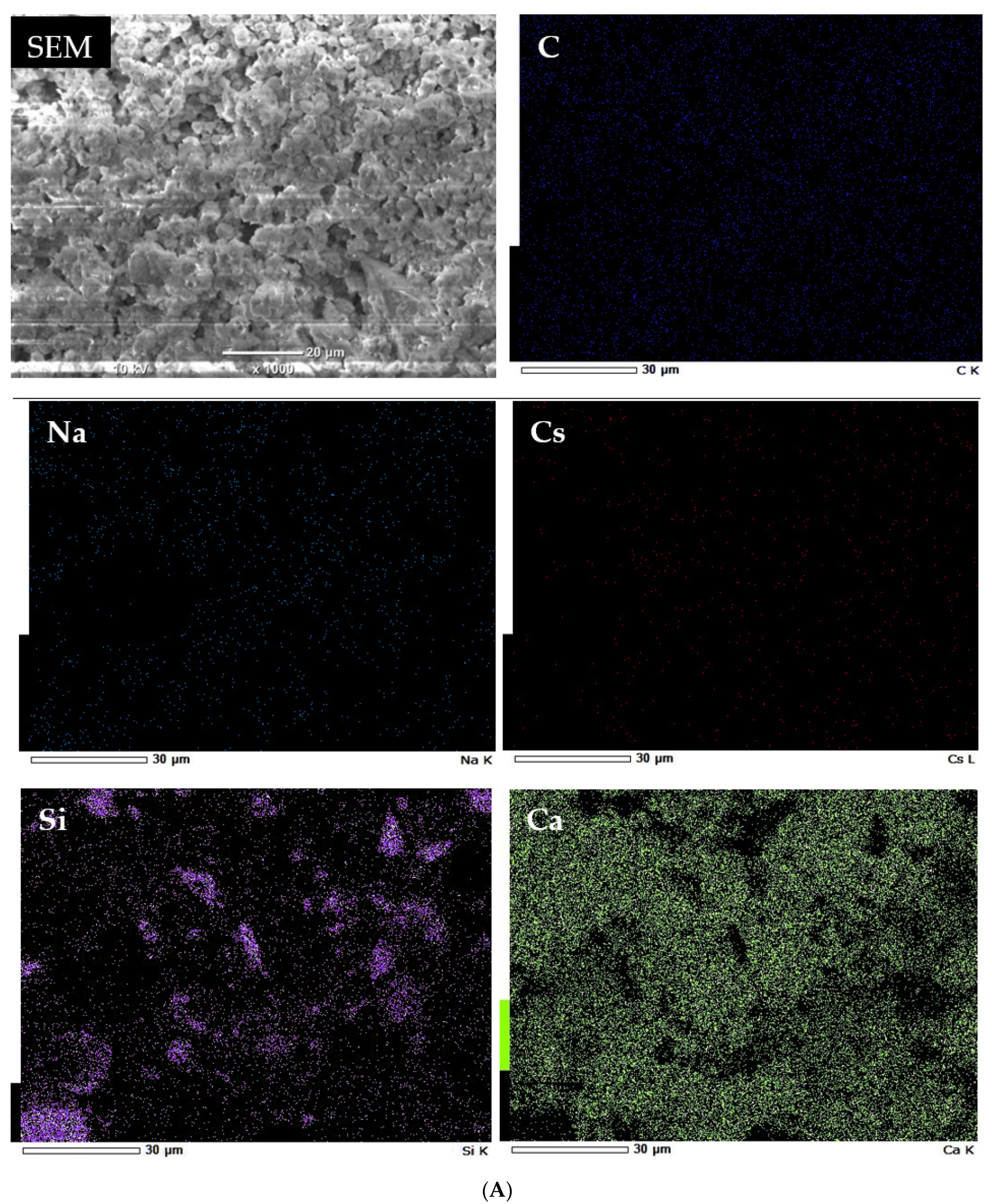
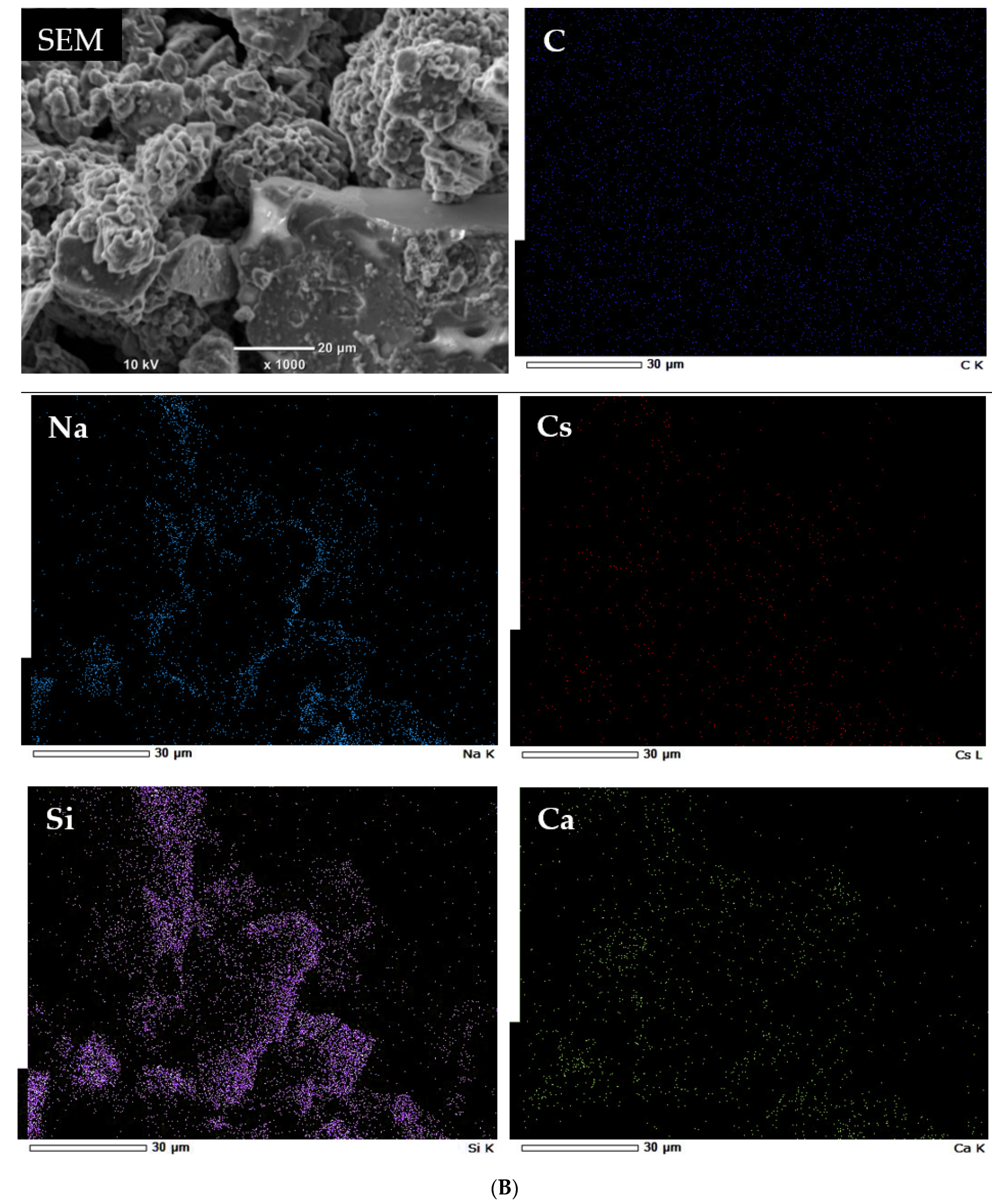
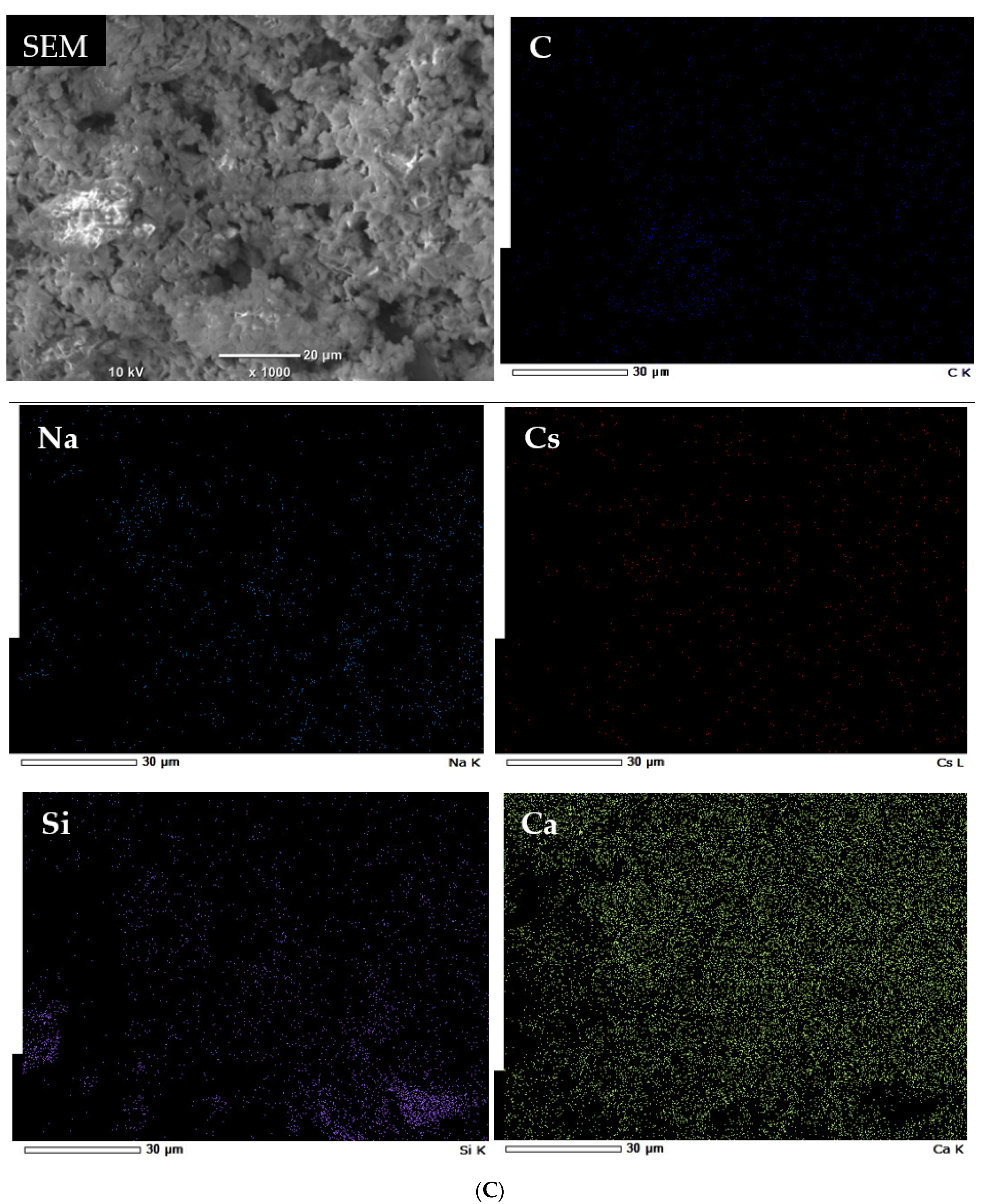
3.2. Characterization of Geomaterials
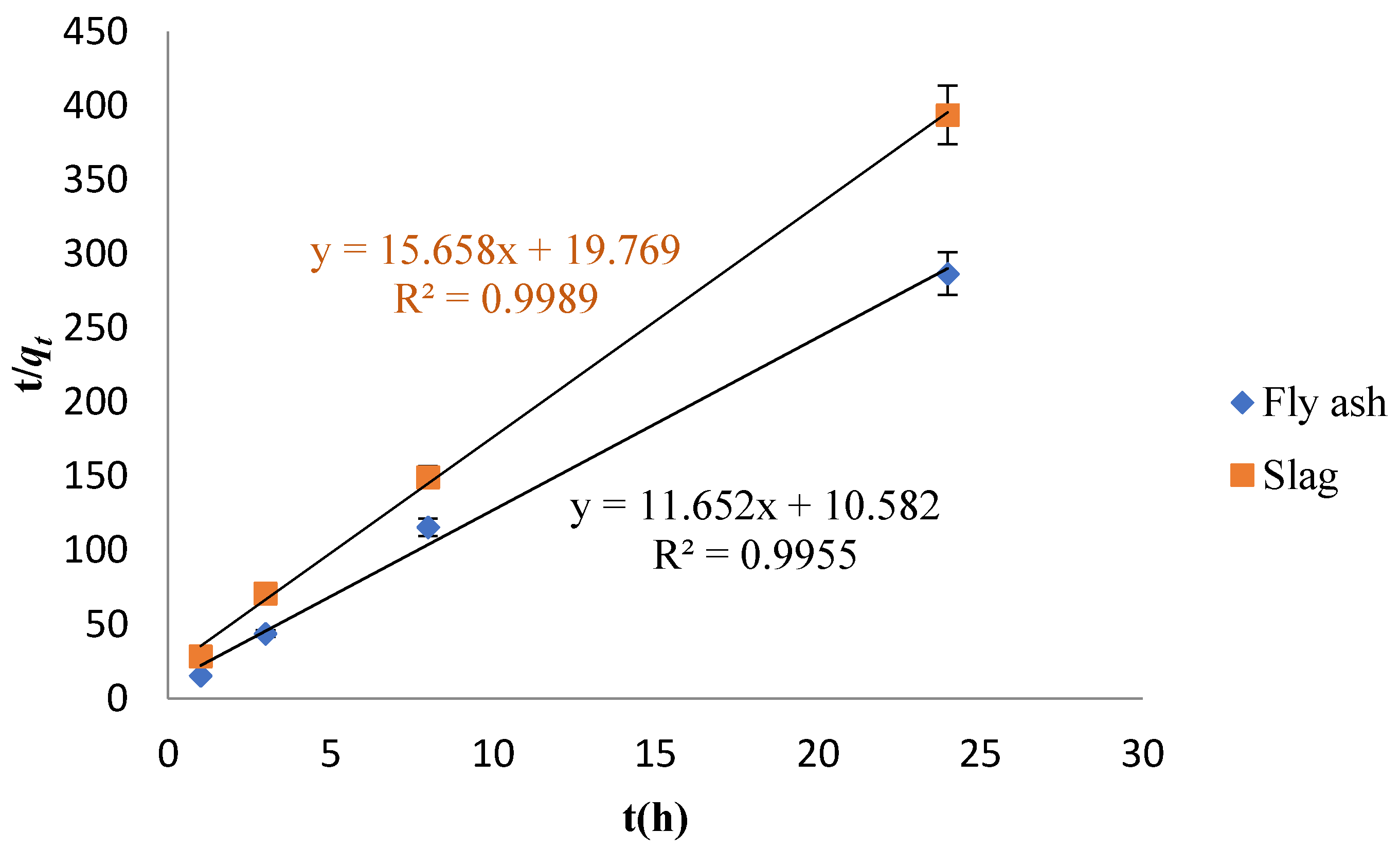
3.3. Adsorption Kinetics Study
3.4. Adsorption Isotherm Study
3.5. Adsorption Mechanism
- (1)
- The adsorption experiment data of Cs on fly ash-based geomaterials and slag-based geomaterials conform to the Langmuir isotherm adsorption equation. The isothermal fitting of the Langmuir model shows that the surface is heterogeneous, which indicates that the adsorption of Cs on the surface of the adsorbent mainly occurred as single-layer adsorption.
- (2)
- The best fit was obtained with the pseudo-second-order kinetic model while investigating the adsorption kinetics of Cs adsorption on the fly ash-based geomaterials, indicating that the adsorption process was mainly chemical adsorption. The R2 was greater than 0.996. On the other hand, Cs adsorption on the slag-based geomaterials was suitably described by the pseudo-first-order model as well as by the pseudo-second-order model (the R2 values were both about 0.999), indicating that physical as well as chemical adsorption were dominant in the adsorption process.
