Biomechanical Evaluation Method to Optimize External Fixator Configuration in Long Bone Fractures—Conceptual Model and Experimental Validation Using Pilot Study
Abstract
:1. Introduction
Configuration Optimization
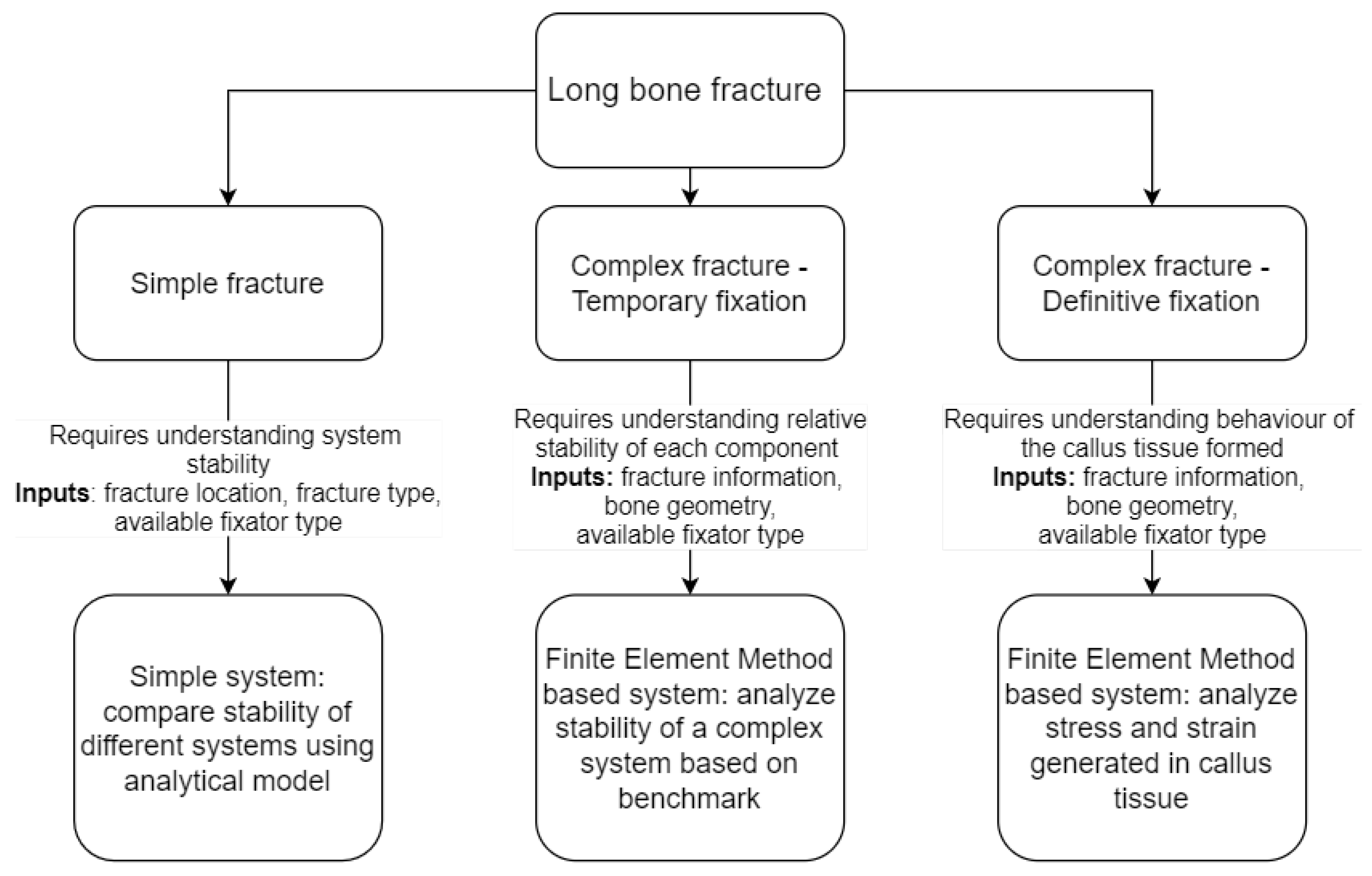
2. Methodology
2.1. Framework Development
2.2. Pilot Study
2.2.1. Mechanical Testing
- Pin–Small Clamp;
- Small Clamp–Large Clamp;
- Large Clamp–Bar;
- Nut–Small Clamp.
