Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses
Abstract
:1. Introduction
2. Physicochemical Properties of Bromelain
| Endopeptidase | Amino Acid Composition% | Molar Mass (kDa) | pI | A1% 280 nm | Presence of Glycoproteins | ||
|---|---|---|---|---|---|---|---|
| Green Fruit | Ripe Fruit | ||||||
| Fruit bromelain | Lysine | 7.8 | 8.3 | 25–31 | 4.6 | 19.2 | Yes |
| Histidine | 1.4 | 1.3 | |||||
| Arginine | 8.6 | 9.1 | |||||
| Aspartic Acid | 29.8 | 29.8 | |||||
| Threonine | 13.8 | 13.8 | |||||
| Serine | 32.2 | 32 | |||||
| Glutamic Acid | 23.2 | 23.4 | |||||
| Proline | 11.6 | 12 | |||||
| Glycine | 32.6 | 32.2 | |||||
| Alanine | 23.8 | 24.4 | |||||
| Cysteine | 10.0 | 10.0 | |||||
| Valine | 19.8 | 20.1 | |||||
| Methionine | 6.0 | 5.8 | |||||
| Isoleucine | 16.4 | 16.2 | |||||
| Leucine | 10.0 | 10.0 | |||||
| Tyrosine | 22.4 | 22.2 | |||||
| Phenylalanine | 7.6 | 8.0 | |||||
| Tryptophan | 5.6 | - | |||||
| Stem bromelain | - | 23.8–27 | 9.5 | 20.1 | Yes | ||
| Ananain | - | 23.4–25 | >10 | 16.5 | No | ||
| Comosain | - | 24.5 | >10 | - | Yes | ||
3. Extraction and Purification Methods
3.1. Unconventional Approaches
- (A).
- Aqueous two-phase systems
- (B).
- Chromatographic techniques
- (C).
- Reversed micellar extraction
- (D).
- Precipitation methods
- (E).
- Novel strategies with recombinant technologies
| Purification Technique | Activity Recovery (%) | Purification Fold/Factor | Reference | |
|---|---|---|---|---|
| Aqueous two-phase extraction | PEG/K2SO4 | 228 | 4.0 | [27] |
| PEG/polyacrylic acid | 335.27 | 25.78 | [55] | |
| PEG/(NH4)2SO4 | - | 11.80 | [38] | |
| PEG/MgSO4 | 113.54–206 | 2.23–62 | [10,39,56,57] | |
| Block copolymers | 79.5 | 1.25 | [36] | |
| Filtration | Microfiltration and ultrafiltration | 85–100 | 10 | [32] |
| Microfiltration, ammonium sulphate precipitation, ultracentrifugation | 50 | - | [30] | |
| Nano-TiO2, ultrafiltration | 64.75 | 5.3 | [31] | |
| Reverse micellar systems | Reverse micelle systems | 85–97.56 | 4–5.2 | [45,46,48,58] |
| Affinity-based reverse micelle system | 185.6 | 12.32 | [59] | |
| Chromatography | High speed counter-current chromatography, reverse micelle system | - | - | [45] |
| Immobilized metal affinity membrane | 94.6 | 15.4 | [60] | |
| Precipitation, ion exchange chromatography | - | 3.3 | [35] | |
| Cation exchange chromatography | - | 10 | [61] | |
3.2. Comparison of Bromelain Extraction Techniques
| Separation Method | Purification Fold | Activity Recovery (%) | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Aqueous two-phase extraction | 16.3 | 55.6 | Aqueous medium Low cost | Poor knowledge on mechanisms; High salt content | [62] |
| Ion exchange chromatography | 10 | 84.5 | Mild operation condition | High cost; Difficult optimization due to the complexity | [35] |
| Precipitation | 4.9 | 85.97 | Low energy needed Many alternative as precipitants | High precipitant (salt) content, hardly reciclable | [20] |
| Ultrafiltration | 10 | 90 | High product throughput Environmentally friendly Easy scaling up | Membrane fouling | [32] |
| Affinity membranes | 2.5 | - | High selectivity | High costs and difficult monitoring | [63] |
4. Bromelain Enzymatic Activity Measurement
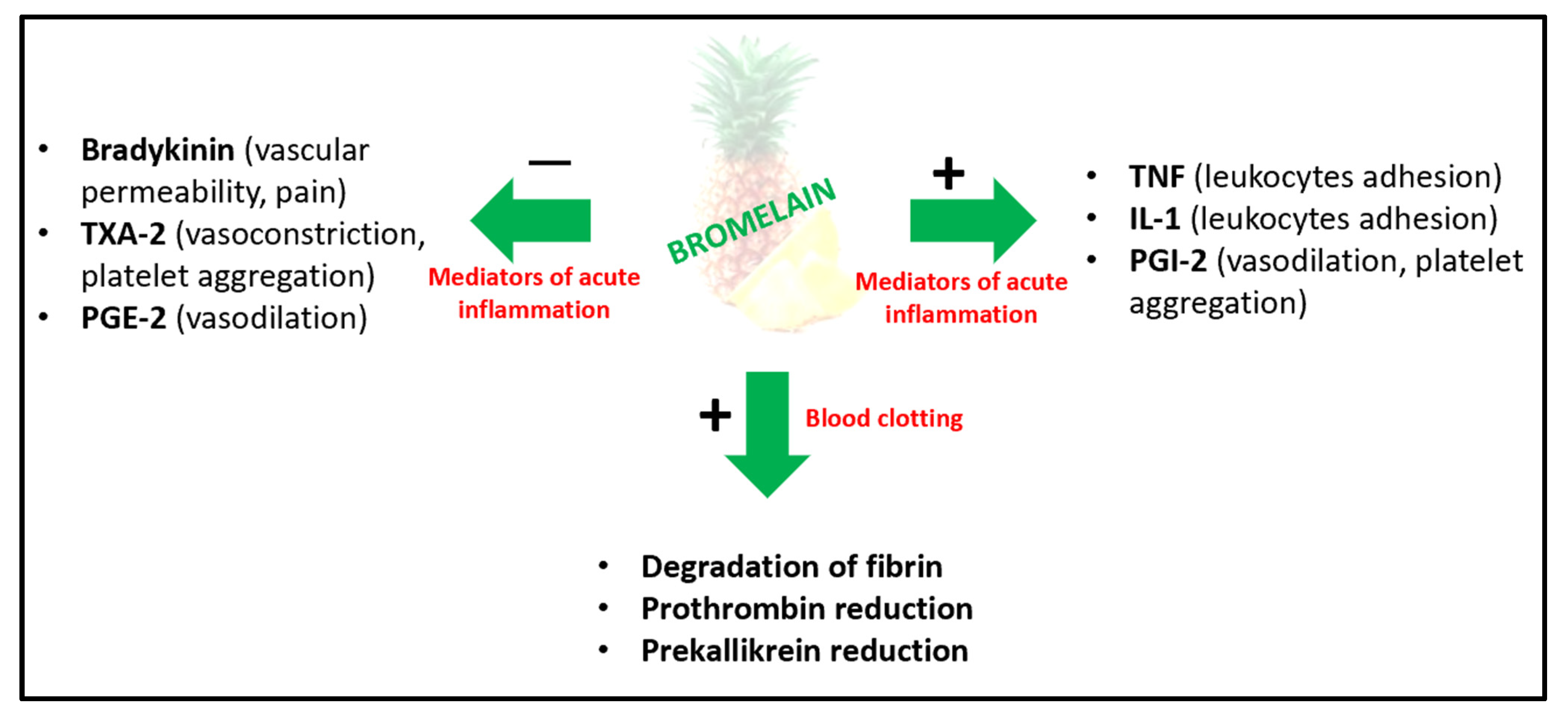
5. Pharmacodynamics and Pharmacokinetics Profiles of Bromelain
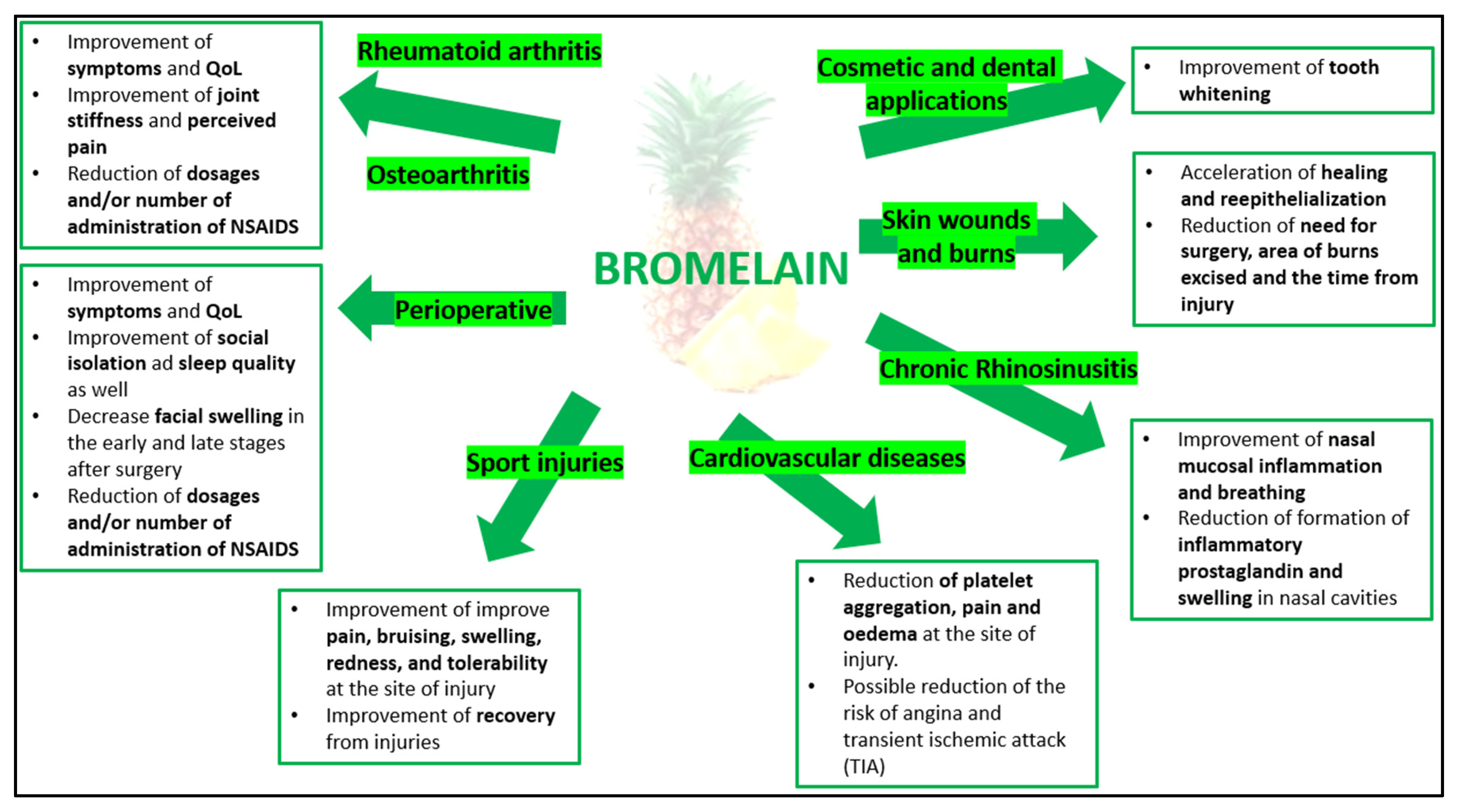
6. Bromelain Studies
6.1. Perioperative
6.2. Osteoarthritis and Rheumatoid Arthritis
Sport Injuries
6.3. Acute Sinusitis, Rhinitis, Rhinosinusitis
6.4. Cancer
6.5. Blood Coagulation and Cardiovascular Disease
6.6. Other Applications
7. Discussion and Future Perspectives
| Purification Approach | Technique | Advantages | Limits |
|---|---|---|---|
| Classic methods | Centrifugation Ultrafiltration Lyophilisation | ↑ yield during pre-purification steps | ↓ enzymatic yields |