3.6. Comparison with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kitto, M.E.; Marrantino, J.C.; Fielman, E.M.; Haines, D.K.; Semkow, T.M.; Bari, A. Long-term monitoring of radioactivity in fish from New York waters. J. Environ. Radioact. 2015, 146, 44–50. [Google Scholar] [CrossRef]
- Ghandhi, S.A.; Weber, W.; Melo, D.; Doyle-Eisele, M.; Chowdhury, M.; Guilmette, R.; Amundson, S.A. Effect of 90Sr internal emitter on gene expression in mouse blood. BMC Genom. 2015, 16, 586. [Google Scholar] [CrossRef] [Green Version]
- Strand, P.; Sundell-Bergman, S.; Brown, J.E.; Dowdall, M. On the divergences in assessment of environmental impacts from ionizing radiation following the Fukushima accident. J. Environ. Radioact. 2017, 169–170, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, A.S.; Nikolaos, E.; Timothy, A.M.; Wu, J.; Ramli, A.T. An overview of current knowledge concerning the health and environmental consequences of the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident. Environ. Int. 2015, 7, 213–228. [Google Scholar] [CrossRef]
- Buesseler, K.O.; Dai, M.; Aoyama, M.; Benitez-Nelson, C.; Charmasson, S.; Higley, K.; Maderich, V.; Masqué, P.; Morris, P.J.; Oughton, D.; et al. Fukushima Daiichiderived radionuclides in the ocean-transport. Annu. Rev. Mar. Sci. 2016, 9, 173–203. [Google Scholar] [CrossRef]
- Buesseler, K.; Aoyama, M.; Fukasawa, M. Impacts of the Fukushima Nclear Power Plants on marine radioactivity. Environ. Sci. Technol. 2011, 45, 9931–9935. [Google Scholar] [CrossRef]
- Hodkin, D.J.; Stewart, D.I.; Graham, J.T.; Burke, I.T. Coprecipitation of 14C and Sr with carbonate precipitates: The importance of reaction kinetics and recrystallization pathways. Sci. Total Environ. 2016, 562, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhang, G.; Wang, Q.; Gu, P. Removal of strontium from liquid waste using a hydraulic pellet co-precipitation microfiltration (HPCMF) process. Desalination 2014, 349, 31–38. [Google Scholar] [CrossRef]
- Rogers, H.; Bowers, J.; Gates-Anderson, D. An isotope dilution-precipitation process for removing radioactive cesium from wastewater. J. Hazard. Mater. 2012, 243, 124–129. [Google Scholar] [CrossRef]
- Lauchnor, E.G.; Schultz, L.N.; Bugni, S.; Mitchell, A.C.; Cunningham, A.B.; Gerlach, R. Bacterially induced calcium carbonate precipitation and strontium coprecipitation in a porous media flow system. Environ. Sci. Technol. 2013, 47, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Zheng, Z.; Chen, J.; Wang, J.; Xu, C. Efficient co-extraction of strontium and cesium from nitric acid medium by mixtures of di-tertbutylcyclohexano-18-crown-6 and 1, 3-di(2 propoxy) calix[4]arenecrown-6 in n-octanol. Sep. Sci. Technol. 2017, 53, 503–512. [Google Scholar] [CrossRef]