2.2.2. Model Generation
Pin Bending Model
Spring Model
Simplified FEA Model
3. Results
3.1. Experimental Results
3.2. Pin Equation Calculation
3.3. Spring Model
3.4. Simplified FEA Model
3.5. Basic FEA Model
3.6. Model Comparison
- Difference in results provided to the actual test results and the comprehensive FEA model;
- Difference in the positioning of each configuration against each other against that of the test results and FEA results.
4. Discussion
4.1. Computational Models
4.2. Workflow for Surgical Assistance
4.3. Limitations
5. Conclusions
6. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amer, K.M.; Congiusta, D.V.; Suri, P.; Merchant, A.M.; Vosbikian, M.M.; Ahmed, I.H. Patient frailty as a risk assessment tool in surgical management of long bone fractures. J. Clin. Orthop. Trauma 2020, 11, S591–S595. [Google Scholar] [CrossRef]
- Court-Brown, C.; Rimmer, S.; Prakash, U.; McQueen, M. The epidemiology of open long bone fractures. Injury 1998, 29, 529–534. [Google Scholar] [CrossRef]
- Bonafede, M.; Espindle, D.; Bower, A.G. The direct and indirect costs of long bone fractures in a working age US population. J. Med. Econ. 2013, 16, 169–178. [Google Scholar] [CrossRef]
- ITF. Road Safety Annual Report 2017; OECD Publishing: Paris, France, 2017. [Google Scholar]
- Sapkota, D.; Bista, B.; Adhikari, S.R. Economic Costs Associated with Motorbike Accidents in Kathmandu, Nepal. Front. Public Health 2016, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León, A.L.; Ascuntar-Tello, J.; Valderrama-Molina, C.O.; Giraldo, N.D.; Constaín, A.; Puerta, A.; Restrepo, C.; Jaimes, F. Grouping of body areas affected in traffic accidents. A cohort study. J. Clin. Orthop. Trauma 2018, 9, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Oluwadiya, K.S.; Oginni, L.M.; Olasinde, A.A.; Fadiora, S.O. Motorcycle limb injuries in a developing country. West Afr. J. Med. 2004, 23, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.; Taj, T.M.; Ali, S.A.; Mirza, W.A.; Badar, N. Non-fatal limb injuries in motorbike accidents. J. Coll. Physicians-Surg.-Pak. JCPSP 2008, 18, 635–638. [Google Scholar] [PubMed]
- Lateef, F. Riding motorcycles: Is it a lower limb hazard? Singap. Med. J. 2002, 43, 566–569. [Google Scholar]
- Mcgee, M.; Sharma, G. The Injury Chart Book: A Graphical Overview of the Global Burden of Injuries; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Meling, T.; Harboe, K.; Søreide, K. Incidence of traumatic long-bone fractures requiring in-hospital management: A prospective age- and gender-specific analysis of 4890 fractures. Injury 2009, 40, 1212–1219. [Google Scholar] [CrossRef]
- Mirhadi, S.; Ashwood, N.; Karagkevrekis, B. Factors influencing fracture healing. Trauma 2013, 15, 140–155. [Google Scholar] [CrossRef]
- Mills, L.A.; Simpson, A.H.R.W. The relative incidence of fracture non-union in the Scottish population (5.17 million): A 5-year epidemiological study. BMJ Open 2013, 3, e002276. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.S.; Waterson, B. Principles of management of long bone fractures and fracture healing. Surgery 2020, 38, 91–99. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Edwards, E.R.; de Steiger, R.; Gabbe, B.J. Incidence, Costs and Predictors of Non-Union, Delayed Union and Mal-Union Following Long Bone Fracture. Int. J. Environ. Res. Public Health 2018, 15, 2845. [Google Scholar] [CrossRef] [Green Version]
