Fast Fabrication of Conductive Copper Structure on Glass Material Using Laser-Induced Chemical Liquid Phase Deposition
Abstract
:1. Introduction
2. Experimental Details
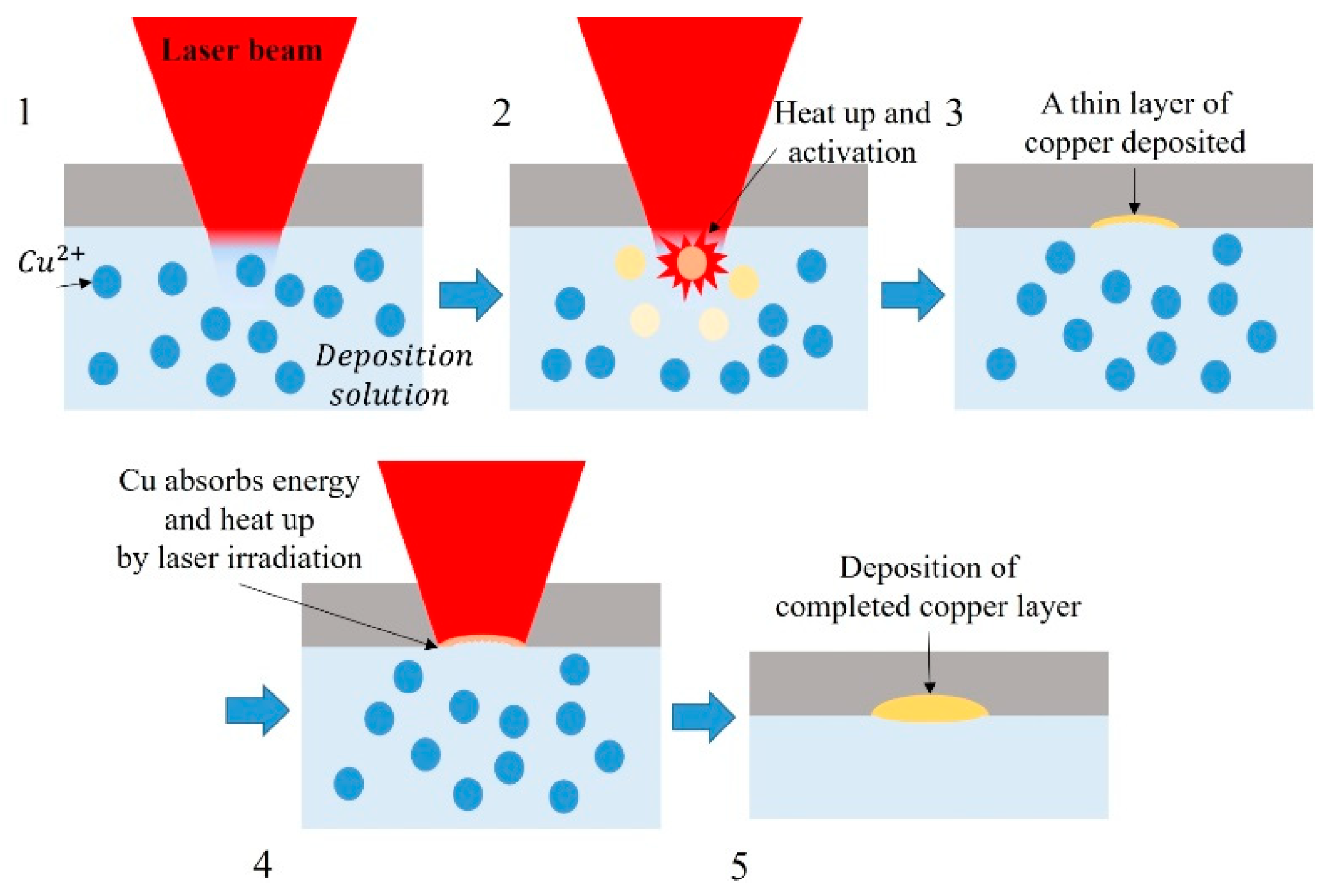
2.1. Laser-Induced Chemical Liquid Phase Deposition
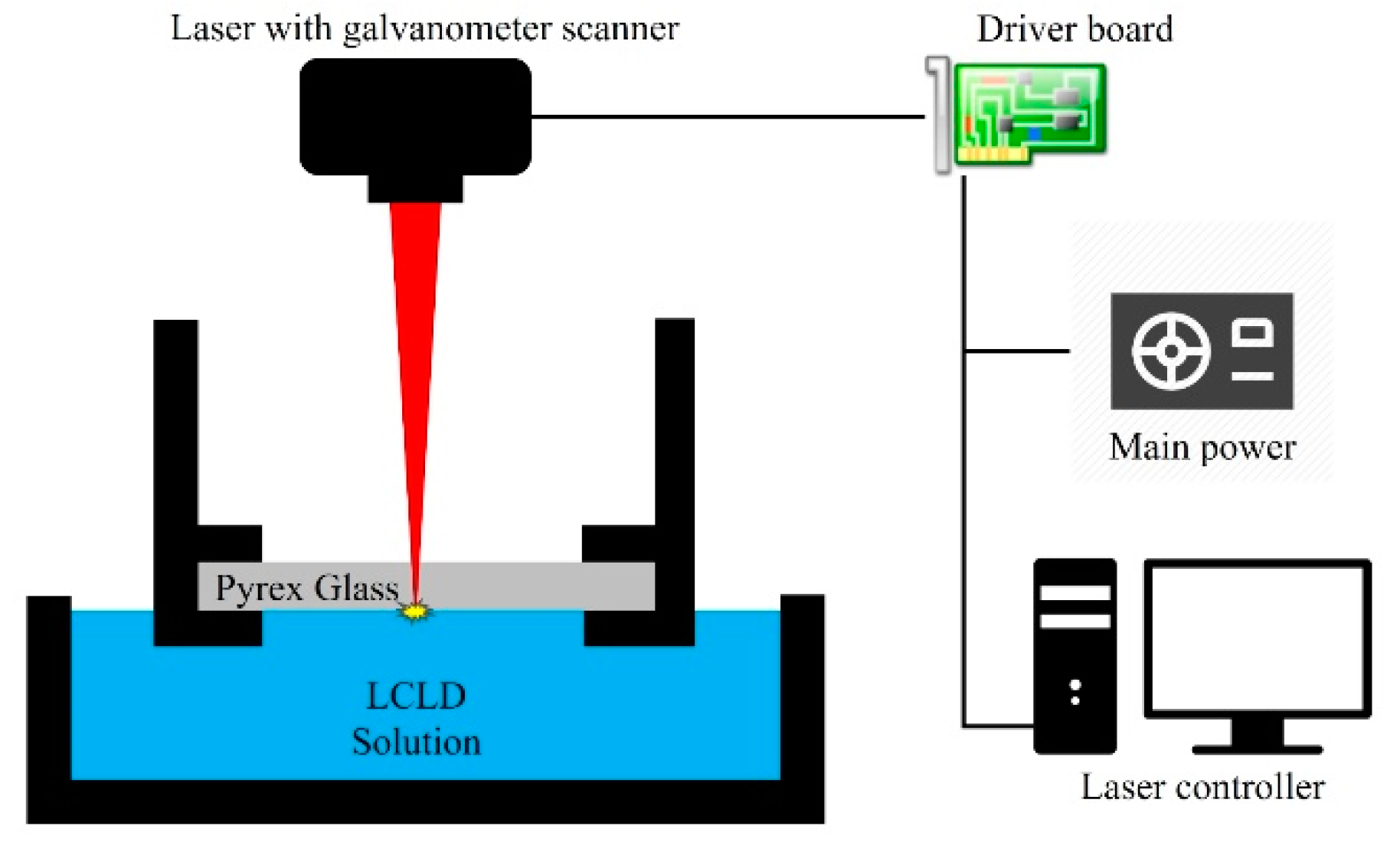
2.2. Experimental Device
3. Results and Discussion
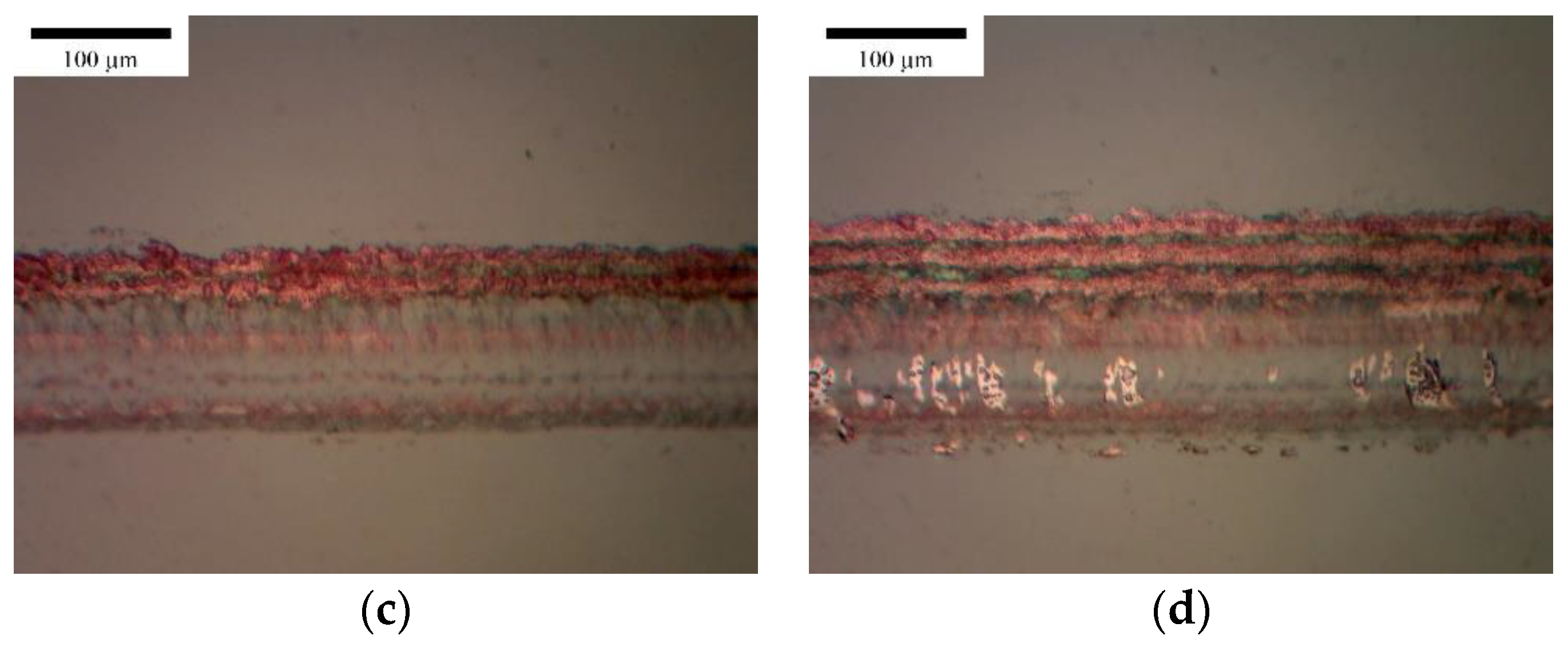
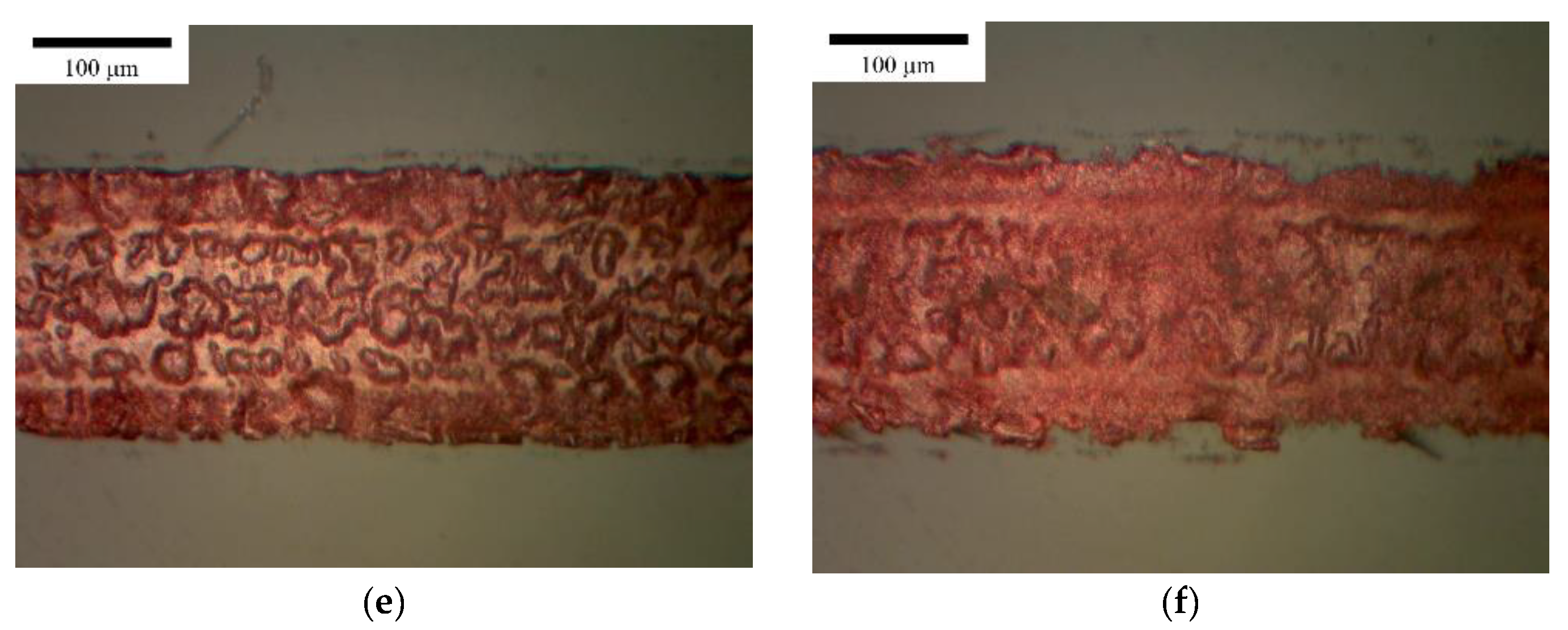
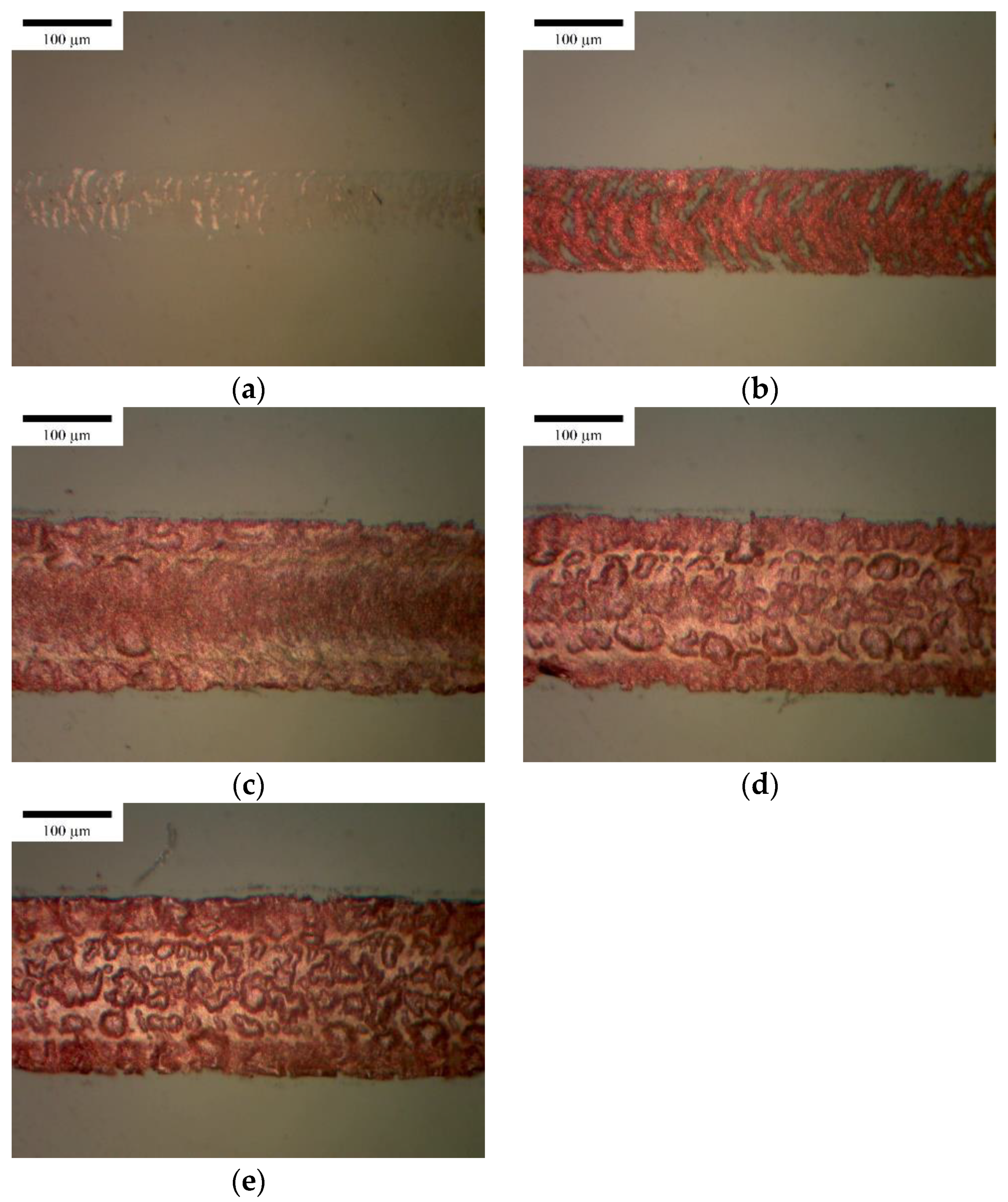
3.1. Horizontal Line
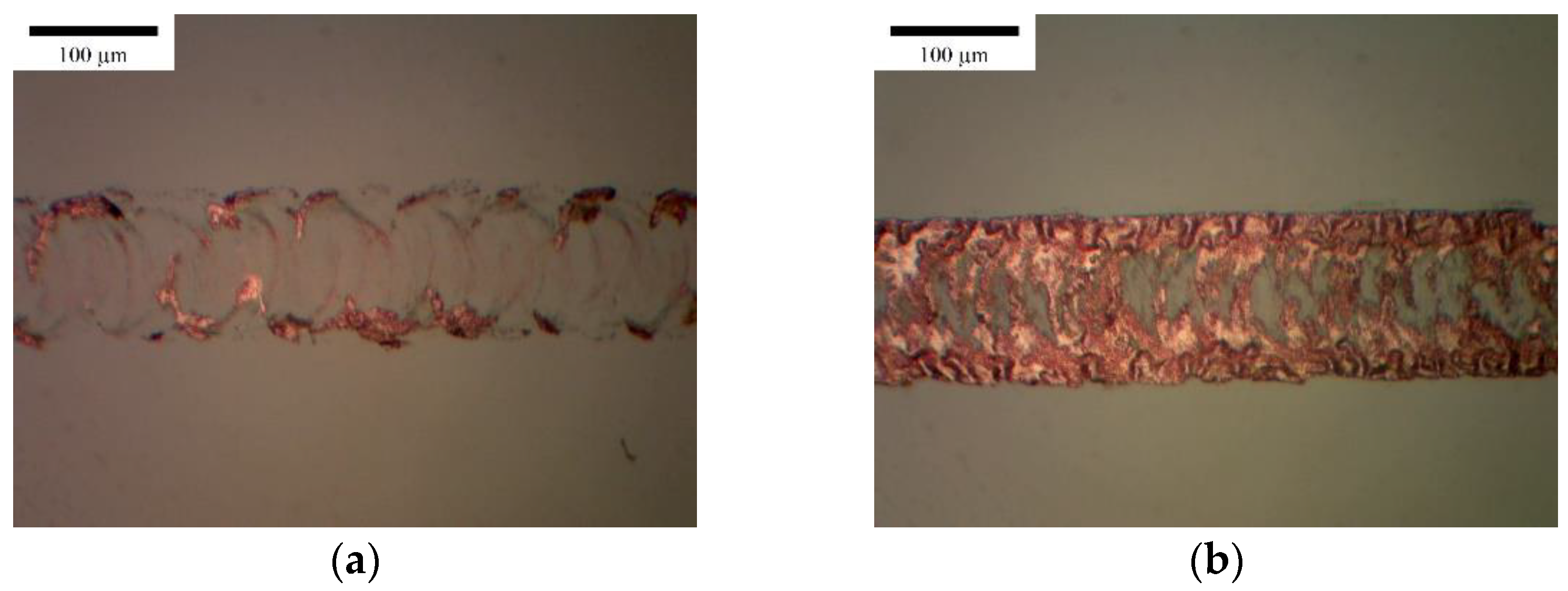
3.2. Vertical Line
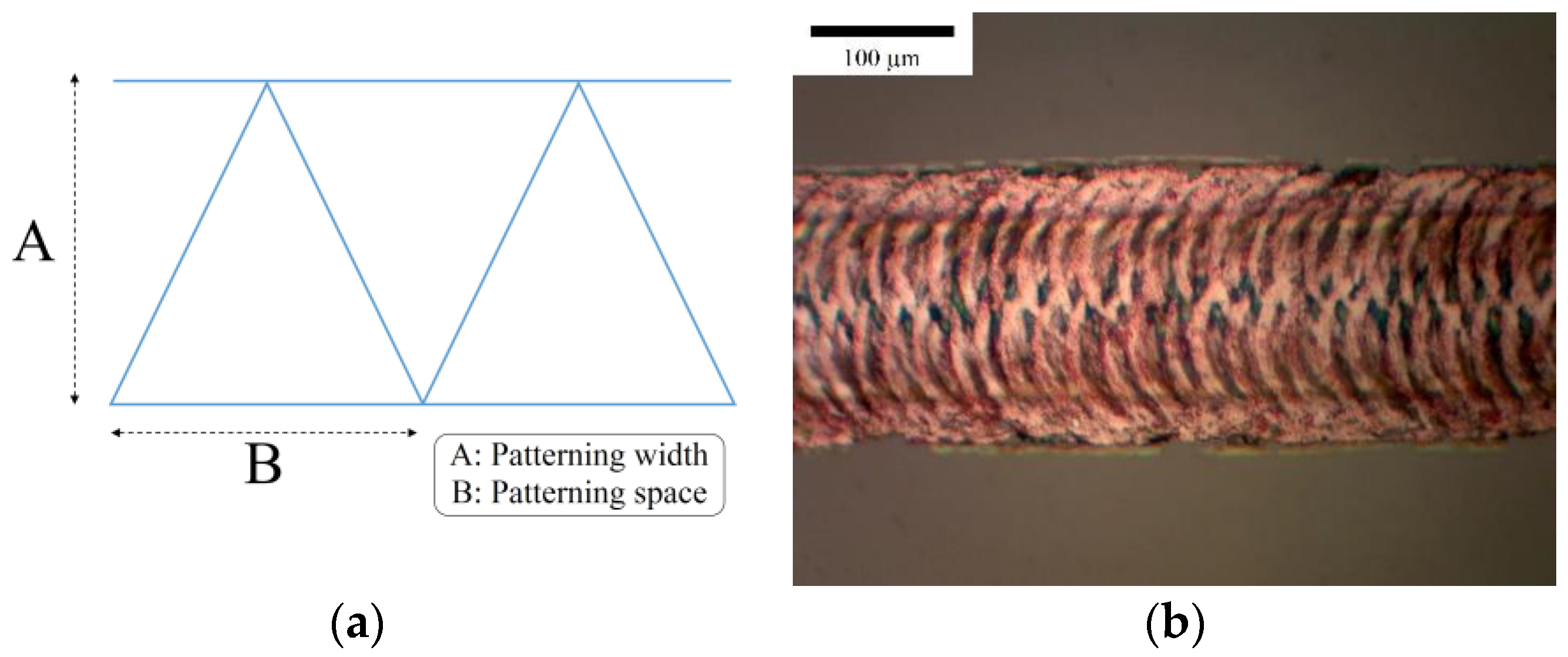
3.2.1. Effect of Patterning Space
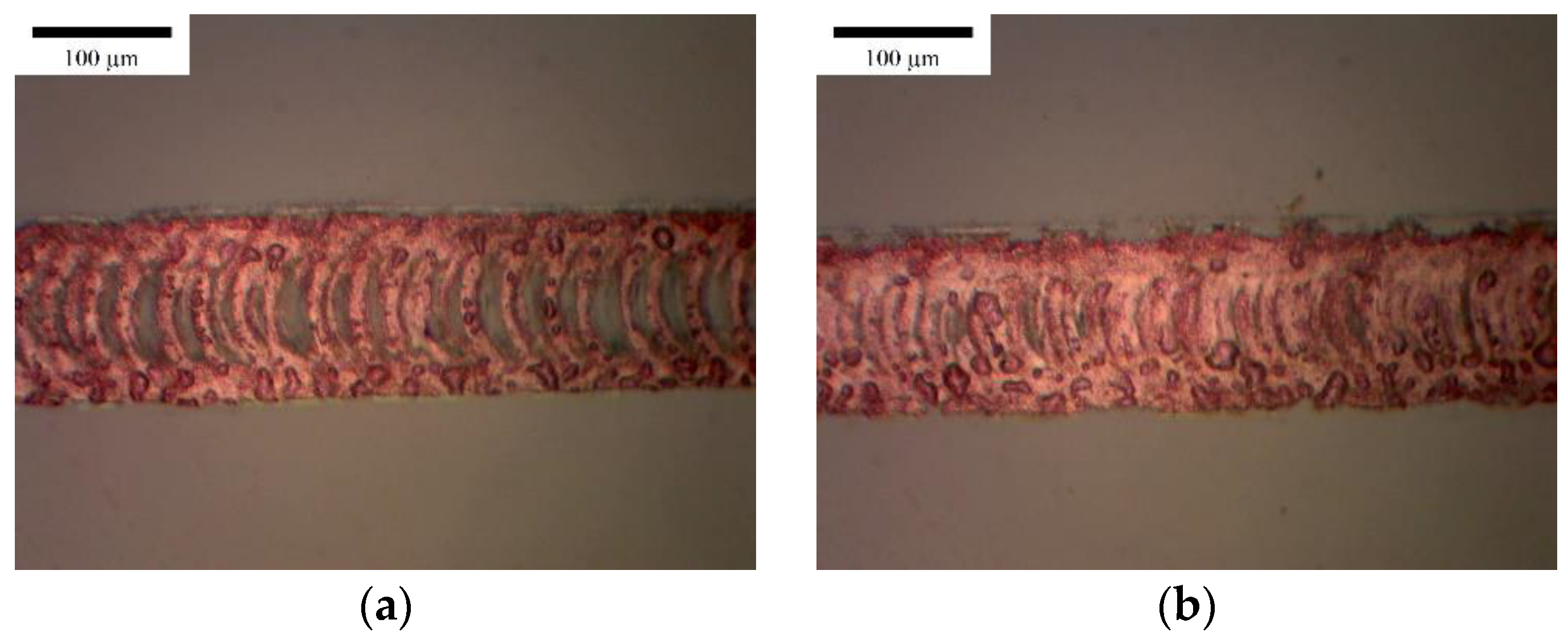
3.2.2. Effect of Frequency
3.2.3. Effect of Patterning Width
3.3. Various Scan Path
3.3.1. Deposition according to Scan Path
3.3.2. Reduction of Line Width
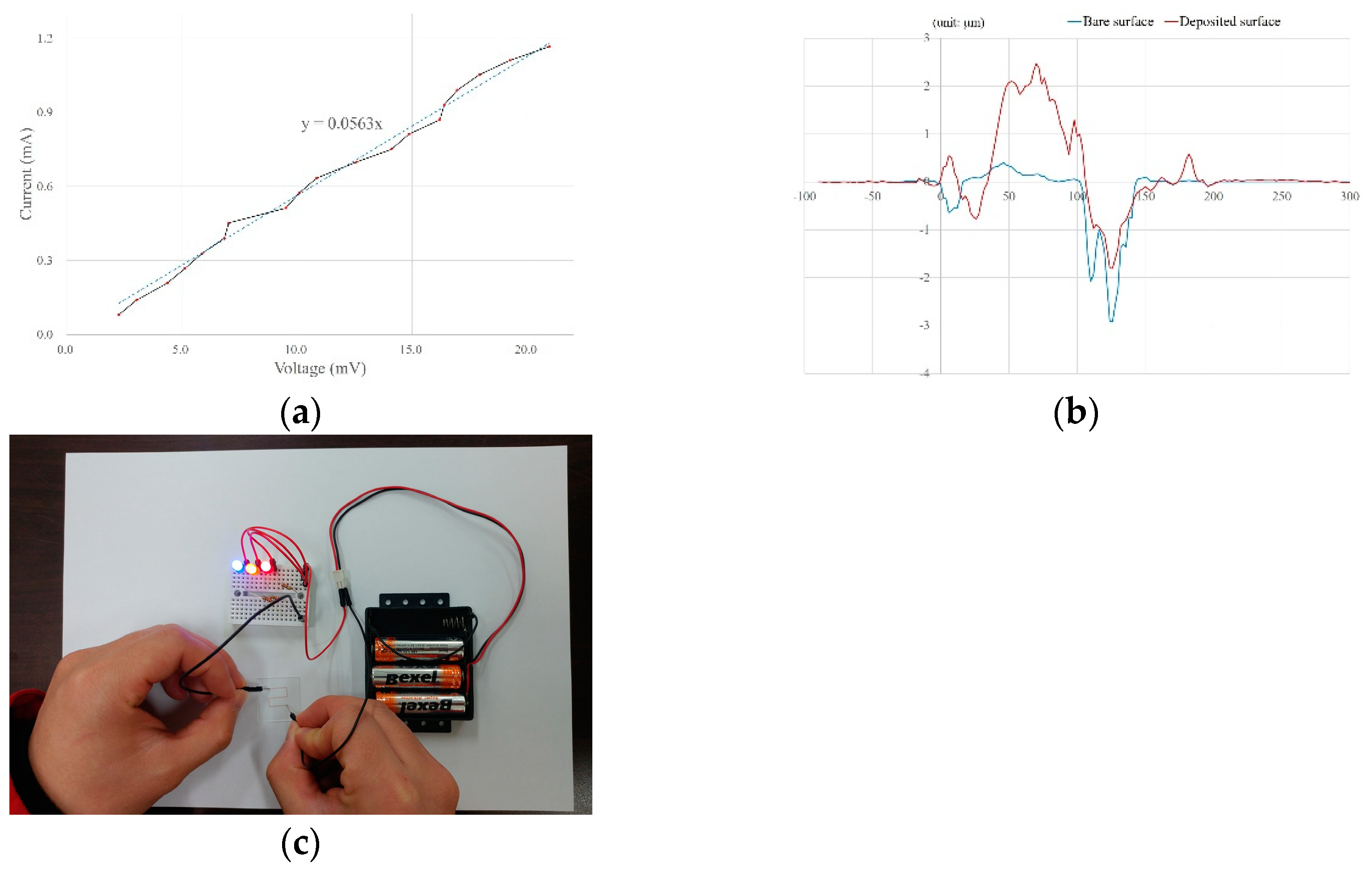
3.3.3. Specific Resistance and Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crawford, G.P. Flexible Flat Panel Displays; John Wiley & Sons, Ltd.: Chichester, UK, 2005; ISBN 9780470870501. [Google Scholar]
