Mechanical Properties and Formaldehyde Release of Particleboard Made with Lignin-Based Adhesives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Wood Particles
2.1.2. Adhesives
2.2. Particleboard Manufacturing
2.3. FTIR
2.4. Mechanical Testing of the Particleboard
2.5. Microscopic Investigation and Density Profile along the Thickness
2.6. Free Formaldehyde Emission
2.7. Statistical Methods
3. Results and Discussions
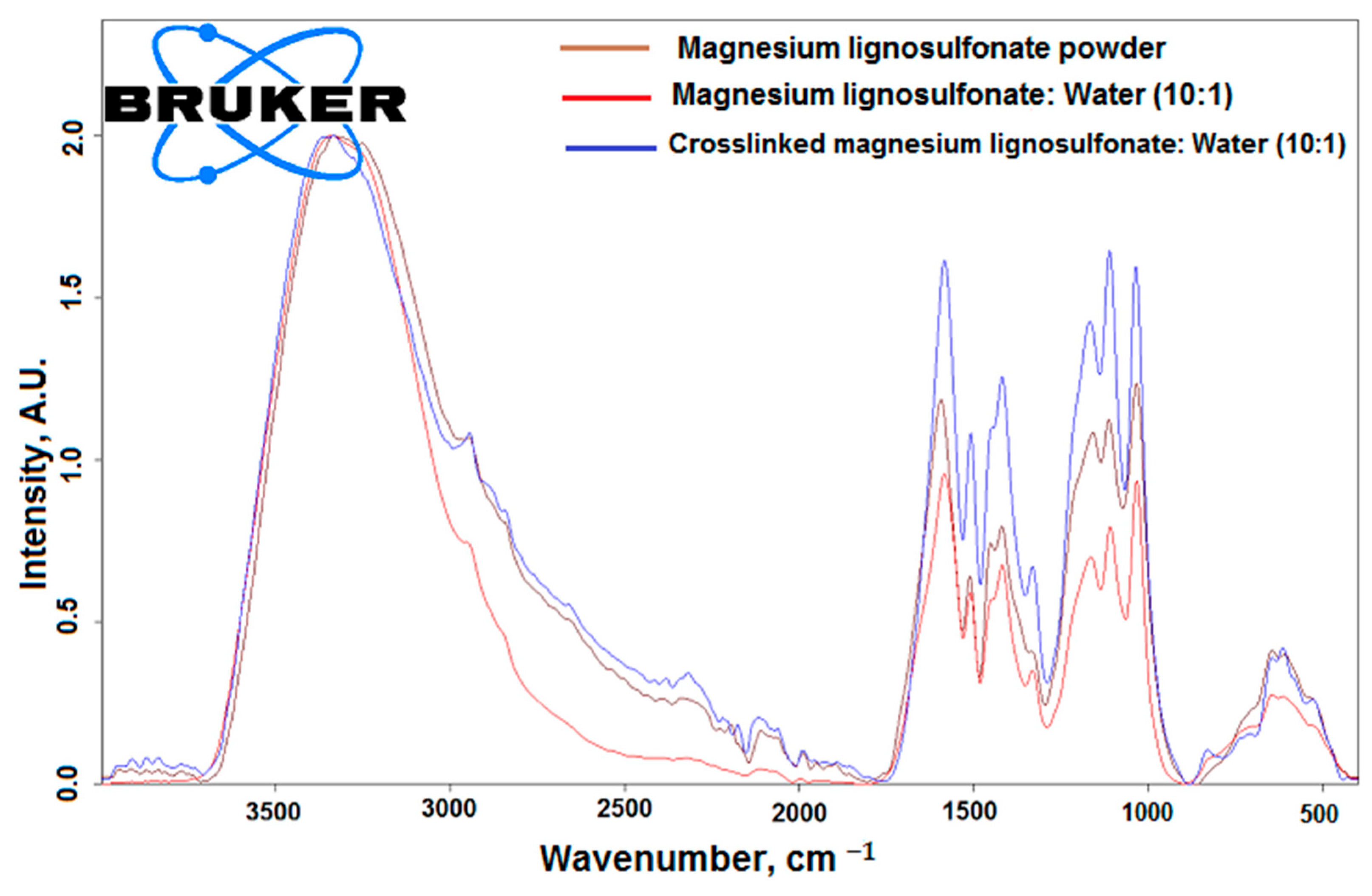
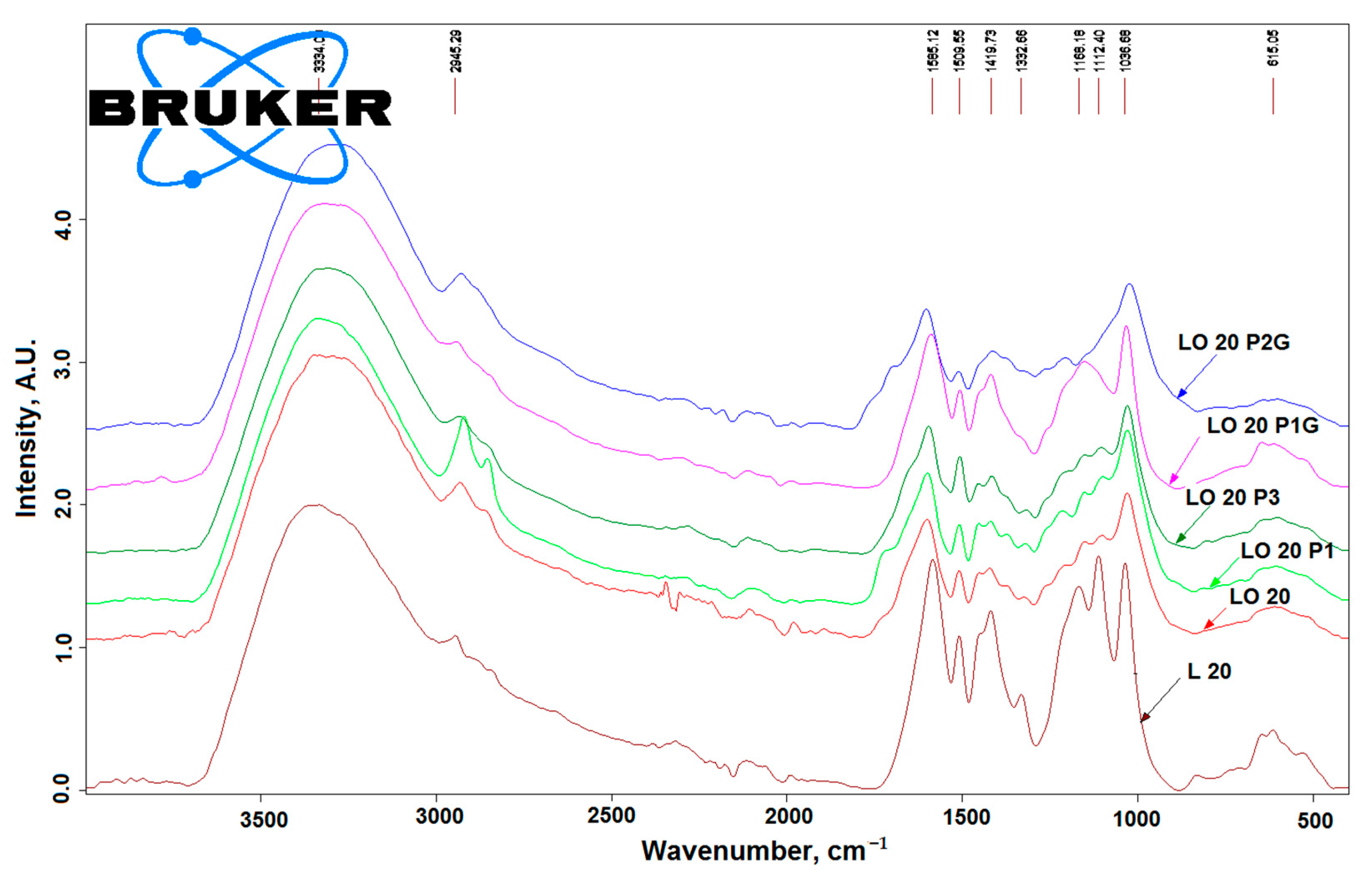
3.1. FTIR
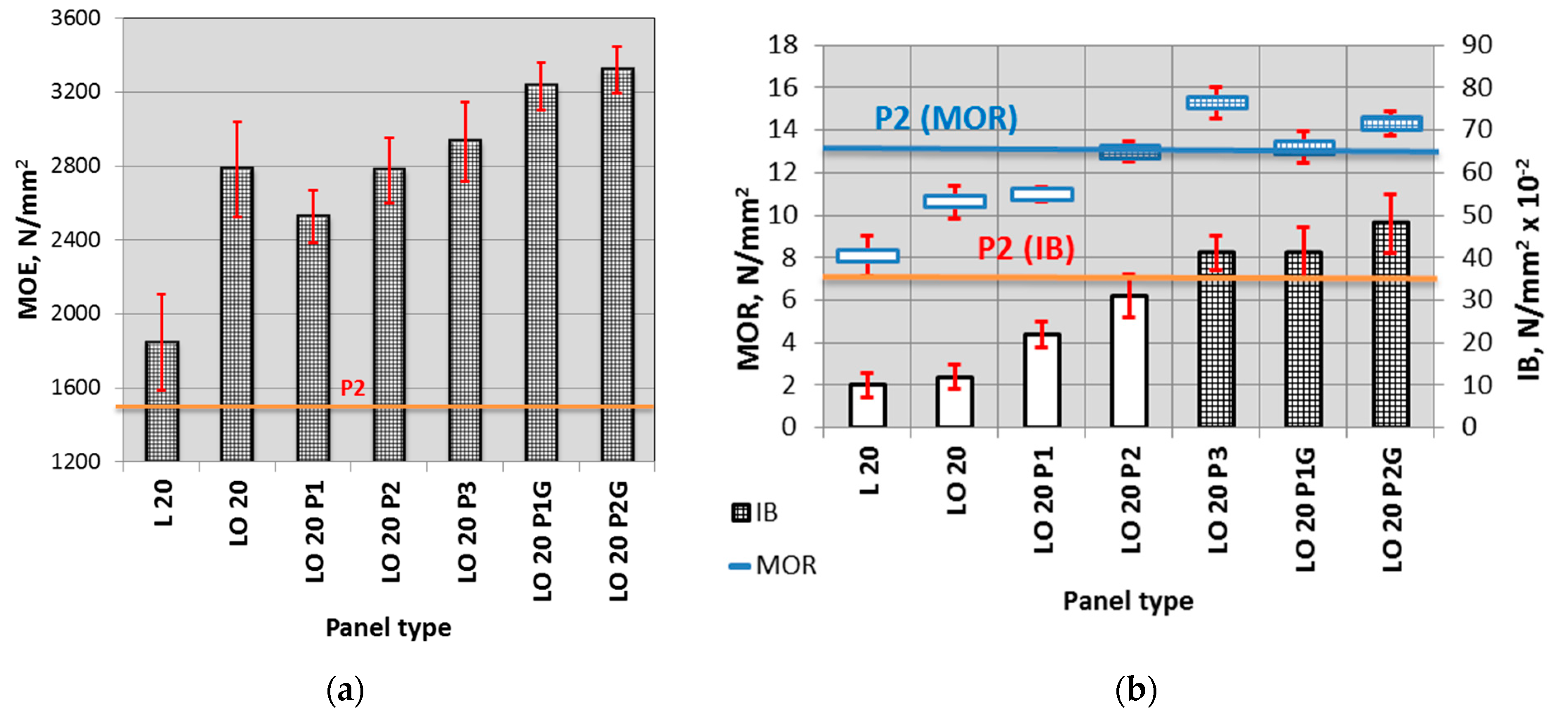
3.2. Mechanical Properties
3.3. Microscopic Investigation and Density Profile along the Thickness
