Microbial Biosynthesis of Lactones: Gaps and Opportunities towards Sustainable Production
Abstract
:1. Introduction
2. Properties, Diversity and Applications/Chemistry and Applications
3. Pathways and Enzymes Involved in the Biosynthesis of Lactones
3.1. Sporidiobolus salmonicolor
| Compound | PubChem CID | Molecular Formula | Odor Descriptor | Microorganisms | Reference |
|---|---|---|---|---|---|
| γ-lactones | |||||
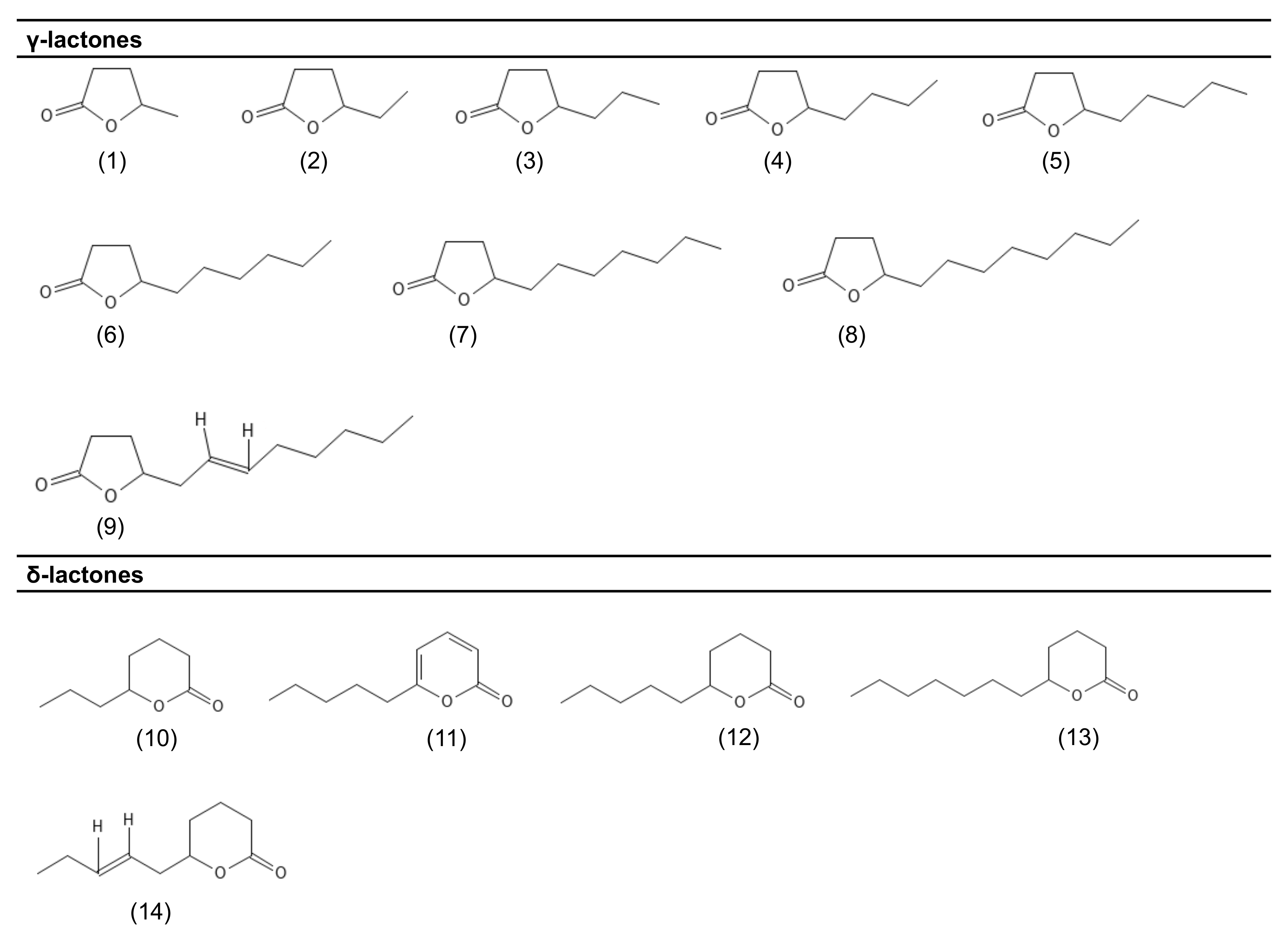
| γ-valerolactone (1) | 7921 | C5H8O2 | Peach | F. poae A. gossypii | [34] [23] |
| γ-caprolactone (2) | 12756 | C6H10O2 | Creamy | F. poae A. gossypii | [34] [23] |
| γ-heptalactone (3) | 7742 | C7H12O2 | Creamy; vanilla; coconut | F. poae | [34] |
| γ-octalactone (4) | 7704 | C8H14O2 | Coconut | F. poae S. salmonicolor A. gossypii | [34] [40] [23] |
| γ-nonalactone (5) | 7710 | C9H16O2 | Coconut | F. poae S. salmonicolor A. gossypii T. viride | [34] [40] [23] [12] |
| γ-decalactone (6) | 12813 | C10H18O2 | Fruity, peach | S. salmonicolor F. poae A. gossypii | [38] [34] [23] |
| γ-undecalactone (7) | 7714 | C11H20O2 | Fruity, peach | F. poae A. gossypii T. viride | [34] [23] [12] |
| γ-dodecalactone (8) | 16821 | C12H22O2 | Peach | F. poae A. gosssypii S. salmonicolor T. viride | [34] [23] [38] [12] |
| cis-6-γ-dodecenolactone (9) | 5352428 | C12H20O2 | Peach | S. salmonicolor F. poae | [38] [34] |
| δ-lactones | |||||
| δ-octalactone (10) | 12777 | C8H14O2 | Coconut | T. viride | [12] |
| 6-pentyl-α-pyrone (11) | 33960 | C10H14O2 | Coconut | T. viride | [35] |
| δ-decalactone (12) | 12810 | C10H18O2 | Creamy | F. poae S. salmonicolor | [34] [39] |
| δ-dodecalactone (13) | 12844 | C12H22O2 | Sweet/fruity | T. viride | [12] |
| δ-jasmin lactone (14) | 5352626 | C10H16O2 | Fruity; sweet; floral | S. salmonicolor | [39] |
3.2. Fruits
4. Microbial Production
4.1. Biotransformation
4.1.1. Yarrowia lipolytica
4.1.2. Other Fungi
4.2. De Novo Biosynthesis
5. Towards Sustainable Production of Lactones: Final Remarks and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gawdzik, B.; Kamizela, A.; Szyszkowska, A. Lactones with a Fragrance Properties. Chemik 2015, 69, 342–349. [Google Scholar]
- Kourist, R.; Hilterhaus, L. Microbial Lactone Synthesis Based on Renewable Resources. In Microorganisms in Biorefineries; Microbiology Monographs; Kamm, B., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2015; Volume 26, pp. 275–301. ISBN 978–3-662–45208–0. [Google Scholar]
- Perestrelo, R.; Fernandes, A.; Albuquerque, F.F.; Marques, J.C.; Câmara, J.S. Analytical Characterization of the Aroma of Tinta Negra Mole Red Wine: Identification of the Main Odorants Compounds. Anal. Chim. Acta 2006, 563, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Tahara, S.; Fujiwara, K.; Ishizaka, H.; Mizutani, J.; Obata, Y. γ-Decalactone—One of Constituents of Volatiles in Cultured Broth of Sporobolomyces Odorus. Agric. Biol. Chem. 1972, 36, 2585–2587. [Google Scholar] [CrossRef] [Green Version]
- Labuda, I. Flavor Compounds. In Encyclopedia of Microbiology, 3rd ed.; Applied Microbiology: Industrial; Academic Press: Cambridge, MA, USA, 2009; pp. 305–320. ISBN 978–0-12–373944–5. [Google Scholar]