- Raut, D.R.; Mohapatra, P.K. Simultaneous extraction of Cs and Sr from synthetic high level waste solutions using a solvent containing chlorinated dicarbollide and PEG-400 in PTMS. J. Radioanal. Nucl. Chem. 2014, 299, 75–80. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Chen, J. Solvent extraction of strontium and cesium: A review of recent progress. Solvent Extr. Ion Exch. 2012, 30, 623–650. [Google Scholar] [CrossRef]
- Sharma, J.N.; Kumar, A.; Kumar, V.; Pahan, S.; Janardanan, C.; Tessi, V.; Wattal, P.K. Process development for separation of cesium from acidic nuclear waste solution using 1,3-dioctyloxycalix[4]arene—crown-6 plus isodecyl alcohol/n-dodecane solvent. Sep. Purif. Technol. 2014, 135, 176–182. [Google Scholar] [CrossRef]
- Combernoux, N.; Schrive, L.; Labed, V.; Wyart, Y.; Carretier, E.; Moulin, P. Treatment of radioactive liquid effluents by reverse osmosis membranes: From labscale to pilot-scale. Water Res. 2017, 123, 311–320. [Google Scholar] [CrossRef]
- Dang, T.T.H.; Li, C.; Choo, K. Comparison of low-pressure reverse osmosis filtration and polyelectrolyte-enhanced ultrafiltration for the removal of Co and Sr from nuclear plant wastewater. Sep. Purif. Technol. 2016, 157, 209–214. [Google Scholar] [CrossRef]
- Ding, S.; Yang, Y.; Li, C.; Huang, H.; Hou, L.A. The effects of organic fouling on the removal of radionuclides by reverse osmosis membranes. Water Res. 2016, 95, 174–184. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y.; Liu, Y.; Li, M.; Zhao, H.Y.; Hou, L.A. High flux MWCNTs-interlinked GO hybrid membranes survived in cross-flow filtration for the treatment of strontium-containing wastewater. J. Hazard. Mater. 2016, 320, 187–193. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J.; Yang, X.; Li, W. Removal of strontium ions by immobilized saccharomyces cerevisiae in magnetic chitosan microspheres. Nucl. Eng. Technol. 2017, 49, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Eapen, S.; Thorat, V.; Kaushik, C.P.; Raj, K.; D’souza, S.F. Phytoremediation of 137cesium and 90strontium from solutions and low-level nuclear waste by Vetiveria zizanoides. Ecotoxicol. Environ. Saf. 2008, 69, 306–311. [Google Scholar] [CrossRef]
- Qi, X.H.; Du, K.Z.; Feng, M.L.; Li, J.R.; Du, C.F.; Zhang, B.; Huang, X.Y. A two-dimensionally microporous thiostannate with superior Cs+ and Sr2+ ion-exchange property. J. Mater. Chem. A 2015, 3, 5665–5673. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, J.Y.; Zhao, X. Adsorption characteristics of strontium on synthesized antimony silicate. Chem. Eng. J. 2015, 277, 378–387. [Google Scholar] [CrossRef]