3.4. Free Formaldehyde Emission
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Mantanis, G.I.; Athanassiadou, E.; Barbu, M.C.; Wijnendaele, K. Adhesive systems used in the European particleboard, MDF and OSB industries. Wood Mater. Sci. Eng. 2018, 13, 104–116. [Google Scholar] [CrossRef]
- Despres, A.; Pizzi, A.; Vu, C.; Delmotte, L. Colourless formaldehyde-free urea resin adhesives for wood panels. Eur. J. Wood Prod. 2010, 68, 13–20. [Google Scholar] [CrossRef]
- Costa, N.A.; Pereira, J.; Ferra, J.; Cruz, P.; Martins, J.; Magalhaes, F.D.; Mendes, A.; Carvalho, L.H. Scavengers for achieving zero formaldehyde emission of wood-based panels. Wood Sci. Technol. 2013, 47, 1261–1272. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang Li, J.; Zhang, S.F. Properties of Particleboard Manufactured with Modified Urea-Formaldehyde Resin. Adv. Mater. Res. 2010, 150–151, 1135–1138. [Google Scholar] [CrossRef]
- Pinkl, S.; van Herwijnen, H.W.G.; Veigel, S.; Gindl-Altmutter, W.; Riegler, M. Urea-formaldehyde microspheres as a potential additive to wood adhesive. J. Wood Sci. 2018, 64, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Agency for Toxic Substances and Disease Registry. Formaldehyde and Your Health. 2016. Available online: https://www.atsdr.cdc.gov/formaldehyde/ (accessed on 10 September 2021).