| Unconventional methods | Gel filtration Ion exchange chromatography Affinity chromatography Aqueous two-phase extraction Reversed micelle extraction | ↑ selectivity and purity of the final extract ↑ enzymatic yields ↓ number of processes for final bromelain extract ↑ efficacy of bromelain purification | ↑ costs of product recovery stability (?) |
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Lencastre Novaes, L.C.; Jozala, A.F.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa Junior, A. Stability, purification, and applications of bromelain: A review. Biotechnol. Prog. 2016, 32, 5–13. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Kumar, A. Properties and Therapeutic Application of Bromelain: A Review. Biotechnol. Res. Int. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ramli, A.N.M.; Aznan, T.N.T.; Illias, R.M. Bromelain: From production to commercialisation. J. Sci. Food Agric. 2017, 97, 1386–1395. [Google Scholar] [CrossRef]
- González-Rábade, N.; Badillo-Corona, J.A.; Aranda-Barradas, J.S.; del Carmen Oliver-Salvador, M. Production of plant proteases in vivo and in vitro—A review. Biotechnol. Adv. 2011, 29, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [Green Version]
- Silveira, E.; Souza, M.E.; Santana, J.C.C.; Chaves, C.; Porto, A.L.F.; Tambourgi, E.B. Expanded bed adsorption of bromelain (E.C. 3.4.22.33) from Ananas comosus crude extract. Braz. J. Chem. Eng. 2009, 26, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Maurer, H. Bromelain: Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Bresolin, I.; Bresolin, I.; Silveira, E.; Tambourgi, E.B.; Mazzola, P.G. Isolation and purification of bromelain from waste peel of pineapple for therapeutic application. Braz. Arch. Biol. Technol. 2013, 56, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Soares, P.A.; Vaz, A.F.; Correia, M.T.; Pessoa, A.; Carneiro-Da-Cunha, M.G. Purification of bromelain from pineapple wastes by ethanol precipitation. Sep. Purif. Technol. 2012, 98, 389–395. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Aqueous Two-phase Extraction of Bromelain from Pineapple Peels (‘PhuLae’ cultv.) and Its Biochemical Properties. Food Sci. Biotechnol. 2011, 20, 1219–1226. [Google Scholar] [CrossRef]
- Costa, H.B.; Fernandes, P.M.; Romao, W.; Ventura, J.A. A new procedure based on column chromatography to purify bromelain by ion exchange plus gel filtration chromatographies. Ind. Crop. Prod. 2014, 59, 163–168. [Google Scholar] [CrossRef]
- Muntari, B.; Ismail, N.A.; Mel, M.; Jami, M.S.; Salleh, H.M.; Amid, A. Bromelain production: Current trends and perspective. Arch. Des Sci. 2012, 65, 369–399. [Google Scholar]
- Ahmad, B.; Ansari, M.A.; Sen, P.; Khan, R.H. Low versus high molecular weight poly(ethylene glycol)-induced states of stem bromelain at low pH: Stabilization of molten globule and unfolded states. Biopolymers 2006, 81, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ritonja, A.; Rowan, A.D.; Buttle, D.J.; Rawlings, N.; Turk, V.; Barrett, A. Stem bromelain: Amino acid sequence and implications for weak binding of cystatin. FEBS Lett. 1989, 247, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Rowan, A.D.; Buttle, D.J. Pineapple cysteine endopeptidases. Methods Enzymol. 1994, 244, 555–568. [Google Scholar] [CrossRef]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase activity and stability of natural bromelain preparations. Int. Immunopharmacol. 2005, 5, 783–793. [Google Scholar] [CrossRef]
- Rowan, A.D.; Buttle, D.J.; Barrett, A. The cysteine proteinases of the pineapple plant. Biochem. J. 1990, 266, 869–875. [Google Scholar] [PubMed]
- Khan, R.H.; Rasheedi, S.; Haq, S.K. Effect of pH, temperature and alcohols on the stability of glycosylated and deglycosylated stem bromelain. J. Biosci. 2003, 28, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Bhattacharyya, D. Preservation of natural stability of fruit “bromelain” from Ananas comosus (pineapple). J. Food Biochem. 2009, 33, 1–19. [Google Scholar] [CrossRef]
- Chaurasiya, R.S.; Hebbar, H.U. Extraction of bromelain from pineapple core and purification by RME and precipitation methods. Sep. Purif. Technol. 2013, 111, 90–97. [Google Scholar] [CrossRef]