- Gatfield, I.L. Biotechnological production of flavour-active lactones. In Biotechnology of Aroma Compounds; Advances in Biochemical Engineering/Biotechnology; Berger, R.G., Babel, W., Blanch, H.W., Cooney, C.L., Enfors, S.-O., Eriksson, K.-E.L., Fiechter, A., Klibanov, A.M., Mattiasson, B., Primrose, S.B., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; Volume 55, pp. 221–238. ISBN 978–3-540–61482–1. [Google Scholar]
- Coelho, E.; Vilanova, M.; Genisheva, Z.; Oliveira, J.M.; Teixeira, J.A.; Domingues, L. Systematic Approach for the Development of Fruit Wines from Industrially Processed Fruit Concentrates, Including Optimization of Fermentation Parameters, Chemical Characterization and Sensory Evaluation. LWT Food Sci. Technol. 2015, 62, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.S. Chapter 3—Olfactory Sensations. In Wine Tasting; Elsevier: Amsterdam, The Netherlands, 2017; pp. 41–101. ISBN 978–0-12–801813–2. [Google Scholar]
- Schrader, J.; Etschmann, M.M.W.; Sell, D.; Hilmer, J.-M.; Rabenhorst, J. Applied Biocatalysis for the Synthesis of Natural Flavour Compounds—Current Industrial Processes and Future Prospects. Biotechnol. Lett. 2004, 26, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Birk, F.; Fraatz, M.A.; Esch, P.; Heiles, S.; Pelzer, R.; Zorn, H. Industrial Riboflavin Fermentation Broths Represent a Diverse Source of Natural Saturated and Unsaturated Lactones. J. Agric. Food Chem. 2019, 67, 13460–13469. [Google Scholar] [CrossRef]
- Romero-Guido, C.; Belo, I.; Ta, T.M.N.; Cao-Hoang, L.; Alchihab, M.; Gomes, N.; Thonart, P.; Teixeira, J.A.; Destain, J.; Waché, Y. Biochemistry of Lactone Formation in Yeast and Fungi and Its Utilisation for the Production of Flavour and Fragrance Compounds. Appl. Microbiol. Biotechnol. 2011, 89, 535–547. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; Mahmoud, M.G.; Asker, M.M.S.; Lotfy, S.N. Characterization and Evaluation of Coconut Aroma Produced by Trichoderma Viride EMCC-107 in Solid State Fermentation on Sugarcane Bagasse. Electron. J. Biotechnol. 2015, 18, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.L.; Christenson, J.K.; Wackett, L.P. Biosynthesis and Chemical Diversity of β-Lactone Natural Products. Nat. Prod. Rep. 2019, 36, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Guerreiro, C.; Belo, I. Generation of Flavors and Fragrances Through Biotransformation and De Novo Synthesis. Food Bioprocess. Technol. 2018, 11, 2217–2228. [Google Scholar] [CrossRef] [Green Version]
- Krzyczkowska, J.; Phan-Thi, H.; Waché, Y. Lactone Formation in Yeast and Fungi. In Fungal Metabolites; Merillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–39. ISBN 978–3-319–19456–1. [Google Scholar]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Gamma-Valerolactone, a Sustainable Platform Molecule Derived from Lignocellulosic Biomass. Green Chem. 2013, 15, 584. [Google Scholar] [CrossRef]
- Braga, A.; Belo, I. Biotechnological Production of γ-Decalactone, a Peach like Aroma, by Yarrowia Lipolytica. World J. Microbiol. Biotechnol. 2016, 32, 169. [Google Scholar] [CrossRef] [Green Version]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin Poisoning: A Comprehensive Review. JAMA J. Am. Med. Assoc. 2005, 294, 2342. [Google Scholar] [CrossRef]
- Patel, V.R.; Dumancas, G.G.; Viswanath, L.C.K.; Maples, R.; Subong, B.J.J. Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 2016, 9, LPI.S40233. [Google Scholar] [CrossRef] [Green Version]