- Han, M.J.; Khang, D.-Y. Glass and Plastics Platforms for Foldable Electronics and Displays. Adv. Mater. 2015, 27, 4969–4974. [Google Scholar] [CrossRef]
- Nam, K.G.; Cho, S.H.; Chang, W.S.; Na, S.J.; Whang, K.H.; Kim, J.G. Micromachining Characteristics inside Transparent Materials using Femtoseocond Laser Pulses. J. Korean Soc. Precis. Eng. 2006, 23, 190–196. [Google Scholar]
- Chen, M.F.; Chen, Y.P.; Hsiao, W.T.; Gu, Z.P. Laser direct write patterning technique of indium tin oxide film. Thin Solid Films 2007, 515, 8515–8518. [Google Scholar] [CrossRef]
- Wang, M.W.; Liu, T.Y.; Pang, D.C.; Hung, J.C.; Tseng, C.C. Inkjet printing of a pH sensitive palladium catalyst patterns of ITO glass for electroless copper. Surf. Coat. Technol. 2014, 259, 340–345. [Google Scholar] [CrossRef]
- Hon, K.K.B.; Li, L.; Hutchings, I.M. Direct writing technology-Advances and developments. CIRP Ann.-Manuf. Technol. 2008, 57, 601–620. [Google Scholar] [CrossRef]
- Choi, S.; Park, M. Fabrication of Conductive Patterns on 3D Printed Structure Using Photo-Polymerization Technology. Phys. Status Solidi 2019, 216, 1801017. [Google Scholar] [CrossRef]