- Murachi, T. Bromelain enzymes. In Methods in Enzymology; Elsevier: New York, NY, USA, 1976; Volume 45, pp. 475–485. [Google Scholar] [CrossRef]
- Bartholomew, D.P.; Paull, R.E.; Rohrbach, K.G. The Pineapple: Botany, Production, and Uses; CABI Publishing: New York, NY, USA, 2002. [Google Scholar]
- Grabowska, E.; Eckert, K.; Fichtner, I.; SchulzeForster, K.; Maurer, H. Bromelain proteases suppress growth, invasion and lung metastasis of B16F10 mouse melanoma cells. Int. J. Oncol. 1997, 11, 243–248. [Google Scholar] [CrossRef]
- Gautam, S.; Mishra, S.; Dash, V.; Goyal, A.K.; Rath, G. Comparative study of extraction, purification and estimation of bromelain from stem and fruit of pineapple plant. Thai J. Pharm. Sci. 2010, 34, 67–76. [Google Scholar]
- Muntari, B.; Salleh, H.M.; Amid, A.; Mel, M.; Jami, M.S. Recovery of recombinant bromelain from Escherichia coli BL21-AI. Afr. J. Biotechnol. 2011, 10, 18829–18832. [Google Scholar]
- Mulyono, N.; Rosmeilia, E.; Moi, J.G.P.; Valentine, B.O.; Suhartono, M.T. Quantity and quality of Bromelain in Some Indonesian Pineapple Fruits. Int. J. Appl. Biol. Pharm. 2013, 4, 235–240. [Google Scholar]
- Babu, B.R.; Rastogi, N.; Raghavarao, K. Liquid–liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chem. Eng. Process. Process. Intensif. 2008, 47, 83–89. [Google Scholar] [CrossRef]
- Szymczyk, A.; Labbez, C.; Fievet, P.; Vidonne, A.; Foissy, A.; Pagetti, J. Contribution of convection, diffusion and migration to electrolyte transport through nanofiltration membranes. Adv. Colloid Interface Sci. 2003, 103, 77–94. [Google Scholar] [CrossRef]
- Braeken, L.; Bettens, B.; Boussu, K.; Van Der Meeren, P.; Cocquyt, J.; Vermant, J.; Van Der Bruggen, B. Transport mechanisms of dissolved organic compounds in aqueous solution during nanofiltration. J. Membr. Sci. 2006, 279, 311–319. [Google Scholar] [CrossRef]
- Doko, B.; Bassani, V.; Casadebaig, J.; Cavailles, L.; Jacob, M. Preparation of proteolytic enzyme extracts from Ananascomosus L. Merr. fruit juice using semi permeable membrane, ammonium sulfate extraction, centrifugation and freeze-drying processes. J. Immunopharmacol. 2005, 4, 783–795. [Google Scholar]
- Chao, M.A.; Wu, M.Y.; Qiao, X.; Song, Y.; Zhao, Y. Study on purification of stem bromelain by nano-TiO2 and ultrafiltration. Food Sci. Technol. 2009, 34, 167–170. [Google Scholar]
- Lopes, F.L.G.; Júnior, J.B.S.; De Souza, R.R.; Ehrhardt, D.D.; Santana, J.; Tambourgi, E.B. Concentration by membrane separation processes of a medicinal product obtained from pineapple pulp. Braz. Arch. Biol. Technol. 2009, 52, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Hebbar, U.H.; Sumana, B.; Hemavathi, A.B.; Raghavarao, K.S.M.S. Separation and Purification of Bromelain by Reverse Micellar Extraction Coupled Ultrafiltration and Comparative Studies with Other Methods. Food Bioprocess Technol. 2012, 5, 1010–1018. [Google Scholar] [CrossRef]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Devakate, R.; Patil, V.; Waje, S.; Thorat, B. Purification and drying of bromelain. Sep. Purif. Technol. 2009, 64, 259–264. [Google Scholar] [CrossRef]
- Rabelo, A.P.B.; Tambourgi, E.B.; Pessoa, A. Bromelain partioning in twophase aqueous systems containing PEO-PPO-PEO block copolymers. J. Chromatogr. B 2004, 807, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Bradoo, S.; Saxena, R.K. Aqueous two-phase systems: An attractive technology for downstream processing of biomolecules. Curr. Sci. 1999, 77, 520–523. [Google Scholar]
- Coelho, D.F.; Silveira, E.; Junior, A.P.; Tambourgi, E.B. Bromelain purification through unconventional aqueous two-phase system (PEG/ammonium sulphate). Bioprocess Biosyst. Eng. 2013, 36, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, K.; Vadanasundari, V.; Hemavathy, R.V. A Comparative Study on Determining the Efficacy of Salt Precipitation and Biphasic System in the Extraction of Bromelain from Ananas comosus. Asian J. Sci. Technol. 2011, 2, 16–22. [Google Scholar]