- Villard, A.; Siboulet, B.; Toquer, G.; Merceille, A.; Grandjean, A.; Dufrêche, J.F. Strontium selectivity in sodium nonatitanate Na4Ti9O20·xH2O. J. Hazard. Mater. 2015, 283, 432–438. [Google Scholar] [CrossRef]
- Naeimi, S.; Faghihian, H. Performance of novel adsorbent prepared by magnetic metal -organic framework (MOF) modified by potassium nickel hexacyanoferrate for removal of Cs+ from aqueous solution. Sep. Purif. Technol. 2017, 175, 255–265. [Google Scholar] [CrossRef]
- Alby, D.; Charnay, C.; Heran, M.; Prelot, B.; Zajac, J. Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review. J. Hazard. Mater. 2018, 344, 511–530. [Google Scholar] [CrossRef]
- Sarma, D.; Malliakas, C.D.; Subrahmanyam, K.S.; Islam, S.M.; Kanatzidis, M.G. K2xSn4−xS8−x (x = 0.65~1): A new metal sulfide for rapid and selective removal of Cs+, Sr2+ and UO22+ ions. Chem. Sci. 2016, 7, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wei, J.; Zhao, X.; Li, F.; Jiang, F.; Zhang, M.; Cheng, X. Removal of strontium(II) and cobalt(II) from acidic solution by manganese antimonate. Chem. Eng. J. 2016, 302, 733–743. [Google Scholar] [CrossRef]
- Sun, D.; Wang, A.; Hu, P. Research and Application prospect of geological polymers. Mater. Bull. 2009, 23, 61–65. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Sarin, V.; Pant, K.K. Removal of chromium from industrial waste by using eucalyptus bark. Bioresour. Technol. 2006, 97, 15–20. [Google Scholar] [CrossRef]
- Duranoğlu, D.; Trochimczuk, A.W.; Beker, U. Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile–divinylbenzene copolymer. Chem. Eng. J. 2012, 187, 193–202. [Google Scholar] [CrossRef]
- Ayawei, N.; Abasi, C.Y.; Wankasi, D.; Dikio, E.D. Layered Double Hydroxide Adsorption of Lead: Equilibrium, Thermodynamic and Kinetic Studies. Int. J. Adv. Res. Chem. Sci. 2015, 2, 22–32. [Google Scholar]
- Ayawei, N.; Ekubo, A.T.; Wankasi, D.; Dikio, E.D. Synthesis and Application of Layered Double Hydroxide for the removal of Copper in Wastewater. Int. J. Chem. 2015, 7, 122–132. [Google Scholar]
- Mahmoodi, N.M.; Hayati, B.; Bahrami, H.; Arami, M. Dye adsorption and desorption properties of Mentha pulegium in single and binary systems. J. Appl. Polym. Sci. 2011, 122, 1489–1499. [Google Scholar] [CrossRef]
- Rahmati, M.M.; Rabbani, P.; Abdolali, A.; Keshtkar, A.R. Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J. Hazard. Mater. 2011, 185, 401–407. [Google Scholar] [CrossRef]
- Pillai, S.S.; Mullassery, M.D.; Fernandez, N.B.; Girija, N.; Geetha, P.; Koshy, M. Biosorption of Cr(VI) from aqueous solution by chemically modified potato starch: Equilibrium and kinetic studies. Ecotoxicol. Environ. Saf. 2013, 92, 199–205. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The AFm phase in Portland cement. Cem. Concr. Res. 2007, 37, 118–130. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Liu, Y.; Pang, Y. Early performance and structure of slag-modified fly ash-based geopolymer materials. J. Yangtze River Sci. Res. Inst. 2019, 36, 113. [Google Scholar]
