In the first part of the study, the pre-formulation studies aimed to establish the properties of the plant raw materials by identifying and dosing the contained active ingredients.
2.2.1. Qualitative Phytochemical Screening
The dried raw plant materials were subjected to repeated hot ethanol extraction. The obtained alcoholic extracts were then concentrated to a certain volume. The active principles of the extracts were identified by means of specific reactions. Qualitative chemical analysis was performed on the initial alcoholic extractive solutions [
47,
48], with particular emphasis on such methods as reduction with Fehling’s and Tollens’ reagents and the use of the Molisch reaction and Styassny’s reagent.
Identification of Reducing Compounds [
47]:
- (i).
The reaction with Fehling’s reagent was based on the ability of these compounds to reduce a solution of Fehling’s reagent to copper oxide in a strongly alkaline medium. A solution of 1 mL of alcoholic extract diluted with 2 mL of distilled water was treated with 1 mL of a mixture of Fehling’s solutions and heated for 30 min. The appearance of a red-brick precipitate of copper oxide indicates the presence of reducing compounds.
- (ii).
Reaction with Tollens’ reagent: 3–4 drops of Tollens’ reagent were added to 1 mL of alcoholic extract solution and heated. We then observed the deposition of the silver mirror on the test tube.
Identification of Carbohydrates
The carbohydrates were identified using the Molisch reaction. A total of 2 mL of alcoholic extract solution was concentrated to dryness. Then, 1–2 drops of concentrated sulfuric acid and 3–4 drops of 1% thymol were added to the residue. The presence of a red-orange color indicated the presence of such compounds.
Identification of Tannins [
49]
According to the chemical nature of tannins and their classification into gallic tannins and catechin tannins [
49], the following methods of identification were used:
- (i).
Identification with 1% ferric chloride: 1 mL of alcoholic extract solution was diluted with 2 mL of distilled water and 2–3 drops of ferric chloride were added until a green (for the catechin tannin) or blue (for the gallic tannin) color was observed.
- (ii).
Identification with Styassny’s reagent (a hydrochloric formaldehyde solution formed by formaldehyde and concentrated hydrochloric acid in a molar ratio of 2:1): 5 mL of alcoholic extract solution was treated with 4–5 mL of Styassny’s reagent; then, the solution was heated for at least 30 min. The appearance of a red-brown precipitate indicates the presence of the catechin tannin.
- (iii).
The final step was the separation of the catechin tannin from the gallic tannin.
- (iv).
For the blue-black color that indicates the presence of the catechin tannin, 10 mL of extract solution was treated with 10 mL of Styassny’s reagent then heated for at least 30 min. The condensed catechin tannin was separated by filtration. The solution was neutralized with Na2CO3. When the blue color appeared, 1–2 drops of Styassny’s reagent were added. The blue color was a signal of the presence of gallic tannins.
Identification of Flavonosides
Flavonosides were identified directly in the alcoholic solution if they were not mixed with anthocyanosides or were identified after hydrolysis [
49].
- (i).
The Shibata reaction (cyanidol reaction): under the reducing action of nascent hydrogen, the pyranic nucleus undergoes a reduction of the ketone group with the redistribution of electrons inside the nucleus and the formation of a pyrrole salt. A 3–5 mL solution was evaporated to a residue. The residue was dissolved in 1–2 mL of hot methanol. The solution was brought to a test tube, and fragments of metallic magnesium and 0.5 mL of concentrated hydrochloric acid were added. Within 5 min, an orange (flavones), a red (flavonols), and a red-purple (flavanones) color appeared.
- (ii).
Chelation reaction with salts of bi- and trivalent metals: 3–5 mL of solution was evaporated to dryness. The residue was dissolved in 1–3 mL of alcohol and then 1–2 mL of aluminum chloride was added. The intensification of the yellow coloration was observed.
- (iii).
Formation of phenoxides: 3–5 mL of solution was evaporated. The residue was dissolved in methanol and then 1–2 mL of sodium hydroxide was added. The intensification of the yellow coloration was observed.
Identification of Coumarins
The identification of coumarins was based on their ability to emit radiation in UV light and the formation of purple salts with hydroxycinnamic acids.
- (i).
Determination of fluorescence in UV light: coumarins in UV light have a blue-green or purple fluorescence. In alkaline aqueous solution (using 0.1 M NaOH), salts of o-hydroxycinnamic acid or its derivatives with an intensification of fluorescence were formed. A total of 3–5 mL of solution was evaporated in a sand bath. The residue was dispersed in 3–4 mL of hot water. The aqueous solution was supplied in two tubes. The contents of one tube were treated with 4–5 drops of 0.1 M NaOH (Merck). Upon examination in UV light, coumarins showed a blue-green or purple fluorescence that was more intense in an alkaline environment.