- Ketnawa, S.; Sai-Ut, S.; Theppakorn, T.; Chaiwut, P.; Rawdkuen, S. Partitioning of bromelain from pineapple peel (Nang Laecultv.) by aqueous two phase system. Asian J. Food Agro-Ind. 2009, 2, 457–468. [Google Scholar]
- Ng, P.K.; He, J.; Synder, M.K. Separation of proteins mixtures using PH-gradient cation exchange chromatography. J. Chromatogr. A 2009, 1216, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.D.; Buttle, D.J.; Barrett, A. Ananain: A novel cysteine proteinase found in pineapple stem. Arch. Biochem. Biophys. 1988, 267, 262–270. [Google Scholar] [CrossRef]
- Hernández, M.; Carvajal, C.; Márquez, M.; Báez, R.; Morris, H.; Santos, R.; de los Ángeles Chávez, M. Obtención de Preparados Enzimáticos a Partir de Tallos de Piña (Ananas Comosus) con Potencialidades de uso en la Biotecnología y la Medicina. Rev. CENIC Cienc. Biológicas 2005, 36. [Google Scholar]
- Bobb, D. Isolation of Stem Bromelain by Affinity Chromatography and its Partial Characterization by Gel Electrophoresis. Prep. Biochem. 1972, 2, 347–354. [Google Scholar] [CrossRef]
- Amid, A.; Ismail, N.A.; Yusof, F.; Mohd-Salleh, H. Expression, purification, and characterization of a recombinant stem bromelain from Ananas comosus. Process Biochem. 2011, 46, 2232–2239. [Google Scholar] [CrossRef]
- Yin, L.; Sun, C.; Han, X.; Xu, L.; Xu, Y.; Qi, Y.; Peng, J. Preparative purification of bromelain (EC 3.4.22.33) from pineapple fruit by high-speed counter-current chromatography using a reverse-micelle solvent system. Food Chem. 2011, 129, 925–932. [Google Scholar] [CrossRef]
- Chaurasiya, R.S.; Sakhare, P.Z.; Bhaskar, N.; Hebbar, H.U. Efficacy of reverse micellar extracted fruit bromelain in meat tenderization. J. Food Sci. Technol. 2014, 52, 3870–3880. [Google Scholar] [CrossRef] [Green Version]
- Hebbar, H.U.; Sumana, B.; Raghavarao, K. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour. Technol. 2008, 99, 4896–4902. [Google Scholar] [CrossRef]
- Silvestre, M.P.C.; Carreira, R.L.; Silva, M.R.; Corgosinho, F.C.; Monteiro, M.R.P.; Morais, H.A. Effect of pH and Temperature on the Activity of Enzymatic Extracts from Pineapple Peel. Food Bioprocess Technol. 2012, 5, 1824–1831. [Google Scholar] [CrossRef]
- Muntari, B.; Amid, A.; Mel, M.; Jami, M.S.; Salleh, H.M. Recombinant bromelain production in Escherichia coli: Process optimization in shake flask culture by response surface methodology. AMB Express 2012, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.-J.; Choi, C.-S.; Park, J.-H.; Kang, H.-W.; Choi, J.-E.; Nou, I.-S.; Lee, S.Y.; Kang, K.-K. Overexpression of the pineapple fruit bromelain gene (BAA) in transgenic Chinese cabbage (Brassica rapa) results in enhanced resistance to bacterial soft rot. Electron. J. Biotechnol. 2008, 11, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Nurul, A.; Azura, A. Differential scanning calorimetry as tool in observing thermal and storage stability of recombinant bromelain. Int. Food Res. J. 2012, 19, 727–731. [Google Scholar]
- George, S.; Bhasker, S.; Madhav, H.; Nair, A.; Chinnamma, M. Functional Characterization of Recombinant Bromelain of Ananas comosus Expressed in a Prokaryotic System. Mol. Biotechnol. 2014, 56, 166–174. [Google Scholar] [CrossRef]
- Manzoor, Z.; Nawaz, A.; Mukhtar, H.; Haq, I. Bromelain: Methods of Extraction, Purification and Therapeutic Applications. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Novaes, L.C.D.L.; Ebinuma, V.D.C.S.; Mazzola, P.G.; Júnior, A.P. Polymer-based alternative method to extract bromelain from pineapple peel waste. Biotechnol. Appl. Biochem. 2013, 60, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Rawdkuen, S.; Chaiwut, P. Two phase partitioning and collagen hydrolysis of bromelain from pineapple peel Nang Laecultivar. J. Biochem. Eng. 2010, 52, 205–211. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Santana, J.C.C.; Tambourgi, E.B. The effect of pH on bromelain partition from Ananascomosus by PEG4000/Phosphate ATPS. Braz. Arch. Biol. Technol. 2011, 54, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Navapara, R.D.; Avhad, D.N.; Rathod, V.K. Application of Response Surface Methodology for Optimization of Bromelain Extraction in Aqueous Two-Phase System. Sep. Sci. Technol. 2011, 46, 1838–1847. [Google Scholar] [CrossRef]