- Antonova, E.; Le, T.K.; Burge, R.; Mershon, J. Tibia shaft fractures: Costly burden of nonunions. BMC Musculoskelet. Disord. 2013, 14, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahagirdar, R.; Scammell, B.E. Principles of fracture healing and disorders of bone union. Surgery 2009, 27, 63–69. [Google Scholar] [CrossRef]
- Wang, M.; Yang, N. A review of bioregulatory and coupled mechanobioregulatory mathematical models for secondary fracture healing. Med. Eng. Phys. 2017, 48, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Betts, D.C.; Müller, R. Mechanical regulation of bone regeneration: Theories, models, and experiments. Front. Endocrinol. 2014, 5, 211. [Google Scholar] [CrossRef] [Green Version]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, J.E.; O’Donnell, J.C. Tibia fractures: What should be fixed? J. Pediatr. Orthop. 2012, 32, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Roseiro, L.M.; Neto, M.A.; Amaro, A.; Leal, R.P.; Samarra, M.C. External fixator configurations in tibia fractures : 1D optimization and 3D analysis comparison. Comput. Methods Programs Biomed. 2013, 113, 360–370. [Google Scholar] [CrossRef]
- Padhi, N.R.; Padhi, P. Use of external fixators for open tibial injuries in the rural third world: Panacea of the poor? Injury 2007, 38, 150–159. [Google Scholar] [CrossRef]
- Nanchahal, J.; Nayagam, S.; Khan, U.; Moran, C.; Barrett, S.; Sanderson, F.; Pallister, I. Standards for the Management of Open Fractures of the Lower Limb; Royal Society of Medicine Press Ltd.: London, UK, 2009. [Google Scholar]
- Moss, D.P.; Tejwani, N.C. Biomechanics of External Fixation: A Review of the literature. Bull. Nyu Hosp. Jt. Dis. 2007, 65, 294–299. [Google Scholar]
- Palomares, K.T.S.; Gleason, R.E.; Mason, Z.D.; Cullinane, D.M.; Einhorn, T.A.; Gerstenfeld, L.C.; Morgan, E.F. Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2009, 27, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.R. Mechanical loading history and skeletal biology. J. Biomech. 1987, 20, 1095–1109. [Google Scholar] [CrossRef]
- Dell’Oca, A.F. External Fixation. In AO Principles of Fracture Management; Rüedi, T.P., Murphy, W.M., Eds.; Thieme: New York, NY, USA, 2000; Chapter 3.3.3; pp. 233–248. [Google Scholar]
- Kempson, G.E.; Campbell, D. The comparative stiffness of external fixation frames. Injury 1981, 12, 297–304. [Google Scholar] [CrossRef]
- Rigal, S.; Merloz, P.; Le Nen, D.; Mathevon, H.; Masquelet, A.C. Bone transport techniques in posttraumatic bone defects. Orthop. Traumatol. Surg. Res. 2012, 98, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salai, M.; Horoszowski, H.; Pritsch, M.; Amit, Y. Primary reconstruction of traumatic bony defects using allografts. Arch. Orthop. Trauma Surg. 1999, 119, 435–439. [Google Scholar] [CrossRef]
- Robinson, C.M.; McLauchlan, G.; Christie, J.; McQueen, M.M.; Court-Brown, C.M. Tibial fractures with bone loss treated by primary reamed intramedullary nailing. J. Bone Jt. Surgery. Br. Vol. 1995, 77, 906–913. [Google Scholar] [CrossRef] [Green Version]
- Keating, J.F. The management of fractures with bone loss. J. Bone Jt.-Surg.-Br. Vol. 2005, 87-B, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, P.; Inzana, J.A.; Gueorguiev, B.; Südkamp, N.P.; Windolf, M. Validated computational framework for efficient systematic evaluation of osteoporotic fracture fixation in the proximal humerus. Med. Eng. Phys. 2018, 57, 29–39. [Google Scholar] [CrossRef]