- Marella, E.R.; Dahlin, J.; Dam, M.I.; ter Horst, J.; Christensen, H.B.; Sudarsan, S.; Wang, G.; Holkenbrink, C.; Borodina, I. A Single-Host Fermentation Process for the Production of Flavor Lactones from Non-Hydroxylated Fatty Acids. Metab. Eng. 2020, 61, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.; Venegas-Calerón, M.; Salas, J.J.; Monforte, A.; Badenes, M.L.; Granell, A. An Integrative “Omics” Approach Identifies New Candidate Genes to Impact Aroma Volatiles in Peach Fruit. BMC Genom. 2013, 14, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Sevilla, J.F.; Cruz-Rus, E.; Valpuesta, V.; Botella, M.A.; Amaya, I. Deciphering Gamma-Decalactone Biosynthesis in Strawberry Fruit Using a Combination of Genetic Mapping, RNA-Seq and EQTL Analyses. BMC Genom. 2014, 15, 218. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.; Aguiar, T.Q.; Coelho, E.; Jiménez, A.; Revuelta, J.L.; Domingues, L. Metabolic Engineering of Ashbya Gossypii for Deciphering the de Novo Biosynthesis of γ-Lactones. Microb. Cell Fact. 2019, 18, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waché, Y.; Aguedo, M.; Nicaud, J.-M.; Belin, J.-M. Catabolism of Hydroxyacids and Biotechnological Production of Lactones by Yarrowia Lipolytica. Appl. Microbiol. Biotechnol. 2003, 61, 393–404. [Google Scholar] [CrossRef]
- Dufossé, L.; Feron, G.; Mauvais, G.; Bonnarme, P.; Durand, A.; Spinnler, H.-E. Production of γ-Decalactone and 4-Hydroxy-Decanoic Acid in the Genus Sporidiobolus. J. Ferment. Bioeng. 1998, 86, 169–173. [Google Scholar] [CrossRef]
- Schulz, S.; Hötling, S. The Use of the Lactone Motif in Chemical Communication. Nat. Prod. Rep. 2015, 32, 1042–1066. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.S.; Jones, R.L. Polyurethanes and Silicone Polyurethane Copolymers. In Comprehensive Biomaterials II.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 570–619. ISBN 978–0-08–100692–4. [Google Scholar]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Singh, G.S. Carbohydrate-based antibiotics: Opportunities and challenges. In Carbohydrates in Drug Discovery and Development; Elsevier: Amsterdam, The Netherlands, 2020; pp. 523–559. ISBN 978–0-12–816675–8. [Google Scholar]
- Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. A Rapid Esterification by Means of Mixed Anhydride and Its Application to Large-Ring Lactonization. Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993. [Google Scholar] [CrossRef] [Green Version]
- Shiina, I. An Effective Method for the Synthesis of Carboxylic Esters and Lactones Using Substituted Benzoic Anhydrides with Lewis Acid Catalysts. Tetrahedron 2004, 60, 1587–1599. [Google Scholar] [CrossRef]
- Corey, E.J.; Nicolaou, K.C. Efficient and Mild Lactonization Method for the Synthesis of Macrolides. J. Am. Chem. Soc. 1974, 96, 5614–5616. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Mifsud, M.; Renz, M.; Susarte, M. A New Environmentally Benign Catalytic Process for the Asymmetric Synthesis of Lactones: Synthesis of the Flavouringδ-Decalactone Molecule. Adv. Synth. Catal. 2004, 346, 257–262. [Google Scholar] [CrossRef]
- Sarris, J.; Latrasse, A. Production of Odoriferous γ Lactones by Fusarium Poae. Agric. Biol. Chem. 1985, 49, 3227–3230. [Google Scholar] [CrossRef]
- Collins, R.P.; Halim, A.F. Characterization of the Major Aroma Constituent of the Fungus Trichoderma Viride. J. Agric. Food Chem. 1972, 20, 437–438. [Google Scholar] [CrossRef]