- Kumar, S.; Hemavathi, A.B.; Hebbar, H.U. Affinity based reverse micellar extraction and purification of bromelain from pineapple (Ananascomosus L. Merryl) waste. Process Biochem. 2011, 46, 1216–1220. [Google Scholar] [CrossRef]
- Nie, H.; Li, S.; Zhou, Y.; Chen, T.; He, Z.; Su, S.; Zhang, H.; Xue, Y.; Zhu, L. Purification of bromelain using immobilized metal affinity membranes. J. Biotechnol. 2008, 136, S402–S459. [Google Scholar] [CrossRef]
- Nadzirah, K.Z.; Zainal, S.; Noriham, A.; Normah, I. Efficacy of selected purification techniques for bromelain. Int. Food Res. J. 2013, 20, 43–46. [Google Scholar]
- Wu, W.-C.; Ng, H.S.; Sun, I.-M.; Lan, J.C.-W. Single step purification of bromelain from Ananas comosus pulp using a polymer/salt aqueous biphasic system. J. Taiwan Inst. Chem. Eng. 2017, 79, 158–162. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, H.; Yu, D.-G.; Wu, C.; Zhang, Y.; White, C.J.B.; Zhu, L. Surface modification of electrospun polyacrylonitrile nanofiber towards developing an affinity membrane for bromelain adsorption. Desalination 2010, 256, 141–147. [Google Scholar] [CrossRef]
- Nor, M.Z.M.; Ramchandran, L.; Duke, M.; Vasiljevic, T. Integrated ultrafiltration process for the recovery of bromelain from pineapple waste mixture. J. Food Process. Eng. 2017, 40, e12492. [Google Scholar] [CrossRef]
- Corzo, C.A.; Waliszewski, K.N.; Welti-Chanes, J. Pineapple fruit bromelain affinity to different protein substrates. Food Chem. 2012, 133, 631–635. [Google Scholar] [CrossRef]
- Murachi, T.; Neurath, H. Fractionation and Specificity Studies on Stem Bromelain. J. Biol. Chem. 1960, 235, 99–107. [Google Scholar] [CrossRef]
- Giles, N.M.; Giles, G.; Jacob, C. Multiple roles of cysteine in biocatalysis. Biochem. Biophys. Res. Commun. 2003, 300, 1–4. [Google Scholar] [CrossRef]
- Shukor, M.Y.; Masdor, N.; Baharom, N.A.; Jamal, J.A.; Abdullah, M.P.A.; Shamaan, N.A.; Syed, M.A. An inhibitive determination method for heavy metals using bromelain, a cysteine protease. Appl. Biochem. Biotechnol. 2008, 144, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Mel, M.; Jami, M.S.; Amid, A.; Salleh, H.M. Kinetic studies on recombinant stem bromelain. Adv. Enzym. Res. 2013, 1, 52–60. [Google Scholar] [CrossRef]
- Esti, M.; Benucci, I.; Liburdi, K.; Garzillo, A.M.V. Effect of Wine Inhibitors on Free Pineapple Stem Bromelain Activity in a Model Wine System. J. Agric. Food Chem. 2011, 59, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Filippova, I.; Lysogorskaya, E.; Oksenoit, E.; Rudenskaya, G.; Stepanov, V. l-Pyroglutamyl-l-phenylalanyl-l-leucine-p-nitroanilide—A chromogenic substrate for thiol proteinase assay. Anal. Biochem. 1984, 143, 293–297. [Google Scholar] [CrossRef]
- Harrach, T.; Eckert, K.; Maurer, H.R.; Machleidt, I.; Machleidt, W.; Nuck, R. Isolation and characterization of two forms of an acidic bromelain stem proteinase. Protein J. 1998, 17, 351–361. [Google Scholar] [CrossRef]
- Napper, A.D.; Bennett, S.P.; Borowski, M.; Holdridge, M.B.; Leonard, M.J.; Rogers, E.E.; Duan, Y.; Laursen, R.A.; Reinhold, B.; Shames, S.L. Purification and characterization of multiple forms of the pineapple-stem-derived cysteine proteinases ananain and comosain. Biochem. J. 1994, 301, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Tochi, B.N.; Wang, Z.; Xu, S.-Y.; Zhang, W. Therapeutic Application of Pineapple Protease (Bromelain): A Review. Pak. J. Nutr. 2008, 7, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, Z.A.; Ahmad, T. Therapeutic uses of pineapple-extracted bromelain in surgical care—A review. J. Pak. Med Assoc. 2017, 67, 121–125. [Google Scholar] [PubMed]