- (ii).
Transformation into hydroxycinnamic acids (the Feigl–Anger–Frehden reaction): in the presence of a hydroxyl amine, in an alkaline medium, hydroxycinnamic acids were formed and their iron (III) salts were colored purple. We then added 4–5 mL of solution, 4–5 drops of hydrochloric hydroxylamine, and 4–5 mL of NaOH (at pH = 9–10) to a porcelain capsule. The mixture was heated to a residue, cooled, and acidified with 1 M HCl solution (at pH = 3–4). Upon adding 3–4 drops of ferric chloride (III), a violet coloration was obtained.
Identification of Anthocyanosides
The identification of anthocyanosides was based on the ability of both the heteroside and the aglycone to present different colors depending on the pH of the solution. At an acidic pH, anthocyanosides were colored in red (due to the presence of oxonium ions); at a neutral pH, anthocyanosides were colored in blue (due to the carbon ion); at a low alkaline pH, anthocyanosides were colored in blue (due to the presence of the phenoxonium ion); and at a strong alkaline pH, anthocyanosides were colored in yellow (due to the formation of chalcone by breakage of the pyran).
A total of 5–6 mL of alcoholic extract solution was treated with 10% sodium hydroxide following the change in the color, which demonstrated the presence of anthocyanosides.
Identification of ODPs
Orthodihydroxyphenols (ODPs) are phenolic compounds of type C
6-C
3 and represented by caffeic acid or monocafeylquinic esters (chlorogenic acid and its isomers) and dicafeylquinic esters (cinarine). These compounds are soluble in polar solvents, and they have marked reducing properties. Their identification was based on their reducing properties, on obtaining nitrosoderivatives with the help of aromatic diazo compounds (the coupling compounds obtained are red or orange), and on the formation of red nitrosoderivatives [
24,
45]. The phenolic compounds form para-nitrosoderivatives with nitric acid. They tautomerize spontaneously, producing the respective red oximes that are soluble in alkaline solutions. A total of 1 mL of test solution was treated with 1 mL of 0.1 M hydrochloric acid, 1 mL of Arnow’s reagent (sodium nitrite), and 1 mL of 1 N sodium hydroxide and then stirred. A red coloration appeared.
2.2.2. Quantitative Phytochemical Screening
Flavones, ODPs, catechin tannins, anthocyanosides, and proanthocyanosides were quantitatively determined. The determinations were repeated three times on different samples.
Quantitative Determination of Flavonoids
Flavones were extracted in hydrophilic solvents and then treated with aluminum chloride to form internal complexes with an intense yellow colorimetric coloration.
A total of 1 g of dried and pulverized vegetable product was treated with 100 mL of 70% methyl alcohol and refluxed for 30 min. The solution (denoted extraction solution A) was cooled and filtered. A total of 10 mL of extraction solution A was diluted with methanol to 25 mL. To 5 mL of filtrate were added 5 mL of 10% sodium acetate, 3 mL of 2.5% aluminum chloride, and methanol to 25 mL. After 45 min, the absorbance of the chelate at λ = 427 nm was determined using as a compensating liquid a solution obtained under the same conditions as the sample solution (5 mL of filtered solution, 8 mL of water, and methanol to 25 mL). The flavonoid concentration in the samples was calculated using a standard curve established on 1 mL, 2 mL, 3 mL, and 4 mL standard rutoside solutions (
Table 1). The results are expressed as the rutoside (%) content of the dried sample.
Quantitative Determination of ODPs
The amount of phenolic derivative was determined based on the ability of caffeic acid to form nitrosoderivatives with nitric acid. Nitrosoderivatives are compounds that spontaneously isomerize to isonitrosoderivatives or oximes. Due to their weak acidic character, they dissolve in alkaline solutions to give red colors.
A total of 1 g of crushed vegetable product was refluxed twice with 10 mL of 50% methanol. The combined solutions were filtered and, after cooling, up to 100 mL of 50% methanol was added. We added 1 mL of 0.5 N HCl, 1 mL of Arnow’s reagent, and 1 mL of 1 N NaOH to 1 mL of solution and then stirred it. After 15 min, the absorbance of the sample at λ = 510 nm was determined. The control sample was prepared by adding to the extraction solution 1 mL of 0.5 N HCl, 1 mL of 1 N NaOH, and 50% methanol to 10 mL.