- Van Der Schaft, P.H.; Ter Burg, N.; Bosch, S.V.D.; Cohen, A.M.; Schaft, P. Microbial Production of Natural δ-Decalactone and δ-Dodecalactone from the Corresponding α, β- Unsaturated Lactones in Massoi Bark Oil. Appl. Microbiol. Biotechnol. 1992, 36, 712–716. [Google Scholar] [CrossRef]
- Berger, R.G.; Neuhäuser, K.; Drawert, F. Biosynthesis of Flavor Compounds by Microorganisms Odorous Constituents of Polyporus durus (Basidiomycetes). Z. Nat. C 1986, 41, 963–970. [Google Scholar] [CrossRef]
- Tahara, S.; Fujiwara, K.; Mizutani, J. Neutral Constituents of Volatiles in Cultured Broth of Sporobolomyces odurus. Agric. Biol. Chem. 1973, 37, 2855–2861. [Google Scholar] [CrossRef]
- Tahara, S.; Mizutani, J. δ-Lactones Produced by Sporobolomyces odorus. Agric. Biological Chem. 1975, 39, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Dufosse, L.; Ron, G.F.; Latrasse, A.; Guichard, E.; Spinnler, H.-E. Chirality of the γ-Lactones Produced by Sporidiobolus salmonicolor Grown in Two Different Media. Chirality 1997, 9, 667–671. [Google Scholar] [CrossRef]
- Hagedorn, S. Microbial Biocatalysis in the Generation of Flavor and Fragrance Chemicals. Annu. Rev. Microbiol. 1994, 48, 773–800. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, W.; Schwarz, M.; Heidlas, J.; Tressl, R. Studies on the Biosynthesis of Aliphatic Lactones in Sporobolomyces odorus. Conversion of (S)- and (R,S)-13-Hydroxy-(Z,E)-9,11-Octadecadienoic Acid into Optically Pure (R)-.Delta.-Decalactone. J. Org. Chem. 1992, 57, 1954–1956. [Google Scholar] [CrossRef]
- Haffner, T.; Nordsieck, A.; Tressl, R. Biosynthesis of ?-Jasmin Lactone ( = (Z)-Dec-7-Eno-5-Lactone) and (Z,Z)-Dodeca-6,9-Dieno-4-Lactone in the Yeast Sporobolomyces odorus. Helv. Chim. Acta 1996, 79, 2088–2099. [Google Scholar] [CrossRef]
- Haffner, T.; Tressl, R. Biosynthesis of (R)-γ-Decanolactone in the Yeast Sporobolomyces Odorus. J. Agric. Food Chem. 1996, 44, 1218–1223. [Google Scholar] [CrossRef]
- Tressl, R.; Haffner, T.; Lange, H.; Nordsieck, A. Formation of γ- δ-lactones by different biochemical pathways. In Flavour Science; Elsevier: Amsterdam, The Netherlands, 1996; pp. 141–150. ISBN 978–1-85573–779–2. [Google Scholar]
- Haffner, T.; Tressl, R. Stereospecific Metabolism of Isomeric Epoxyoctadecanoic Acids in the Lactone-Producing Yeast Sporidiobolus salmonicolor. Lipids 1998, 33, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Feron, G.; Dufosse, L.; Pierard, E.; Bonnarme, P.; Quere, J.L.; Spinnler, H. Production, Identification, and Toxicity of (Gamma)-Decalactone and 4-Hydroxydecanoic Acid from Sporidiobolus spp. Appl. Environ. Microbiol. 1996, 62, 2826–2831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöttler, M.; Boland, W. Biosynthesis of Dodecano-4-Lactone in Ripening Fruits: Crucial Role of an Epoxide-Hydrolase in Enantioselective Generation of Aroma Components of the Nectarine (Prunus Persica Var. Nucipersica) and the Strawberry (Fragaria ananassa). HCA 1996, 79, 1488–1496. [Google Scholar] [CrossRef]
- Van de Loo, F.J.; Broun, P.; Turner, S.; Somerville, C. An Oleate 12-Hydroxylase from Ricinus Communis, L. Is a Fatty Acyl Desaturase Homolog. Proc. Natl. Acad. Sci. USA 1995, 92, 6743–6747. [Google Scholar] [CrossRef] [Green Version]