- Fitzhugh, D.J.; Shan, S.; Dewhirst, M.W.; Hale, L.P. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin. Immunol. 2008, 128, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Moss, J.N.; Frazier, C.V.; Martin, G.J. Bromelains: The Pharmacology of the Enzymes. Arch. Int. Pharmacodyn. Ther. 1963, 145, 166–189. [Google Scholar]
- Hamdy, S. Bromelain. Monograph. Altern. Med. Rev. 2010, 15, 61–68. [Google Scholar]
- Liu, S.; Zhao, H.; Wang, Y.; Zhao, H.; Ma, C. Oral Bromelain for the Control of Facial Swelling, Trismus, and Pain after Mandibular Third Molar Surgery: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2019, 77, 1566–1574. [Google Scholar] [CrossRef]
- Mendes, M.; Nascimento-Junior, E.D.; Reinheimer, D.; Martins-Filho, P. Efficacy of proteolytic enzyme bromelain on health outcomes after third molar surgery. Systematic review and meta-analysis of randomized clinical trials. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e61–e69. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.A.; Lima, F.D.S.; Vasconcelos, B.D.E. Is bromelain an effective drug for the control of pain and inflammation associated with impacted third molar surgery? Systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 651–658. [Google Scholar] [CrossRef] [PubMed]
- De Souza, G.M.; Fernandes, I.A.; Dos Santos, C.R.R.; Falci, S.G.M. Is bromelain effective in controlling the inflammatory parameters of pain, edema, and trismus after lower third molar surgery? A systematic review and meta-analysis. Phytother. Res. 2019, 33, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Kamenícek, V.; Holán, P.; Franĕk, P. Systemic enzyme therapy in the treatment and prevention of post-traumatic and postoperative swelling. Acta Chir. Orthop. Traumatol. Cechoslov. 2001, 68, 45–49. [Google Scholar]
- Golezar, S. Ananas comosus Effect on Perineal Pain and Wound Healing after Episiotomy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Iran. Red Crescent Med. J. 2016, 18, e21019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howat, R.C.L.; Lewis, G.D. The Effect of Bromelain Therapy on Episiotomy Wounds-A Double Blind Controlled Clinical Trial. BJOG Int. J. Obstet. Gynaecol. 1972, 79, 951–953. [Google Scholar] [CrossRef]
- Seltzer, A.P. Minimizing post-operative edema and ecchymoses by the use of an oral enzyme preparation (bromelain). A controlled study of 53 rhinoplasty cases. Eye Ear Nose Throat Mon. 1962, 41, 813–817. [Google Scholar] [PubMed]
- Cohen, A.; Goldman, J. Bromelains Therapy in Rheumatoid Arthritis. Pa. Med. J. (1928) 1964, 67, 27–30. [Google Scholar]
- Klein, G.; Kullich, W.; Schnitker, J.; Schwann, H. Efficacy and tolerance of an oral enzyme combination in painful osteoarthritis of the hip. A double-blind, randomised study comparing oral enzymes with non-steroidal anti-inflammatory drugs. Clin. Exp. Rheumatol. 2006, 24, 25–30. [Google Scholar] [PubMed]
- Kasemsuk, T.; Saengpetch, N.; Sibmooh, N.; Unchern, S. Improved WOMAC score following 16-week treatment with bromelain for knee osteoarthritis. Clin. Rheumatol. 2016, 35, 2531–2540. [Google Scholar] [CrossRef]
- Naeem, H.; Naqvi, S.N.-U.; Perveen, R.; Ishaque, F.; Bano, R.; Abrar, H.; Arsalan, A.; Malik, N. Efficiency of proteolytic enzymes in treating lumbar spine osteoarthritis (low back pain) patients and its effects on liver and kidney enzymes. Pak. J. Pharm. Sci. 2020, 33, 371–378. [Google Scholar]
- Conrozier, T.; Mathieu, P.; Bonjean, M.; Marc, J.-F.; Renevier, J.-L.; Balblanc, J.-C. A complex of three natural anti-inflammatory agents provides relief of osteoarthritis pain. Altern. Ther. Health Med. 2014, 20, 32–37. [Google Scholar]
- Henriksson, K.; From, J.; Strateli, G. Patient-reported adherence to coprescribed proton pump inhibitor gastroprotection in osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis patients using nonsteroidal anti-inflammatory drugs. Patient Prefer. Adherence 2014, 8, 1611–1617. [Google Scholar] [CrossRef] [Green Version]
- Vital Produx. Enzymes for Athletic Injuries. Available online: http://www.vitalprodux.com/enzymes_for_athetic_injuries.html (accessed on 20 December 2001).