- Shin, I.J.; Park, M.S. Direct Conductive Patterning on 3D Printed Structure Using Laser. Phys. Status Solidi 2018, 215, 1700597. [Google Scholar] [CrossRef]
- Shin, I.; Park, M. 3D printed conductive patterns based on laser irradiation. Phys. Status Solidi 2017, 214, 1600943. [Google Scholar] [CrossRef]
- Hanada, Y.; Sugioka, K.; Midorikawa, K. Selective metallization of photostructurable glass by femtosecond laser direct writing for biochip application. Appl. Phys. A Mater. Sci. Process. 2008, 90, 603–607. [Google Scholar] [CrossRef]
- Sugioka, K.; Masuda, M.; Hongo, T.; Cheng, Y.; Shihoyama, K.; Midorikawa, K. Three-dimensional microfluidic structure embedded in photostructurable glass by femtosecond laser for lab-on-chip applications. In Proceedings of the Applied Physics A: Materials Science and Processing; Springer: Berlin/Heidelberg, Germany, 2004; Volume 79, pp. 815–817. [Google Scholar]
- Kim, H.-G.; Park, M.S. Circuit patterning using laser on transparent material. Surf. Coatings Technol. 2017, 315, 377–384. [Google Scholar] [CrossRef]
- Kochemirovsky, V.A.; Fateev, S.A.; Logunov, L.S.; Tumkin, I.I.; Safonov, S.V. Laser-induced copper deposition with weak reducing agents. Int. J. Electrochem. Sci. 2014, 9, 644–658. [Google Scholar] [CrossRef] [Green Version]
- Kochemirovsky, V.A.; Khairullina, E.M.; Safonov, S.V.; Logunov, L.S.; Tumkin, I.I.; Menchikov, L.G. The influence of non-ionic surfactants on laser-induced copper deposition. Appl. Surf. Sci. 2013, 280, 494–499. [Google Scholar] [CrossRef]
- Manshina, A.A.; Povolotskiy, A.V.; Ivanova, T.Y.; Tver’Yanovich, Y.S.; Tunik, S.P.; Kim, D.; Kim, M.; Kwon, S.C. Effect of salt precursor on laser-assisted copper deposition. Appl. Phys. A Mater. Sci. Process. 2007, 89, 755–759. [Google Scholar] [CrossRef]
- Tumkin, I.I.; Kochemirovsky, V.A.; Bal’makov, M.D.; Safonov, S.V.; Zhigley, E.S.; Logunov, L.S.; Shishkova, E.V. Laser-induced deposition of nanostructured copper microwires on surfaces of composite materials. Surf. Coat. Technol. 2015, 264, 187–192. [Google Scholar] [CrossRef]