- Elmedin, M.; Vahid, A.; Nedim, P.; Nedžad, R. Finite Element Analysis and Experimental Testing of Stiffness of the Sarafix External Fixator. Procedia Eng. 2015, 100, 1598–1607. [Google Scholar] [CrossRef] [Green Version]
- Oken, O.F.; Yildirim, A.O.; Asilturk, M. Finite element analysis of the stability of AO/OTA 43-C1 type distal tibial fractures treated with distal tibia medial anatomic plate versus anterolateral anatomic plate. Acta Orthop. Traumatol. Turc. 2017, 51, 404–408. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Lu, Y. Three-dimensional finite element analysis of mini-external fixation and Kirschner wire internal fixation in Bennett fracture treatment. Orthop. Traumatol. Surg. Res. 2013, 99, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternick, M.B.; Dallacosta, D. Relationship between rigidity of external fixator and number of pins: Computer analysis using finite elements. Rev. Bras. Ortop. 2012, 47, 646–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toumanidou, T.; Spyrou, L.A.; Aravas, N. A Finite Element Model of the Ilizarov Fixator System. In Proceedings of the 10th International Workshop on Biomedical Engineering, Kos, Greece, 5–7 October 2011; pp. 11–14. [Google Scholar]
- Roland, M.; Tjardes, T.; Otchwemah, R.; Bouillon, B.; Diebels, S. An optimization algorithm for individualized biomechanical analysis and simulation of tibia fractures. J. Biomech. 2015, 48, 1119–1124. [Google Scholar] [CrossRef]
- Dahmen, T.; Roland, M.; Tjardes, T.; Bouillon, B.; Slusallek, P.; Diebels, S. An automated workflow for the biomechanical simulation of a tibia with implant using computed tomography and the finite element method. Comput. Math. Appl. 2015, 70, 903–916. [Google Scholar] [CrossRef]
- Anderson, D.D.; Thomas, T.P.; Campos Marin, A.; Elkins, J.M.; Lack, W.D.; Lacroix, D. Computational techniques for the assessment of fracture repair. Injury 2014, 45, S23–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Benito, M.J.; García-Aznar, J.M.; Kuiper, J.H.; Doblaré, M. Influence of fracture gap size on the pattern of long bone healing: A computational study. J. Theor. Biol. 2005, 235, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chang, S.H.; Jung, H.J. The finite element analysis of a fractured tibia applied by composite bone plates considering contact conditions and time-varying properties of curing tissues. Compos. Struct. 2010, 92, 2109–2118. [Google Scholar] [CrossRef]
- Zamani, A.R.; Oyadiji, S.O. Theoretical and Finite Element Modeling of Fine Kirschner Wires in Ilizarov External Fixator. J. Med. Devices 2010, 4, 031001. [Google Scholar] [CrossRef] [Green Version]
- Kellam, J. Fracture Classification. In Techniques and Principles for the Operating Room; Porteous, M., Susanne, B., Eds.; Principles of Trauma Care; AO Publishing: Davos, Switzerland, 2010; Chapter 2; pp. 114–121. [Google Scholar]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef] [PubMed]
- ASTM Standard F1541. Specification and Test Methods for External Skeletal Fixation Devices; ASTM International: West Conshohocken, PA, USA, 2003; Available online: https://www.astm.org/Standards/F1541.htm (accessed on 11 September 2021).