- Okui, S.E.; Uchtvama, M.; Mizugaki, M. Metabolism of Hydroxy Fatty Acids: II. Intermediates of the Oxidative Breakdown of Ricinoleic Acid by Genus Candida. J. Biochem. 1963, 54, 5. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Biotechnological Production of Flavours and Fragrances. Appl. Microbiol. Biotechnol. 1998, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Nicaud, J.-M. Yeast: A New Oil Producer? OCL 2012, 19, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Pagot, Y.; Wache, Y. Peroxisomal Beta-Oxidation Activities and Gamma-Decalactone Production by the Yeast Yarrowia Lipolytica. Appl. Microbiol. Biotechnol. 1998, 49, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Le Dall, M.-T.; Waché, Y.; Laroche, C.; Belin, J.-M.; Gaillardin, C.; Nicaud, J.-M. Evaluation of Acyl Coenzyme A Oxidase (Aox) Isozyme Function in the n -Alkane-Assimilating Yeast Yarrowia Lipolytica. J. Bacteriol. 1999, 181, 5140–5148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wache, Y.; Laroche, C.; Bermark, K.; Møller-Andersen, C.; Aguedo, M.; Le Dall, M.-T.; Wang, H.; Nicaud, J.M.; Belin, J.-M. Involvement of Acyl Coenzyme a Oxidase Isozymes in Biotransformation of Methyl Ricinoleate into Gamma-Decalactone by Yarrowia Lipolytica. Appl. Environ. Microbiol. 2000, 66, 1233–1236. [Google Scholar] [CrossRef] [Green Version]
- Wache, Y.; Aguedo, M.; Choquet, A.; Gatfield, I.L.; Nicaud, J.-M.; Belin, J.-M. Role of β-Oxidation Enzymes in γ-Decalactone Production by the Yeast Yarrowia Lipolytica. Appl. Environ. Microbiol. 2001, 67, 5700–5704. [Google Scholar] [CrossRef] [Green Version]
- Waché, Y.; Aguedo, M.; Le Dall, M.-T.; Nicaud, J.-M.; Belin, J.-M. Optimization of Yarrowia Lipolytica’s β-Oxidation Pathway for γ-Decalactone Production. J. Mol. Catal. B Enzym. 2002, 19–20, 347–351. [Google Scholar] [CrossRef]
- Guo, Y.; Song, H.; Wang, Z.; Ding, Y. Expression of POX2 Gene and Disruption of POX3 Genes in the Industrial Yarrowia Lipolytica on the γ-Decalactone Production. Microbiolog. Res. 2012, 167, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Gomes, N.; Belo, I. Lipase Induction in Yarrowia Lipolytica for Castor Oil Hydrolysis and Its Effect on γ-Decalactone Production. J. Am. Oil Chem. Soc. 2012, 89, 1041–1047. [Google Scholar] [CrossRef] [Green Version]
- Gomes, N.; Braga, A.; Teixeira, J.A.; Belo, I. Impact of Lipase-Mediated Hydrolysis of Castor Oil on γ-Decalactone Production by Yarrowia Lipolytica. J. Am. Oil Chem. Soc. 2013, 90, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Aguedo, M.; Beney, L.; Wache, Y.; Belin, J.-M.; Gervais, P. Interaction of Odorous Lactones with Phospholipids: Implications in Toxicity towards Producing Yeast Cells. Biotechnol. Lett. 2002, 24, 1975–1979. [Google Scholar] [CrossRef]
- Aguedo, M.; Beney, L.; Waché, Y.; Belin, J.-M. Interaction of an Odorant Lactone with Model Phospholipid Bilayers and Its Strong Fluidizing Action in Yeast Membrane. Int. J. Food Microbiol. 2003, 80, 211–215. [Google Scholar] [CrossRef]