- WHO. International Agency for Research on Cancer. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2006; Volume 88, Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono88.pdf (accessed on 10 September 2021).
- Formacare. Formaldehyde and CLP. 2014. Available online: https://www.formacare.eu/about-formaldehyde/regulatory-status/clp/ (accessed on 10 September 2021).
- Formaldehyde Emission Standards for Composite Wood Products. Posted by the Environmental Protection Agency on Dec 12, 2016. Available online: https://www.regulations.gov/document/EPA-HQ-OPPT-2016-0461-0001 (accessed on 31 July 2021).
- Boon, J.G.; Hashim, R.; Danish, M.; Wan Nadhari, W.N.A. Physical and Mechanical Properties of Binderless Particleboard Made from Steam-Pretreated Oil Palm Trunk Particles. J. Compos. Sci. 2019, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Boran, S.; Usta, M.; Gümüşkaya, E. Decreasing formaldehyde emission from medium density fiberboard panels produced by adding different amine compounds to urea formaldehyde resin. Int. J. Adhes. Adhes. 2011, 31, 674–678. [Google Scholar] [CrossRef]
- Boran, S.; Usta, M.; Ondaral, S.; Gümüşkaya, E. The efficiency of tannin as a formaldehyde scavenger chemical in medium density fibreboard. Compos. Part B Eng. 2012, 43, 2487–2491. [Google Scholar] [CrossRef]
- Neimsuwan, T.; Siramon, P.; Hengniran, P.; Punsuvon, V. Effect of Tannin Addition as a Bio-Scavenger on Formaldehyde Content in Particleboard. J. Trop. For. Res. 2017, 1, 45–56. [Google Scholar]
- Antov, P.; Savov, V.; Neykov, N. Reduction of formaldehyde emission from engineered wood panels by formaldehyde scavengers—A review. In Proceedings of the Joint 13th International Scientific Conference Wood EMA 2020 and the 31st International Scientific Conference ICWST 2020, Vinkovci, Croatia, 28–30 September 2020; pp. 289–294. [Google Scholar]
- Park, B.-D.; Kang, E.C.; Lee, S.-M.; Park, J.Y. Formaldehyde emission of wood-based composite panels with different surface lamination materials using desiccator method. J. Korean Wood Sci. Technol. 2016, 44, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Shen, J.; Xia, X. Effect of the surface finishing methods on particleboard volatile organic compounds and formaldehyde emission. BioResources 2020, 15, 5450–5463. [Google Scholar] [CrossRef]
- Viikari, L.; Hase, A.; Qvintus-Leino, P.; Kataja, K.; Tuominen, S.; Gädda, L. Lignin-Based Adhesives for Particle Board Manufacture. Patent PCT/FI98/00026, 23 July 1998. [Google Scholar]
- Li, D.L.; Wu, J.Q.; Peng, W.X.; Xiao, W.F.; Wu, J.G.; Zhuo, J.Y.; Yuan, T.Q.; Sun, R.C. Effect of Lignin on Bamboo Biomass Self-Bonding During Hot-Pressing: Lignin Structure and Characterization. BioResources 2015, 10, 6769–6782. [Google Scholar] [CrossRef] [Green Version]
- Yotov, N.; Valchev, I.; Petrin, S.; Savov, V. Lignosulphonate and Waste Hydrolysis Lignin as Adhesives for Eco-friendly Fiberboard. Bulg. Chem. Commun. 2017, 49, 93–98. [Google Scholar]
- Kariuki, S.W.; Wachira, J.; Kawira, M.; Murithi, G. Formalhehyde Use and Alternative Biobased Binders for Particleboard Formulation: A Review. Hindawi J. Chem. 2019, 5256897. [Google Scholar]