The calculation of the amount of polyphenolic derivative was performed in relation to a standard curve established on caffeic acid (
Table 1).
Quantitative Determination of Tannins
The determination of tannins was based on the ability of catechin tannins to precipitate with Styassny’s reagent (formaldehyde and concentrated hydrochloric acid in a 2:1 molar ratio). The precipitate was weighed after filtration and drying [
49,
50].
A total of 1 g of crushed vegetable product was extracted repeatedly with hot distilled water to 10 mL. A total of 5 mL of Styassny’s reagent was added. The solution was heated at 90 °C for 30 min, after which it was cooled and then filtered. The precipitate was kept for 10–15 min in the heated oven. After drying, the precipitate was weighed.
Quantitative Determination of Anthocyanins
A 0.15 g sample of dried raw plant materials was treated with a 50 mL mixture of 95% v/v. ethyl alcohol and 1.5 N hydrochloric acid (85:15) and kept at 4 °C for 24 h, after which it was filtered. The absorbances at 535 nm were measured in comparison with a 1% cyanidol solution.
The anthocyanin content was calculated according to Equation (1):
where
A is the absorbance of the solution at 535 nm and d is the dilution factor (50). The absorbance value in the given solvent was measured for a concentration of 1% cyanidol.
Quantitative Determination of Proanthocyanins
A 0.1 g sample of dried vegetable product was treated with 50 mL of 2 N HCl and heated for 40 min. After cooling and filtering, the solution was extracted twice with 20–25 mL of n-butyl alcohol. The absorbance at 550 nm was immediately determined.
The amount of proanthocyanin was calculated according to Equation (2):
where 6 is the correction coefficient of the efficiency of transformation of proanthocyanins into anthocyanins,
A is the absorbance of the sample at 550 nm,
e is the molecular absorbance of the compound under consideration (347 nm),
M is the molecular weight of the compound under consideration (306 g/mol),
V is the volume of the solution, and
P is the mass of the analyzed sample (g).
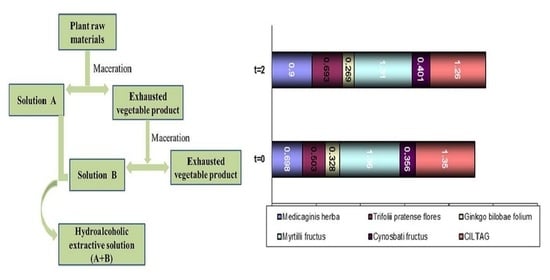
2.2.5. Obtaining the “CILTAG” Hydroalcoholic Extract
After establishing the plant extracts’ characteristics, a new pharmaceutical product, conventionally named “CILTAG”, was prepared. The compound contains a mixture in equal parts of 10% hydroalcoholic extracts of Medicaginis herba, Trifolii pratense flores, Ginkgo bilobae folium, Myrtilli fructus, and Cynosbati fructus. The pharmaceutical form of the hydroalcoholic extract was chosen due to its high stability over time. As the performance of bioactive compounds from plant materials depends on the extraction process, it is crucial to select a proper extraction method by considering different parameters with an important influence on the process: the mean particle diameter and the pre-extraction wetting of the vegetable product, the nature of the extraction solvent, the vegetable product to solvent ratio, the stirring time, the extraction time, and the temperature.
The raw plant materials were crushed and passed through a no. 4 mesh size sieve (4.75 mm). As mixed mucilage-type polyholosides were identified in the aqueous extraction solution, they were first precipitated in the plant cell by wetting the plant products with absolute alcohol.
Based on the solubility of the active compounds contained in the studied plant products, 50% ethanol (v/v) as an extraction solvent (named the menstrum) and a 1:10 vegetable product to solvent ratio were chosen. The double maceration method was selected.
The dried and crushed raw plant materials were successively treated with various amounts of the total volume of menstruum and remained in a closed container for a well-established period of time. The extraction solution (A) was separated, and the pressed plant product was treated with the next portion of solvent, resulting in another extracted solution (B). The extraction fractions (A and B) were finally reunited.
The vegetable product was placed with the menstrum in a closed vessel for seven days at 20 °C and protected from light. During this period, the samples were shaken daily 3–4 times. After 7 days, the liquid was strained then filtered and the solid residue (the marc) was pressed. The obtained hydroalcoholic extract was kept in the refrigerator. The partially depleted vegetable product was subjected to a new depletion after 4 days, following the same procedure. The final hydroalcoholic extract was combined with the previously obtained one.