- Martins, A.A.; FÃtima, P.M.; Manuel, R.L.; Augusta, N.M. The Effect of External Fixator Configurations on the Dynamic Compression Load: An Experimental and Numerical Study. Appl. Sci. 2020, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- AvÅŸar, E.; Ãœn, K. Automatic 3D modeling and simulation of bone-fixator system in a novel graphical user interface. Inform. Med. Unlocked 2016, 2, 78–91. [Google Scholar] [CrossRef] [Green Version]
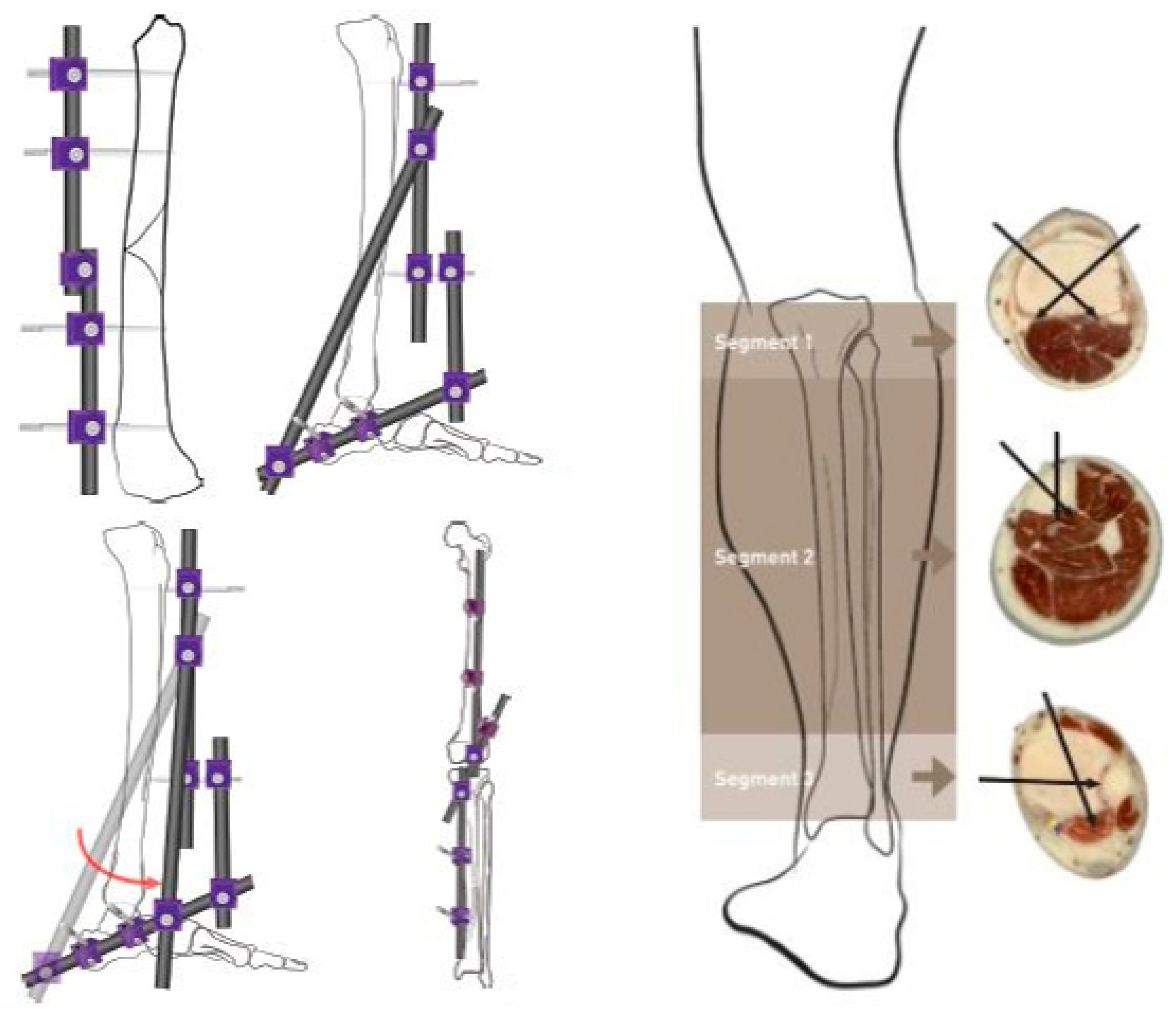



















| Configuration | Bone Segment 1 | Bone Segment 2 | Representation |
|---|---|---|---|
| Configuration 1 | Near-Mid (1 and 2) | Near-Mid (1 and 2) | 2-1–1-2 |
| Configuration 2 | Near-Far (1 and 3) | Near-Far (1 and 3) | 3-1–1-3 |
| Configuration 3 | Mid-Far (2 and 3) | Mid-Far (2 and 3) | 3-2–2-3 |
| Configuration 4 | Near-Mid (1 and 2) | Near-Far (1 and 3) | 2-1–1-3 |
| Configuration 5 | Near-Mid (1 and 2) | Mid-Far (2 and 3) | 2-1–2-3 |
| Configuration 6 | Near-Far (1 and 3) | Mid-Far (2 and 3) | 3-1–2-3 |
| Component | Material Type | Material Properties Used |
|---|---|---|
| Clamps | AISI 1215 Steel | E = 210 GPa , G = 80 GPa , = 0.3 |
| Shaft | AISI 1215 Steel | E = 210 GPa , G = 80 GPa , = 0.33 |
| Pins | AISI 316L Stainless Steel | E = 200 GPa , G = 79.05 GPa , = 0.265 |
| Bone analogous | Homopolymer Acetal (Delrin ©) | E = 3.1 GPa , G = 2.9 GPa , = 0.32 |
| Pin + Clamp simplification (for simplified model) | Defined bi-linear isotropic hardening metal | E1 = 96.88 GPa , Yield Strength = 134 MPa, E2 = 52 GPa , = 0.3 |
| Spring Constant | Segment | Deformation Type Considered | Function of | Calculation |
|---|---|---|---|---|