- Hotfiel, T.; Freiwald, J.; Hoppe, M.W.; Lutter, C.; Forst, R.; Grim, C.; Bloch, W.; Hüttel, M.; Heiss, R. Advances in Delayed-Onset Muscle Soreness (DOMS): Part I: Pathogenesis and Diagnostics. Sportverletz. Sportschaden 2018, 32, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Cerny, F.; Cotter, J. Attenuation of contraction induced skeletal muscle injury by bromelain. Med. Sci. Sports Exerc. 1992, 24, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.B.; Merrick, M.A.; Ingersoll, C.D.; Edwards, J.E. Preliminary Comparison of Bromelain and Ibuprofen for Delayed Onset Muscle Soreness Management. Clin. J. Sport Med. 2002, 12, 373–378. [Google Scholar] [CrossRef]
- Grabs, V.; Kersten, A.; Haller, B.; Braun, S.; Nieman, D.C.; Halle, M.; Scherr, J. Rutoside and Hydrolytic Enzymes Do Not Attenuate Marathon-Induced Inflammation. Med. Sci. Sports Exerc. 2017, 49, 387–395. [Google Scholar] [CrossRef]
- Kerkhoffs, G.M.M.J.; Struijs, P.A.A.; De Wit, C.; Rahlfs, V.W.; Zwipp, H.; Van Dijk, C.N. A double blind, randomised, parallel group study on the efficacy and safety of treating acute lateral ankle sprain with oral hydrolytic enzymes. Br. J. Sports Med. 2004, 38, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Baumuller, M. The application of hydrolytic enzymes in blunt woundsto the soft tissue and distortion of the ankle joint: A double blind clinic al trial (Translated from German). Allgemeinmedizin 1990, 19, 178–182. [Google Scholar]
- Zuschlag, J.M. Double-blind clinical study using certain proteolytic enzyme mixtures in karate fighters: Working paper. Gerestsried Ger. Mucos Pharma GmbH 1988, 1–5. [Google Scholar]
- Deitrick, R.E. Oral proteolytic enzymes in the treatment of athletic injuries: A double-blind study. Pa. Med. 1965, 68, 35–37. [Google Scholar]
- Rathgeber, W.F. The use of proteolytic enzymes (chymoral) in sporting injuries. South Afr. Med. J. 1971, 45, 181–183. [Google Scholar]
- Masson, M. Bromelain in blunt injuries of the locomotor system. A study of observed applications in general practice. Fortschr. Med. 1995, 113, 303–306. [Google Scholar]
- Woolf, R.M.; Snow, J.W.; Walker, J.H.; Broadbent, T.R. Resolution of an Artifically Induced Hematoma and the Influence of a Proteolytic Enzyme. J. Trauma Acute Care Surg. 1965, 5, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Büttner, L.; Achilles, N.; Böhm, M.; Shah-Hosseini, K.; Mösges, R. Efficacy and tolerability of bromelain in patients with chronic rhinosinusitis—A pilot study. B-ENT 2013, 9, 217–225. [Google Scholar] [PubMed]
- Helms, S.; Miller, A. Natural treatment of chronic rhinosinusitis. Altern. Med. Rev. A J. Clin. Ther. 2006, 11, 196–207. [Google Scholar]
- Seltzer, A.P. Adjunctive use of bromelains in sinusitis: A controlled study. Eye Ear Nose Throat Mon. 1967, 46, 1281–1285. [Google Scholar]
- Ryan, R.E. A Double-Blind Clinical Evaluation of Bromelains in the Treatment of Acute Sinusitis. Headache J. Head Face Pain 1967, 7, 13–17. [Google Scholar] [CrossRef]
- Bhui, K.; Tyagi, S.; Prakash, B.; Shukla, Y. Pineapple bromelain induces autophagy, facilitating apoptotic response in mammary carcinoma cells. BioFactors 2010, 36, 474–482. [Google Scholar] [CrossRef]
- Bhui, K.; Tyagi, S.; Srivastava, A.K.; Singh, M.; Roy, P.; Singh, R.; Shukla, Y. Bromelain inhibits nuclear factor kappa-B translocation, driving human epidermoid carcinoma A431 and melanoma A375 cells through G2/M arrest to apoptosis. Mol. Carcinog. 2011, 51, 231–243. [Google Scholar] [CrossRef]
- Amini, A.; Moghaddam, S.M.; Ehteda, A.; Morris, L. Bromelain and N-acetylcysteine inhibit proliferation and survival of gastrointestinal cancer cells in vitro: Significance of combination therapy. J. Exp. Clin. Cancer Res. 2014, 33, 92. [Google Scholar]
- Amini, A.; Moghaddam, S.M.; Morris, D.L. Bromelain. In Utility of Bromelain and N-Acetylcysteine in Treatment of Peritoneal Dissemination of Gastrointestinal Mucin-Producing Malignancies; Springer International Publishing: New York, NY, USA, 2016; pp. 63–80. [Google Scholar]
- Pillai, K.; Akhter, J.; Chua, T.C.; Morris, D.L. Anticancer Property of Bromelain with Therapeutic Potential in Malignant Peritoneal Mesothelioma. Cancer Investig. 2013, 31, 241–250. [Google Scholar] [CrossRef]
- Beuth, J.; Van Leendert, R.; Schneider, B.; Uhlenbruck, G. Complementary medicine on side-effects of adjuvant hormone therapy in patients with breast cancer. In Vivo 2013, 27, 869–871. [Google Scholar] [PubMed]
- Uhlenbruck, G.; VAN Leendert, R.; Schneider, B.; Beuth, J. Reduced side-effects of adjuvant hormone therapy in breast cancer patients by complementary medicine. In Vivo 2010, 24, 799–802. [Google Scholar] [PubMed]
- Eckert, K.; Grabowska, E.; Stange, R.; Schneider, U.; Eschmann, K.; Maurer, H.R. Effects of oral bromelain administration on the impaired immunocytotoxicity of mononuclear cells from mammary tumor patients. Oncol. Rep. 1999, 6, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, S.; Perez, H.D.; Paroulek, A.; Chinnakkannu, P.; Kandalam, U.; Jaffe, M.; Rathinavelu, A. Bromelain-Induced Apoptosis in GI-101A Breast Cancer Cells. J. Med. Food 2012, 15, 344–349. [Google Scholar] [CrossRef]
- Desideri, I.; Francolini, G.; Becherini, C.; Terziani, F.; Paoli, C.D.; Olmetto, E.; Loi, M.; Perna, M.; Meattini, I.; Scotti, V.; et al. Use of an alpha lipoic, methylsulfonylmethane and bromelain dietary supplement (Opera®) for chemotherapy-induced peripheral neuropathy management, a prospective study. Med. Oncol. 2017, 34, 46. [Google Scholar] [CrossRef] [Green Version]
- Juhasz, B.; Thirunavukkarasu, M.; Pant, R.; Zhan, L.; Penumathsa, S.V.; Secor, E.R.; Srivastava, S.; Raychaudhuri, U.; Menon, V.P.; Otani, H.; et al. Bromelain induces cardioprotection against ischemia-reperfusion injury through Akt/FOXO pathway in rat myocardium. Am. J. Physiol. Circ. Physiol. 2008, 294, H1365–H1370. [Google Scholar] [CrossRef] [Green Version]
- Ley, C. A review of the use of bromelain in cardiovascular diseases. J. Chin. Integr. Med. 2011, 9, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, M.G.; Smyth, R.D. Effects of Bromelain Anti-Edema Therapy on Coagulation, Bleeding, and Prothrombin Times. J. New Drugs 1963, 3, 37–39. [Google Scholar] [CrossRef]
- European Medicines Agency-EMA/113587/2014. 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/PIP_decision/WC500166523.pdf (accessed on 14 December 2015).