- Ferdosian, F.; Pan, Z.; Gao, G.; Zhao, B. Bio-Based Adhesives and Evaluation for Wood Composites Application. Polymers 2017, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norström, E.; Demircan, D.; Fogelström, L.; Khabbaz, F.; Malmström, E. Green Binders for Wood Adhesives. In Applied Adhesive Bonding in Science and Technology; INTECH Open Science/Open Minds: London, UK, 2018; pp. 49–71. [Google Scholar]
- Antov, P.; Savov, V.; Neykov, N. Sustainable Bio-Based Adhesives for Eco-friendly Wood Composites. A Review. Wood Res. 2020, 65, 51–62. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Dos Santos, P.V.; Silva, G.C.; Lelis, R.C.C.; Do Nascimento, A.M.; Brito, E.O. Using lignosulfonate and Phenol-Formaldehyde adhesive in particleboard manufacturing. Sci. For. 2017, 45, 423–433. [Google Scholar]
- Geng, X.; Li, K. Investigation of wood adhesives from kraft lignin and polyethylenimine. J. Adhes. Sci. Technol. 2006, 20, 847–858. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Pizzi, A.; Salvado, J. Lignin-based polycondensation resins for wood adhesives. J. Appl. Polym. Sci. 2007, 103, 1690–1699. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, F.; Lv, C.; Tang, L.; Wei, K.; Liu, X. Properties of formaldehyde-free environmentally friendly lignocellulosic composites made from poplar fibres and oxygen-plasma-treated enzymatic hydrolysis lignin. Compos. Part B Eng. 2013, 53, 369–375. [Google Scholar] [CrossRef]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Tahir, P.M.; Halis, R. Lignin-based copolymer adhesives for composite wood panel—A review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Khan, M.A.; Ashraf, S.M.; Malhotra, V.P. Eucalyptus bark lignin substituted phenol formaldehyde adhesives: A study on optimization of reaction parameters and characterization. J. Appl. Polym. Sci. 2004, 92, 3514–3523. [Google Scholar] [CrossRef]
- Hu, L.; Pan, H.; Zhou, Y.; Zhang, M. Methods to Improve Lignin’s Reactivity as a Phenol Substitute and as a Replacement for Other Phenolic Compounds: A Brief Review. BioResources 2011, 6, 3515–3525. [Google Scholar] [CrossRef]
- Malutan, T.; Nicu, R.; Popa, V.I. Contribution to the Study of Hydroxymetylation Reaction of Alkali Lignin. BioResources 2008, 3, 13–20. [Google Scholar]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Hemmilä, V.; Trischler, J.; Sandberg, D. Lignin—An adhesive raw material of the future or waste of research energy. In Proceedings of the 9th Meeting of the Northern European Network for Wood Science and Engineering (WSE), Hannover, Germany, 11–12 September 2013; pp. 98–103. [Google Scholar]
- Klapiszewski, L.; Jamrozik, A.; Strzemiecka, B.; Matykiewicz, D.; Voelkel, A.; Jesionowski, T. Activation of Magnesium Lignosulfonate and Kraft Lignin: Influence on the Properties of Phenolic Resin-Based Composites for Potential Applications in Abrasive Materials. Int. J. Mol. Sci. 2017, 18, 1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, M.R.C.; Huang, X.; Abbenhuis, H.C.I.; Hensen, E.J.M. Lignin oxidation with an organic peroxide and subsequent aromatic ring opening. Int. J. Biol. Macromol. 2019, 123, 1044–1051. [Google Scholar] [CrossRef]
- Junghans, U.; Bernhardt, J.J.; Wollnik, R.; Triebert, D.; Unkelbach, G.; Pufky-Heinrich, D. Valorization of Lignin via Oxidative Depolymerization with Hydrogen Peroxide: Towards Carboxyl-Rich Oligomeric Lignin Fragments. Molecules 2020, 25, 2717. [Google Scholar] [CrossRef]