- Aguedo, M.; Beney, L.; Wache, Y.; Belin, J.-M. Mechanisms Underlying the Toxicity of Lactone Aroma Compounds towards the Producing Yeast Cells. J. Appl. Microbiol. 2003, 94, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, N.; Teixeira, J.A.; Belo, I. Fed-Batch versus Batch Cultures of Yarrowia Lipolytica for γ-Decalactone Production from Methyl Ricinoleate. Biotechnol. Lett. 2012, 34, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga, A.; Belo, I. Production of γ-Decalactone by Yarrowia lipolytica: Insights into Experimental Conditions and Operating Mode Optimization: Production of γ-Decalactone by Yarrowia Lipolytica. J. Chem. Technol. Biotechnol. 2015, 90, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Aguedo, M.; Gomes, N.; Garcia, E.E.; Waché, Y.; Mota, M.; Teixeira, J.A.; Belo, I. Decalactone Production by Yarrowia Lipolytica under Increased O2 Transfer Rates. Biotechnol. Lett. 2005, 27, 1617–1621. [Google Scholar] [CrossRef] [Green Version]
- Gomes, N.; Aguedo, M.; Teixeira, J.; Belo, I. Oxygen Mass Transfer in a Biphasic Medium: Influence on the Biotransformation of Methyl Ricinoleate into γ-Decalactone by the Yeast Yarrowia lipolytica. Biochem. Eng. J. 2007, 35, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Carreon, L.; Flores, C.; Galindo, E. γ-Decalactone Production by Trichoderma Harzianum in Stirred Bioreactors. Biotechnol. Prog. 1997, 13, 205–208. [Google Scholar] [CrossRef]
- Lee, S.-L.; Lin, S.-J.; Chou, C.-C. Growth of and Production of γ-Decalactone by Sporobolomyces Odorus in Jar Fermentors as Affected by PH, Aeration and Fed-Batch Technique. J. Ferment. Bioeng. 1995, 80, 195–199. [Google Scholar] [CrossRef]
- Feron, G.; Dufossé, L.; Mauvais, G.; Bonnarme, P.; Spinnler, H.-E. Fatty Acid Accumulation in the Yeast Sporidiobolus salmonicolor during Batch Production of γ-Decalactone. FEMS Microbiol. Lett. 1997, 149, 17–24. [Google Scholar] [CrossRef]
- Iacazio, G. Isolation and Characterisation of 8-Hydroxy-3Z,5Z-Tetradecadienoic Acid, a Putative Intermediate in Pichia Guilliermondii γ-Decalactone Biosynthesis from Ricinoleic Acid. FEMS Microbiol. Lett. 2002, 209, 55–60. [Google Scholar] [CrossRef]
- Ercoli, B.; Fuganti, C.; Grasselli, P.; Servi, S.; Allegrone, G.; Barbeni, M.; Pisciotta, A. Stereochemistry of the Biogeneration of C-10 and C-12 Gamma Lactones in Yarrowia lipolytica and Pichia ohmeri. Biotechnol. Lett. 1992, 14, 665–668. [Google Scholar] [CrossRef]
- Rong, S.; Yang, S.; Li, Q.; Cai, B.; Guan, S.; Wang, J.; Zhou, Y.; Chen, Y. Improvement of γ-Decalactone Production by Stimulating the Import of Ricinoleic Acid and Suppressing the Degradation of γ-Decalactone in Saccharomyces Cerevisiae. Biocatal. Biotransform. 2017, 35, 96–102. [Google Scholar] [CrossRef]
- Alchihab, M.; Destain, J.; Aguedo, M.; Majad, L.; Ghalfi, H.; Wathelet, J.-P.; Thonart, P. Production of γ-Decalactone by a Psychrophilic and a Mesophilic Strain of the Yeast Rhodotorula aurantiaca. Appl. Biochem. Biotechnol. 2009, 158, 41–50. [Google Scholar] [CrossRef]
- Alchihab, M.; Destain, J.; Aguedo, M.; Wathelet, J.-P.; Thonart, P. The Utilization of Gum Tragacanth to Improve the Growth of Rhodotorula aurantiaca and the Production of γ-Decalactone in Large Scale. Appl. Biochem. Biotechnol. 2010, 162, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Holic, R.; Yazawa, H.; Kumagai, H.; Uemura, H. Engineered High Content of Ricinoleic Acid in Fission Yeast Schizosaccharomyces pombe. Appl. Microbiol. Biotechnol. 2012, 95, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.-C.; Seo, E.-S.; Kim, Y.-S.; Kim, K.-R.; Park, J.-B.; Oh, D.-K. Production of 10-Hydroxystearic Acid from Oleic Acid by Whole Cells of Recombinant Escherichia Coli Containing Oleate Hydratase from Stenotrophomonas maltophilia. J. Biotechnol. 2012, 158, 17–23. [Google Scholar] [CrossRef] [PubMed]