| KN1, KN2, KN3, KN4 | Bone analogous | Compression | Material type (compression modulus-B), Cross sectional area-A, Length of segment – l | K = (B*A)/l |
| KP1, KP2, KP3, KP4 | Pin + Clamp | Bending | Pin clamp assembly behavior is modeled to a function of load based on the experimental results | K = F(f) |
| KS1, KS2, KS3 | Shaft | Bending and compression | Material type (Young’s modulus-E), Second moment of area across the cross-section-I, Length of segment – l, conversion coefficient-t |
| Method | Computational Cost and Computation Time | Development Complexity | Accuracy | Simplicity in Being Extended to Other Fixator Forms |
|---|---|---|---|---|
| Pin bending model | Low | High | Low | Complex |
| Spring model | Low | Medium | Medium | Simple |
| Simplified FEA Model | Medium | Medium | High | Complex |
| FEA Model | High | Low | High | Medium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sellahewa, T.; Weerasinghe, C.; Silva, P. Biomechanical Evaluation Method to Optimize External Fixator Configuration in Long Bone Fractures—Conceptual Model and Experimental Validation Using Pilot Study. Appl. Sci. 2021, 11, 8481. https://doi.org/10.3390/app11188481
Sellahewa T, Weerasinghe C, Silva P. Biomechanical Evaluation Method to Optimize External Fixator Configuration in Long Bone Fractures—Conceptual Model and Experimental Validation Using Pilot Study. Applied Sciences. 2021; 11(18):8481. https://doi.org/10.3390/app11188481
Chicago/Turabian StyleSellahewa, Thiran, Charitha Weerasinghe, and Pujitha Silva. 2021. "Biomechanical Evaluation Method to Optimize External Fixator Configuration in Long Bone Fractures—Conceptual Model and Experimental Validation Using Pilot Study" Applied Sciences 11, no. 18: 8481. https://doi.org/10.3390/app11188481