The technological process is summarized in
Figure 1.
2.2.7. Determination of Antioxidant Action
- (1)
Determination of antioxidant action by chemiluminescence
The correlation between the characteristics of active principles for the studied plant products and their antioxidant activity (in the H
2O
2–luminol system) is useful for estimating the antioxidant capacity with potential use in the pharmaceutical industry [
52,
53].
For good functioning of the chemiluminescent system (a LH
2 + H
2O
2 (luminol and hydrogen peroxide) system as a chemiluminescence -generating system for determining the oxidation capacity of biological molecules), it was important to establish the optimal measurement parameters of the system [
54,
55].
The parameters that characterize the chemiluminescence are the intensity, color, and speed of the chemiluminescence emission, the decrease in light intensity, and the polarization of light. Several factors must be considered, including the signal’s reproducibility, the amplitude and possible existence of inflection points, the maximum and minimum emission points, and areas of characteristic length.
From the reproducibility point of view, this means obtaining for the same reactions, at the same component concentrations, similar signals, both in terms of kinetics and intensity. Achieving this reproducibility depends on several factors, including the nature of the buffer used, the pH of the sample, the homogenization of the sample, the duration of the reaction, and the ambient temperature.
To determine the antioxidant activity, we used a TD 20/20 TUNER DESIGNES chemiluminometer (Turner BioSystems, California, USA) coupled to a computer equipped with 1.5 mL encapsulated glass vats, a luminol chemiluminescence-generating system, and hydrogen peroxide in TRIS-HCl buffer at a pH = 8.6 with a final volume of 1 mL.
An initial unstable compound resulted from the reaction using the chemiluminescence-generating system, but it was stabilized by the formation of a bi-anion and a transformation accompanied by the emission of a quantum of light with a maximum wavelength of 430 nm.
When an antioxidant molecule is introduced into this system, it causes a decrease in the intensity of the light signal by producing the consumption of free radicals. When the molecule is pro-oxidant, there is an increase in the number of free radicals in the system.
The behavior of some molecules, as well as comparisons between the pro- and antioxidant properties, can be analyzed when the chemiluminescence increases knowing that some diseases can be produced by reactive oxygen species and free radicals.
All measurements were made on three parallel samples, obtaining a relative spread of the results of up to 2–3% compared with the average value.
The recording of the chemiluminescent signal was done at 5 s and the results obtained are expressed in relative luminescence values in comparison with the standard values. It is important to vary the concentration of the solutions by using concentrations of 2 × 10−3 g%/mL.
In the study of the peroxidation behavior of the system, we determined: (i) the antioxidant activity (AA%); (ii) the speed of inhibition/amplification of the signal according to Equations (3) and (4):
where I
0 represents the intensity of the chemiluminescent signal of the control (luminol) at t = 5 s, and I
p represents the intensity of the chemiluminescent signal in the presence of the sample at t = 5 s; and (iii) the initial speed at different times according to the relation:
where v represents the reaction speed expressed in time
−1, I
5 represents the intensity of the chemiluminescent signal after the first 5 s, and t represents the reaction time corresponding to the change in the signal intensity.
- (2)
Determination of antioxidant action by the electrochemical method [
56,
57,
58]
Voltametric studies were performed with a 273 A EG&G (potentiostat/galvanostat) electrochemical system (Princeton Applied Research/EG&G, USA) with a type KO264 three-electrode microcell with a total working volume of 3 to 15 mL. Millielectrodes (effective electrode area: 3.14E-2 cm2) of solid gold (Au) were used as working electrodes, depending on the electroactivity manifested by each compound investigated, with a Pt wire auxiliary electrode and a Ag/AgCl (3M KCl) reference electrode.
The used electrochemical techniques were cyclic voltammetry (CV), linear potential sweep voltammetry (LSV), and square wave voltammetry (SWV). The inert electrolyte was H2SO4 0.12 mol L−1 in methanol solution.
The used reagents were methyl alcohol, sulfuric acid, Trolox, and 2,2’-diphenyl-1-picrylhydrazyl (DPPH). They were of analytical purity and obtained from Sigma-Aldrich, Darmstadt, Germany.
All voltametric measurements were made at 25 °C in an inert atmosphere after deaeration of the analyzed solutions.
The Trolox stock solutions were 10−3 mol L−1 methanolic solutions freshly prepared and maintained at 4 °C and in the dark so as to avoid the formation of oxidative degradation compounds. The stock solutions of DPPH were 3 × 10−3 mol L−1 alcoholic solutions.