- An, J.-U.; Joo, Y.-C.; Oh, D.-K. New Biotransformation Process for Production of the Fragrant Compound γ-Dodecalactone from 10-Hydroxystearate by Permeabilized Waltomyces Lipofer Cells. Appl. Environ. Microbiol. 2013, 79, 2636–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.-U.; Oh, D.-K. Increased Production of γ-Lactones from Hydroxy Fatty Acids by Whole Waltomyces Lipofer Cells Induced with Oleic Acid. Appl. Microbiol. Biotechnol. 2013, 97, 8265–8272. [Google Scholar] [CrossRef]
- Joo, Y.-C.; Jeong, K.-W.; Yeom, S.-J.; Kim, Y.-S.; Kim, Y.; Oh, D.-K. Biochemical Characterization and FAD-Binding Analysis of Oleate Hydratase from Macrococcus caseolyticus. Biochimie 2012, 94, 907–915. [Google Scholar] [CrossRef]
- Soares, G.P.A.; Souza, K.S.T.; Vilela, L.F.; Schwan, R.F.; Dias, D.R. γ-Decalactone Production by Yarrowia Lipolytica and Lindnera Saturnus in Crude Glycerol. Prep. Biochem. Biotechnol. 2017, 47, 633–637. [Google Scholar] [CrossRef]
- Guichard, E.; Mosandl, A.; Hollnagel, A.; Latrasse, A.; Henry, R. Chiral γ-Lactones from Fusarium Poae. Z Lebensm Unters Forch 1991, 193, 26–31. [Google Scholar] [CrossRef]
- Latrasse, A.; Guichard, E.; Piffaut, C.; Fournier, N.; Dufosse, L. Chirality of the γ-Lactones Formed by Fusarium Poae INRA. Chirality 1993, 5, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Chalier, P.; Crouzet, J. Enantiodifferentiation of Four γ-Lactones Produced by Penicillium roqueforti. Chirality 1998, 10, 786–790. [Google Scholar] [CrossRef]
- Ravasio, D.; Wendland, J.; Walther, A. Major Contribution of the Ehrlich Pathway for 2-Phenylethanol/Rose Flavor Production in Ashbya Gossypii. FEMS Yeast Res. 2014, 14, 833–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledesma-Amaro, R.; Santos, M.-A.; Jiménez, A.; Revuelta, J.L. Tuning Single-Cell Oil Production in Ashbya Gossypii by Engineering the Elongation and Desaturation Systems: Engineering of Single-Cell Oil in Ashbya Gossypii. Biotechnol. Bioeng. 2014, 111, 1782–1791. [Google Scholar] [CrossRef]
- Aguiar, T.Q.; Silva, R.; Domingues, L. Ashbya gossypii beyond Industrial Riboflavin Production: A Historical Perspective and Emerging Biotechnological Applications. Biotechnol. Adv. 2015, 33, 1774–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, T.Q.; Silva, R.; Domingues, L. New Biotechnological Applications for Ashbya Gossypii: Challenges and Perspectives. Bioengineered 2017, 8, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Fernández, D.; Aguiar, T.Q.; Martín, V.I.; Romaní, A.; Silva, R.; Domingues, L.; Revuelta, J.L.; Jiménez, A. Microbial Lipids from Industrial Wastes Using Xylose-Utilizing Ashbya gossypii Strains. Bioresour. Technol. 2019, 293, 122054. [Google Scholar] [CrossRef] [PubMed]
- Christenson, J.K.; Richman, J.E.; Jensen, M.R.; Neufeld, J.Y.; Wilmot, C.M.; Wackett, L.P. β-Lactone Synthetase Found in the Olefin Biosynthesis Pathway. Biochemistry 2017, 56, 348–351. [Google Scholar] [CrossRef]