- Jeong, C.H. Mineralogical and hydrochemical effects on adsorption removal of cesium-137 and strontium-90 by kaolinite. J. Environ. Sci. Health A 2001, 36, 1089–1099. [Google Scholar] [CrossRef]
- Lu, N.; Mason, C.F.V. Sorption-desorption behavior of strontium-85 onto montmorillonite and silica colloids. Appl. Geochem. 2001, 16, 1653–1662. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eljamal, O.; Shubair, T.; Tahara, A.; Sugihara, Y.; Matsunaga, N. Iron based nanoparticles-zeolite composites for the removal of cesium from aqueous solutions. Molliq 2018, 12, 115. [Google Scholar] [CrossRef]
- Smičiklas, I.; Dimović, S.; Plećaš, I. Removal of Cs+, Sr2+ and Co2+ from aqueous solutions by adsorption on natural clinoptilolite. Appl. Clay Sci. 2007, 35, 139–144. [Google Scholar] [CrossRef]
- Lee, K.Y.; Park, M.; Kim, J.; Oh, M.; Lee, E.H.; Kim, K.W.; Moon, J.K. Equilibrium, kinetic and thermodynamic study of cesium adsorption onto nanocrystalline mordenite from high-salt solution. Chemosphere 2016, 150, 765–771. [Google Scholar] [CrossRef]
- Vipin, A.K.; Sun, L.B.F. Removal of Cs+ and Sr2+ from water using MWCNT reinforced zeolite-A beads. Microporous Mesoporous Mater. 2016, 224, 84–88. [Google Scholar] [CrossRef]
- De Haro-Del Rio, D.A.; Al-Joubori, S.; Kontogiannis, O.; Papadatos-Gigantes, D.; Ajayi, O.; Li, C.; Holmes, S.M. The removal of caesium ions using supported clinoptilolite. J. Hazard. Mater. 2015, 289, 181. [Google Scholar] [CrossRef]
- Borai, E.; Harjula, R.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef]
- Wang, T.-H.; Li, M.-H.; Wei, Y.-Y.; Teng, S.-P. Desorption of cesium from granite under various aqueous conditions. Appl. Radiat. Isot. 2010, 68, 2140–2146. [Google Scholar] [CrossRef]
- Long, H.; Wu, P.; Yang, L.; Huang, Z.; Zhu, N.; Hu, Z. Efficient removal of cesium from aqueous solution with vermiculite of enhanced adsorption property through surface modification by ethylamine. J. Colloid. Interface Sci. 2014, 428, 295–301. [Google Scholar] [CrossRef]
- Yang, S.; Han, C.; Wang, X.; Nagatsu, M. Characteristics of cesium ion sorption from aqueous solution on bentonite-and carbon nanotube-based composites. J. Hazard. Mater. 2014, 274, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Sangvanich, T.; Sukwarotwat, V.; Wiacek, R.J.; Grudzien, R.M.; Fryxell, G.E.; Addleman, R.S.; Yantasee, W. Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica. J. Hazard. Mater. 2010, 182, 225–231. [Google Scholar] [CrossRef] [Green Version]