- Kochemirovsky, V.A.; Skripkin, M.Y.; Tveryanovich, Y.S.; Mereshchenko, A.S.; Gorbunov, A.O.; Panov, M.S.; Tumkin, I.I.; Safonov, S. V Laser-induced copper deposition from aqueous and aqueous–organic solutions: State of the art and prospects of research. Russ. Chem. Rev. 2015, 84, 1059–1075. [Google Scholar] [CrossRef]
- Seo, J.M.; Kwon, K.-K.; Song, K.Y.; Chu, C.N.; Ahn, S.-H. Deposition of Durable Micro Copper Patterns into Glass by Combining Laser-Induced Backside Wet Etching and Laser-Induced Chemical Liquid Phase Deposition Methods. Materials 2020, 13, 2977. [Google Scholar] [CrossRef]
- Chong, E.K.; Stevens, M.G.; Nissen, K.E. Effect of surface roughness on the adhesion of electrolessly plated platinum to poly(ethylene terephthalate) films. J. Adhes. 2003, 79, 667–681. [Google Scholar] [CrossRef]
- Bugaev, S.P.; Sochugov, N.S. Production of large-area coatings on glasses and plastics. In Proceedings of the Surface and Coatings Technology; Elsevier: Amsterdam, The Netherlands, 2000; Volume 131, pp. 474–480. [Google Scholar]
- Kochemirovsky, V.A.; Menchikov, L.G.; Kuz’Min, A.G.; Safonov, S.V.; Tumkin, I.I.; Tver’Yanovich, Y.S. Side reactions during laser-induced deposition of copper from aqueous solutions of CuII complexes. Russ. Chem. Bull. 2012, 61, 1041–1047. [Google Scholar] [CrossRef]