- Hiblot, J.; Gotthard, G.; Elias, M.; Chabriere, E. Differential Active Site Loop Conformations Mediate Promiscuous Activities in the Lactonase SsoPox. PLoS ONE 2013, 8, e75272. [Google Scholar] [CrossRef] [Green Version]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The Emergence of Adaptive Laboratory Evolution as an Efficient Tool for Biological Discovery and Industrial Biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, S.K.; Becker, J.; Peyriga, L.; Portais, J.-C.; Sauer, D.; Müller, R.; Hoff, B.; Haefner, S.; Schröder, H.; Zelder, O.; et al. Improved Riboflavin Production with Ashbya Gossypii from Vegetable Oil Based on 13C Metabolic Network Analysis with Combined Labeling Analysis by GC/MS, LC/MS, 1D, and 2D NMR. Metab. Eng. 2018, 47, 357–373. [Google Scholar] [CrossRef]

| Cultured Microorganisms | Lactones Produced | Substrate | Production Levels | Culture Time | Reference |
|---|---|---|---|---|---|
| A. gossypii | |||||
| Non-engineered strains | γ-decalactone γ-nonalactone γ-dodecalactone γ-undecalactone γ-octalactone γ-valerolactone γ-caprolactone | Glucose | Up to 18 mg/L Up to 0.2 mg/L Up to 82 μg/L Up to 40 μg/L Up to 24 μg/L Up to 12 μg/L Up to 3.5 μg/L | 2 days | [23] |
| Engineered strains | γ-decalactone γ-octalactone | Up to 31 mg/L Up to 0.5 mg/L | 3 days | ||
| F. poae | cis-6-γ-dodecenolactone γ-decalactone γ-nonalactone γ-octalactone γ-dodecalactone γ-valerolactone | Glucose | Up to 23 mg/L Up to 1.0 mg/L Up to 0.6 mg/L Up to 0.6 mg/L Up to 0.1 mg/L Up to 0.1 mg/L | 8 days | [82] |
| cis-6-γ-dodecenolactone | Malt extract | 2.0 mg/L | - | [34] | |
| γ-dodecalactone γ-decalactone δ-decalactone γ-caprolactone γ-octalactone γ-heptalactone γ-nonalactone γ-undecalactone γ-valerolactone | Malt extract | - | - | [34] | |
| S. salmonicolor | cis-6-γ-dodecenolactone | Glucose | Up to 11 mg/L | 12 days | [40] |
| Fructose Sucrose | Up to 1.9 mg/L 1.5 mg/L | 8 days | [38] [38] | ||
| γ-decalactone | Mannitol; sucrose; fructose | Up to 4.5 mg/L Up to 2.7 mg/L Up to 1.9 mg/L | 8 days | [38] [38] [38] | |
| Glucose | Up to 3.0 mg/L | 6–9 days | [40] | ||
| γ-dodecalactone γ-nonalactone γ-octalactone | Glucose | Up to 0.4 mg/L Up to 0.3 mg/L Up to 25 μg/L | 6–9 days | [40] | |
| δ-jasmin lactone δ-decalactone | Fructose | - | - | [39] | |
| T. viride | 6-pentyl-α-pyrone | Potato dextrose | 170 mg/L | 3–4 days | [35] |
| 6-pentyl-α-pyrone γ-nonalactone γ-undecalactone δ-octalactone δ-dodecalactone γ-dodecalactone | Sugarcane bagasse | Up to 3.6 mg/g Up to 0.3 mg/g Up to 0.1 mg/g Up to 52 μg/g Up to 59 μg/g Up to 36 μg/g | 5 days 7 days 9 days 5 days 9 days 9 days | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.; Coelho, E.; Aguiar, T.Q.; Domingues, L. Microbial Biosynthesis of Lactones: Gaps and Opportunities towards Sustainable Production. Appl. Sci. 2021, 11, 8500. https://doi.org/10.3390/app11188500
Silva R, Coelho E, Aguiar TQ, Domingues L. Microbial Biosynthesis of Lactones: Gaps and Opportunities towards Sustainable Production. Applied Sciences. 2021; 11(18):8500. https://doi.org/10.3390/app11188500
Chicago/Turabian StyleSilva, Rui, Eduardo Coelho, Tatiana Q. Aguiar, and Lucília Domingues. 2021. "Microbial Biosynthesis of Lactones: Gaps and Opportunities towards Sustainable Production" Applied Sciences 11, no. 18: 8500. https://doi.org/10.3390/app11188500
APA StyleSilva, R., Coelho, E., Aguiar, T. Q., & Domingues, L. (2021). Microbial Biosynthesis of Lactones: Gaps and Opportunities towards Sustainable Production. Applied Sciences, 11(18), 8500. https://doi.org/10.3390/app11188500