UV-VIS spectrometric determinations were performed using a JASCO V-530 spectrometer (JASCO INTERNATIONAL CO., LTD., Japan).
In the first step, a calibration curve was made for Trolox alone, without free radicals in the system, in the concentration range between 10−6 mol L−1 and 10−4 mol L−1 to ensure the accuracy of the method.
The square wave voltammetry (SWV) investigation technique and the Trolox peak response with a potential of +0.785 ± 0.015 V were used. The obtained calibration line (
Figure S1 in the Supplementary Materials File), with a good correlation coefficient value (0.9698), defines a linear range of the response in two intervals between 3 × 10
−6 mol L
−1 and 1 × 10
−4 mol L
−1. The subsequent experimental step was the electroanalytical characterization of the free radical DPPH, which has a peak potential of around 0.915 ± 0.015 V and whose signal response is extremely sensitive to concentration (
Figure S2 in the Supplementary Materials File).
Once the characteristic electroanalytical parameters of the compounds were established, the antioxidant capacity of Trolox was determined against the DPPH free radical. For this, the linear dependencies between the decrease in the current intensity of the DPPH peak and the increase in the Trolox concentration in the working cell were obtained and considered the calibration lines for the antioxidant activity.
A total of 5 mL of inert electrolyte and 0.12 M H
2SO
4 in CH
3OH were deaerated for 4 min by purging Argon in the system; then, 1 mL of DPPH from the 10
−3 M stock solution was introduced, again purging the Argon for 30 s. The corresponding DPPH voltammogram was registered, then volumes between 10 µL and 70 µL of the Trolox stock solution were added to the system in order to obtain the dependence between the decrease in the anodic peak current intensity of the DPPH and the Trolox concentration in the system. The corresponding voltammogram was displayed 2 min and 4 min after the addition of Trolox. The obtained lines are shown in
Figure S3 in the Supplementary Materials File.
In
Figure S3, it can be observed that for 2 min the sensitivity of the slope was satisfactory and, also, the correlation coefficient that was obtained for the linear dependence, which gives the equivalent value of the antioxidant capacity, was satisfactory. Values are the average of three determinations.
After obtaining the linear dependencies, the antioxidant capacity of the 10% hydroalcoholic extractive solutions of Medicaginis herba, Trifolii pratense flores, Ginkgo bilobae folium, Myrtilli fructus, and Cynosbati fructus and the CILTAG antioxidant preparation was determined.
- (3)
Determination of antioxidant action by the superoxide dismutase (SOD) method
SOD is an enzyme that catalyzes the dismutation of superoxide radicals to hydrogen peroxide and molecular oxygen. To determine the superoxide dismutase (SOD)-like activity, a reaction system to produce (-O
2−) and an indicator substance to measure it are required. The production system (-O
2−) is a mixture of methylthioninium chloride–methylene blue (AM) and tetramethylenediamine (TMED), and the indicator substance is nitroblue tetrazolium (NBT). In the presence of (-O
2−), NBT is reduced to formazan, a colored compound whose maximum absorbance is 560 nm. Thus, the color intensity is directly proportional to the concentration of (-O
2−) in the reaction mixture. So, SOD and substances with SOD-like activity cause the consumption of (-O
2−) in the system and thus prevent the formation of formazan [
59].
The hydroalcoholic extractive solutions of Medicaginis herba, Trifolii pratense flores, Ginkgo bilobae folium, Myrtilli fructus, and Cynosbati fructus and the antioxidant product (CILTAG) were tested for their antioxidant action. To ensure the presence of oxygen in the reaction mixture, the phosphate buffer used was previously purged with air for 20 min. Both the sample and the standard were placed for 15 min under a 17 W fluorescent lamp in an enclosed space and away from other light sources. All samples (including the control) were read at 560 nm after having previously been extracted in 2 mL of chloroform.
All used reagents were of analytical grade and obtained from Fluka® Analytical, Aesch, Switzerland.
The schemes shown in
Table 2 were used. The ingredient quantities and the used reagents are also listed in
Table 2.
A SOD unit was considered to be the amount of enzyme that reduces by 50% the amount of formazan formed in the control reaction [
49]. SOD activity is expressed in units/mL of sample and was calculated considering the dilution of the sample.
The calculation was considered to be valid if the proportion of formazan reduced in the control reaction was 50%. This amount was defined as one unit of enzymatic activity and is expressed as unit/mg protein.