- Wang, X.C.; Zheng, H.Y.; Lim, G.C. Laser induced copper electroless plating on polyimide with Q-switch Nd:YAG laser. Appl. Surf. Sci. 2002, 200, 165–171. [Google Scholar] [CrossRef]
- Kochemirovsky, V.A.; Khairullina, E.M.; Safonov, S.V.; Logunov, L.S.; Tumkin, I.I.; Menchikov, L.G. Influence of surfactants on laser-induced copper deposition from solution. Russ. Chem. Bull. 2014, 62, 1570–1578. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Hong, M.H.; Do, T.B.M.; Lin, Q.Y. Laser etching of glass substrates by 1064 nm laser irradiation. Appl. Phys. A 2008, 93, 159–163. [Google Scholar] [CrossRef]
- Xie, X.; Huang, X.; Jiang, W.; Wei, X.; Hu, W.; Ren, Q. Three dimensional material removal model of laser-induced backside wet etching of sapphire substrate with CuSO4 solutions. Opt. Laser Technol. 2017, 89, 59–68. [Google Scholar] [CrossRef]
- Zehnder, S.; Schwaller, P.; Arx, U.V.; Neuenschwander, B. Laser-Induced Chemical Liquid-Phase Deposition of Copper on Transparent Substrates. In Proceedings of the International Conference on Advanced Laser Technologies, Thun, Switzerland, 2–6 September 2012. [Google Scholar]