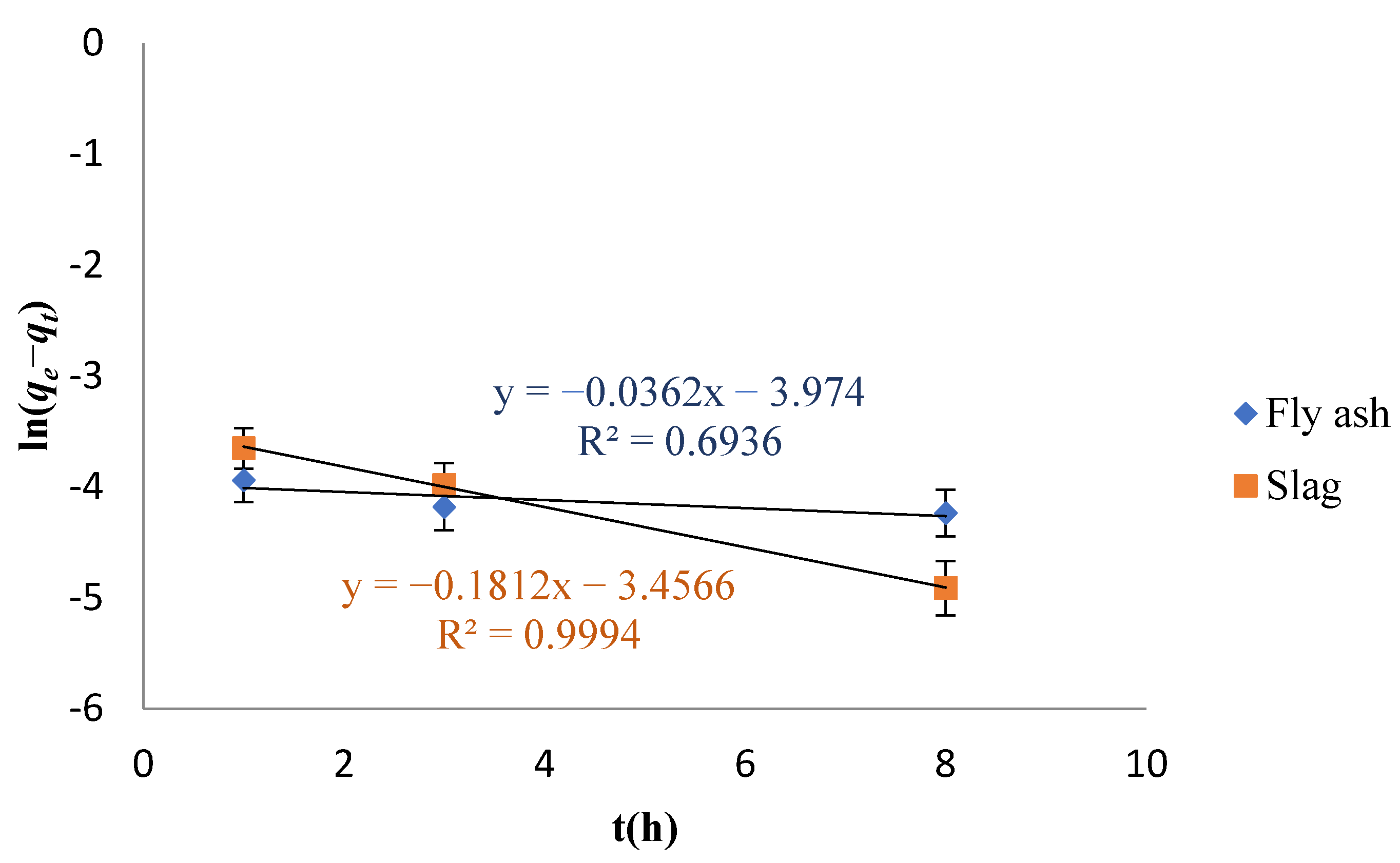
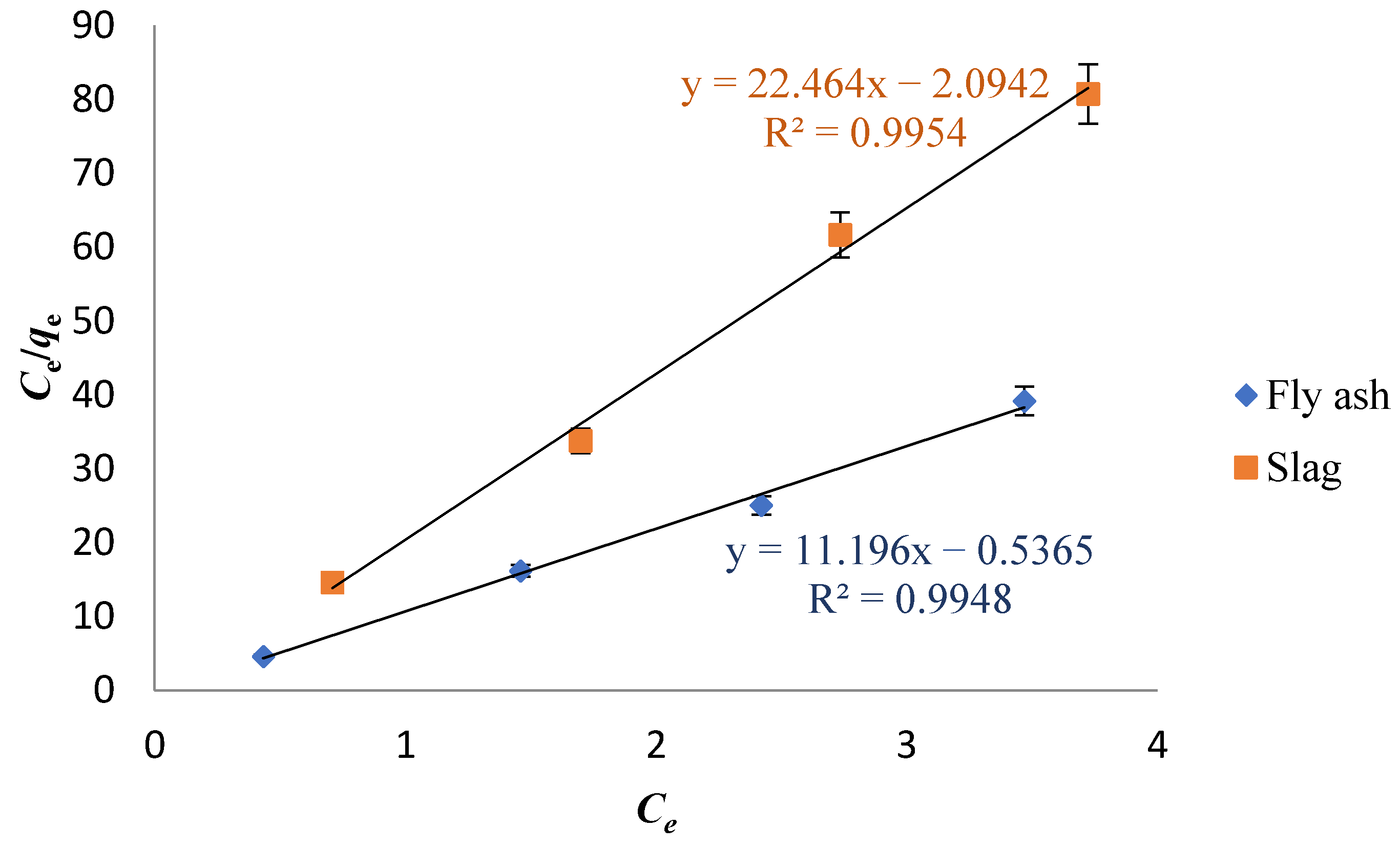
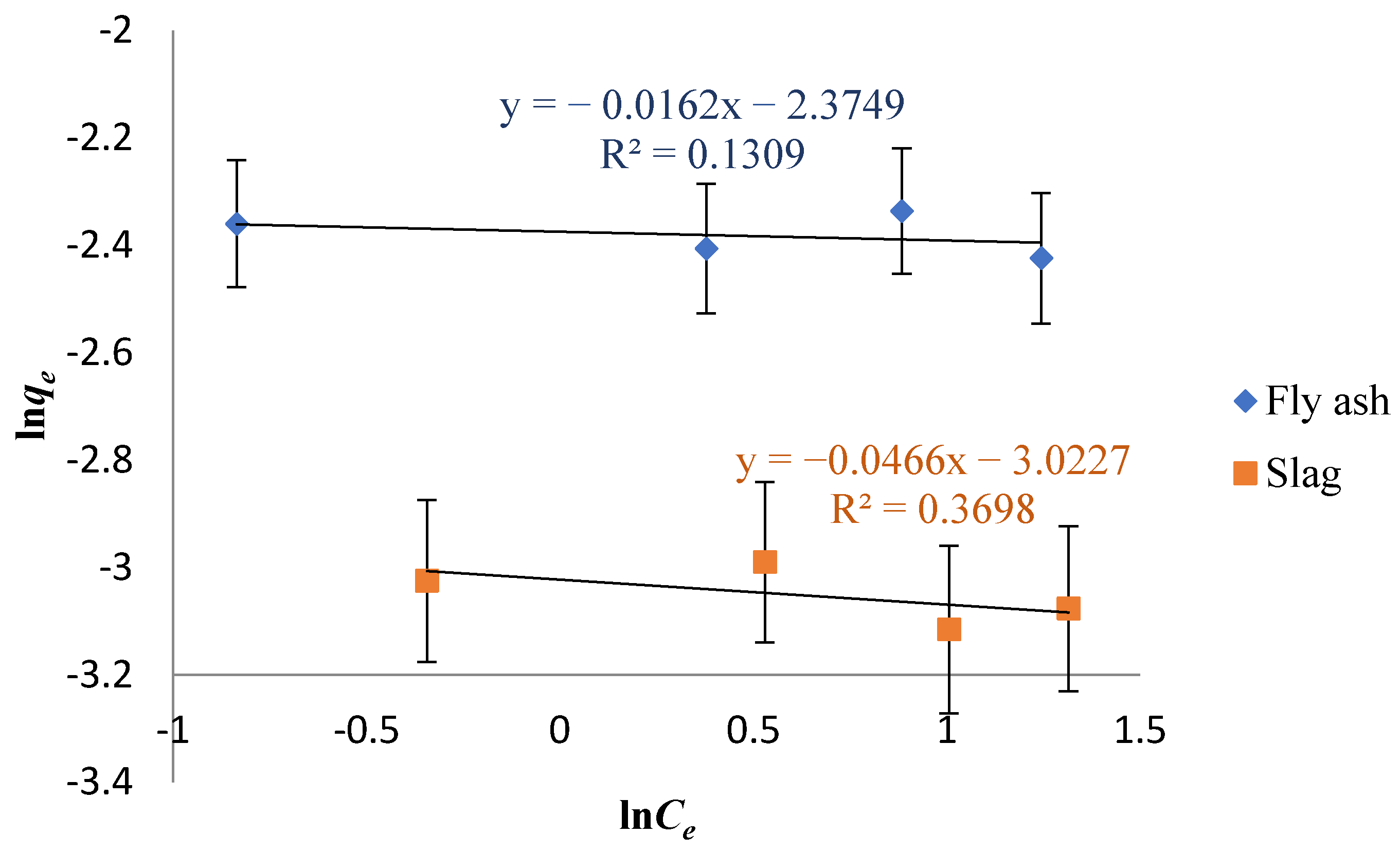
| Weight of Mixture (g) | |||
|---|---|---|---|
| Chemical Compositions | Portland Cement | Fly Ash | Slag |
| CaO | 15.6 | 2.65 | 23.9 |
| SiO2 | 5.25 | 25.8 | 16.2 |
| Al2O3 | 1.48 | 11.0 | 5.75 |
| Fe2O3 | 0.800 | 5.40 | 0.300 |
| MgO | 0.700 | 1.00 | 1.50 |
| TiO2 | - | 0.800 | 0.250 |
| Sample | BET Surface Area [m2·g−1] | Pore Volume [cm3·g−1] | Pore Size [nm] |
|---|---|---|---|
| Portland Cement | 0.9582 | 0.002652 | 11.07 |
| Slag | 1.388 | 0.004432 | 12.77 |
| Fly Ash | 1.869 | 0.006359 | 13.61 |
| Sample | qexp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| qe (mg·g−1) | k1 (h−1) | R2 | qe (mg·g−1) | k2 (g·mg−1·h−1) | R2 | ||
| Fly ash | 83.68 | 53.20 | 0.03620 | 0.6936 | 85.82 | 12.83 | 0.9955 |
| Slag | 60.93 | 60.67 | 0.1812 | 0.9994 | 31.71 | 12.40 | 0.9989 |
| Sample | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qmax (mg·g−1) | KL (L·mg−1) | R2 | KF ((mg·g−1)· (dm−3·mg−1)1/n) | 1/n | R2 | |
| Fly ash | 89.32 | 0.1665 | 0.9948 | 10.75 | 0.01620 | 0.1309 |
| Slag | 44.52 | 0.02126 | 0.9954 | 20.55 | 0.04660 | 0.3698 |
| Adsorbents | Final Concentration (mg·L−1) | Adsorption Capacity (mg·g−1) | Partition Coefficient (mg·g−1·mM−1) | Reference |
|---|---|---|---|---|
| nFe/Cu–Z | 26.28 | 77.51 | 2.949 | [43] |
| Natural clinoptilolite from Serbia | 76.00 | 49.26 | 0.6482 | [44] |
| Nanocrystalline mordenite (pulverized) | 4.000 | 37.30 | 9.325 | [45] |
| MWCNT reinforced zeolite-A beads | 30.00 | 113.0 | 3.767 | [46] |
| Carbon–zeolite | 156.0 | 120.9 | 0.7750 | [47] |
| Natural mordenite | 9.574 | 254.8 | 26.61 | [48] |
| Synthetic mordenite | 9.508 | 220.4 | 23.18 | [48] |
| Phosphoric acid activated montmorillonite | 13.30 | 208 | 15.64 | [49] |
| Ethyl-VER | 2.161 | 43.96 | 20.34 | [50] |
| Bentonite | 8.700 | 177.4 | 20.39 | [51] |
| Clinoptilolite | 12.50 | 12.50 | 1.000 | [52] |
| Fly ash-based geomaterials | 2.879 | 89.32 | 31.02 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, M.; Du, X.; Feng, S.; Miyamoto, N.; Kano, N. Removal of Cesium from Radioactive Waste Liquids Using Geomaterials. Appl. Sci. 2021, 11, 8407. https://doi.org/10.3390/app11188407
Zhang H, Zhu M, Du X, Feng S, Miyamoto N, Kano N. Removal of Cesium from Radioactive Waste Liquids Using Geomaterials. Applied Sciences. 2021; 11(18):8407. https://doi.org/10.3390/app11188407
Chicago/Turabian StyleZhang, Haixin, Mingze Zhu, Xiaoyu Du, Sihan Feng, Naoto Miyamoto, and Naoki Kano. 2021. "Removal of Cesium from Radioactive Waste Liquids Using Geomaterials" Applied Sciences 11, no. 18: 8407. https://doi.org/10.3390/app11188407