- Errasti, M.E.; Prospitti, A.; Viana, C.A.; Gonzalez, M.M.; Ramos, M.V.; Rotelli, A.E.; Caffini, N.O. Effects on fibrinogen, fibrin, and blood coagulation of proteolytic extracts from fruits of Pseudananas macrodontes, Bromelia balansae, and B. hieronymi (Bromeliaceae) in comparison with bromelain. Blood Coagul. Fibrinolysis 2016, 27, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Barboza, A.D.S.; Cuevas-Suárez, C.E.; Da Silva, A.F.; Piva, E.; Lund, R.G. Novel in-office peroxide-free tooth-whitening gels: Bleaching effectiveness, enamel surface alterations, and cell viability. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Parravano, M.; Tedeschi, M.; Manca, D.; Costanzo, E.; di Renzo, A.; Giorno, P.; Barbano, L.; Ziccardi, L.; Varano, M.; Parisi, V. Effects of Macuprev® Supplementation in Age-Related Macular Degeneration: A Double-Blind Randomized Morpho-Functional Study Along 6 Months of Follow-Up. Adv. Ther. 2019, 36, 2493–2505. [Google Scholar] [CrossRef] [Green Version]
- Lete, I.; Mendoza, N.; De La Viuda, E.; Carmona, F. Effectiveness of an antioxidant preparation with N-acetyl cysteine, alpha lipoic acid and bromelain in the treatment of endometriosis-associated pelvic pain: LEAP study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.P.; Chichlowski, M.; Trinh, C.T.; Greer, P.K. Dietary supplementation with fresh pineapple juice decreases inflammation and colonic neoplasia in IL-10-deficient mice with colitis. Inflamm. Bowel Dis. 2010, 16, 2012–2021. [Google Scholar] [CrossRef]
- Secor, E.R.; Szczepanek, S.M.; Castater, C.A.; Adami, A.J.; Matson, A.P.; Rafti, E.T.; Guernsey, L.; Natarajan, P.; McNamara, J.T.; Schramm, C.M.; et al. Bromelain Inhibits Allergic Sensitization and Murine Asthma via Modulation of Dendritic Cells. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.R.; Carson, W.F.; Singh, A.; Pensa, M.; Guernsey, L.A.; Schramm, C.M.; Thrall, R.S. Oral Bromelain Attenuates Inflammation in an Ovalbumin-Induced Murine Model of Asthma. Evidence-Based Complement. Altern. Med. 2008, 5, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordts, T.; Horter, J.; Vogelpohl, J.; Kremer, T.; Kneser, U.; Hernekamp, J.-F. Enzymatic debridement for the treatment of severely burned upper extremities—Early single center experiences. BMC Dermatol. 2016, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, L.; Shoham, Y.; Krieger, Y.; Rubin, G.; Sander, F.; Koller, J.; David, K.; Egosi, D.; Ahuja, R.; Singer, A. Minimally invasive burn care: A review of seven clinical studies of rapid and selective debridement using a bromelain-based debriding enzyme (Nexobrid®). Ann. Burn. Fire Disasters 2015, 28, 264–274. [Google Scholar]
- Singer, A.J.; Boyce, S.T. Burn Wound Healing and Tissue Engineering. J. Burn. Care Res. 2017, 38, e605–e613. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, L.; Krieger, Y.; Bogdanov-Berezovski, A.; Silberstein, E.; Shoham, Y.; Singer, A.J. A novel rapid and selective enzymatic debridement agent for burn wound management: A multi-center RCT. Burns 2014, 40, 466–474. [Google Scholar] [CrossRef]
- Hirche, C.; Almeland, S.K.; Dheansa, B.; Fuchs, P.; Governa, M.; Hoeksema, H.; Korzeniowski, T.; Lumenta, D.B.; Marinescu, S.; Martinez-Mendez, J.R.; et al. Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Burns 2020, 46, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Massimiliano, R.; Pietro, R.; Paolo, S.; Sara, P.; Michele, F. Role of bromelain in the treatment of patients with pityriasis lichenoides chronica. J. Dermatol. Treat. 2007, 18, 219–222. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, C.; Geng, L.; Chen, G.; Wang, X.; Chen, W.; Sa, R.; Zhang, J.; Zhang, X. Purification and characterization of bromelain from pineapple (Ananas comosus L.) peel waste. J. Food Sci. 2021, 86, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-M.; Xia, M.-F.; Wang, Y.; Chang, X.-X.; Yao, X.-Z.; Rao, S.-X.; Zeng, M.-S.; Tu, Y.-F.; Feng, R.; Jia, W.-P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colletti, A.; Li, S.; Marengo, M.; Adinolfi, S.; Cravotto, G. Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses. Appl. Sci. 2021, 11, 8428. https://doi.org/10.3390/app11188428
Colletti A, Li S, Marengo M, Adinolfi S, Cravotto G. Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses. Applied Sciences. 2021; 11(18):8428. https://doi.org/10.3390/app11188428
Chicago/Turabian StyleColletti, Alessandro, Shuyi Li, Mauro Marengo, Salvatore Adinolfi, and Giancarlo Cravotto. 2021. "Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses" Applied Sciences 11, no. 18: 8428. https://doi.org/10.3390/app11188428
APA StyleColletti, A., Li, S., Marengo, M., Adinolfi, S., & Cravotto, G. (2021). Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses. Applied Sciences, 11(18), 8428. https://doi.org/10.3390/app11188428