- Chen, Q.J.; Imen, K.; Allen, S.D. Laser Enhanced Electroless Plating of Micron-Scale Copper Wires. J. Electrochem. Soc. 2000, 147, 1418. [Google Scholar] [CrossRef]
- Li, J.; Shi, T.; Feng, C.; Liang, Q.; Yu, X.; Fan, J.; Cheng, S.; Liao, G.; Tang, Z. The novel Cu nanoaggregates formed by 5 nm Cu nanoparticles with high sintering performance at low temperature. Mater. Lett. 2018, 216, 20–23. [Google Scholar] [CrossRef]



| Reagents | Contents |
|---|---|
| CuSO4 | 15 g/L |
| EDTA | 35 g/L |
| HCHO | 10 g/L |
| NaOH | pH 12.6 |
| 2.2 dipyridyl | 10 mg/L |
| Characteristics | Typical | Unit |
|---|---|---|
| Working material | Nd:YAG | |
| Mode of operation | Pulsed/Continuous wave | |
| Wavelength | 1064 | nm |
| Maximum output power | 20 | W |
| Pulse width | 200 | ns |
| Pulse repetition rate | 1–500 | kHz |
| Beam quality, M2 | 2 | |
| Focused beam diameter | 55 | μm |
| Bulk Copper | Common Electroless Plating | Cu Nanoparticles after Sintering [28] | Previous LCLD Process | Proposed Fast LCLD Process | |
|---|---|---|---|---|---|
| Specific resistance [μΩ·cm] | 1.68 | 2.00 | 4.37 | 10~300 | 26.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-G.; Park, M.-S. Fast Fabrication of Conductive Copper Structure on Glass Material Using Laser-Induced Chemical Liquid Phase Deposition. Appl. Sci. 2021, 11, 8695. https://doi.org/10.3390/app11188695
Kim H-G, Park M-S. Fast Fabrication of Conductive Copper Structure on Glass Material Using Laser-Induced Chemical Liquid Phase Deposition. Applied Sciences. 2021; 11(18):8695. https://doi.org/10.3390/app11188695
Chicago/Turabian StyleKim, Han-Guel, and Min-Soo Park. 2021. "Fast Fabrication of Conductive Copper Structure on Glass Material Using Laser-Induced Chemical Liquid Phase Deposition" Applied Sciences 11, no. 18: 8695. https://doi.org/10.3390/app11188695
APA StyleKim, H.-G., & Park, M.-S. (2021). Fast Fabrication of Conductive Copper Structure on Glass Material Using Laser-Induced Chemical Liquid Phase Deposition. Applied Sciences, 11(18), 8695. https://doi.org/10.3390/app11188695