- Antov, P.; Krišták, L.; Réh, R.; Savov, V.; Papadopoulos, A.N. Eco-Friendly Fiberboard Panels from Recycled Fibers Bonded with Calcium Lignosulfonate. Polymers 2021, 13, 639. [Google Scholar] [CrossRef]
- Bekhta, P.; Noshchenko, G.; Réh, R.; Kristak, L.; Sedliačik, J.; Antov, P.; Mirski, R.; Savov, V. Properties of Eco-Friendly Particleboards Bonded with Lignosulfonate-Urea-Formaldehyde Adhesives and pMDI as a Crosslinker. Materials 2021, 14, 4875. [Google Scholar] [CrossRef]
- Hu, J.-P.; Guo, M.-H. Influence of ammonium lignosulfonate on the mechanical and dimensional properties of wood fiber biocomposites reinforced with polylactic acid. Ind. Crop. Prod. 2015, 78, 48–57. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Krišťák, Ľ.; Réh, R.; Mantanis, G.I. Eco-Friendly, High-Density Fiberboards Bonded with Urea-Formaldehyde and Ammonium Lignosulfonate. Polymers 2021, 13, 220. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Trichkov, N.; Krišták, L.; Réh, R.; Papadopoulos, A.N.; Taghiyari, H.R.; Pizzi, A.; Kunecová, D.; Pachikova, M. Properties of High-Density Fiberboard Bonded with Urea–Formaldehyde Resin and Ammonium Lignosulfonate as a Bio-Based Additive. Polymers 2021, 13, 2775. [Google Scholar] [CrossRef]
- Yuan, Y.; Guo, M.; Liu, F. Preparation and evaluation of green composites using modified ammonium lignosulfonate and polyethylenimine as a binder. BioResources 2014, 9, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, A.; Lutfullah, G.; Zahoorulah. Lignosulfonate-phenolformaldehyde Adhesive: A Potential Binder for Wood Panel Industry. J. Chem. Soc. Pak. 2011, 33, 535–538. [Google Scholar]
- Ghorbani, M.; Liebner, F.; van Herwijnen, H.W.G.; Pfungen, L.; Krahofer, M.; Budjav, E.; Konnerth, J. Lignin Phenol Formaldehyde Resoles: The Impact of Lignin Type on Adhesive Properties. BioResources 2016, 11, 6727–6741. [Google Scholar] [CrossRef] [Green Version]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Antov, P.; Mantanis, G.I.; Savov, V. Development of Wood Composites from Recycled Fibres Bonded with Magnesium Lignosulfonate. Forests 2020, 11, 613. [Google Scholar] [CrossRef]
- Cetin, S.; Özmen, N. Studies on Lignin-Based Adhesives for Particleboard Panels. Turk. J. Agric. For. 2003, 27, 183–189. [Google Scholar]
- Antov, P.; Jivkov, V.; Savov, V.; Simeonova, R.; Yavorov, N. Structural Application of Eco-Friendly Composites from Recycled Wood Fibres Bonded with Magnesium Lignosulfonate. Appl. Sci. 2020, 10, 7526. [Google Scholar] [CrossRef]
- NTL Chemical Consulting. Resins in Panel Production. Available online: https://www.ntl-chemicals.com/wp-content/uploads/2019/02/MDF_Yearbook_2017_2018_Article_NTL_Chemical_Print-1.pdf (accessed on 10 September 2021).
- Hemmilä, V.; Adamopoulos, S.; Hosseinpourpia, R.; Ahmed, S.I. Ammonium Lignosulfonate Adhesives for Particleboards with pMDI and Furfuryl Alcohol as Crosslinkers. Polymers 2019, 11, 1633. [Google Scholar] [CrossRef] [Green Version]
- Solt, P.; Konnerth, J.; Gindl-Altmutter, W.; Kantner, W.; Moser, J.; Mitter, R.; van Herwijnen, H.W.G. Technological Performance of formaldehyde-free adhesive alternatives for particleboard industries. Int. J. Adhes. Adhes. 2019, 94, 99–131. [Google Scholar] [CrossRef]
- Lengyel, K. Contributions to the Manufacturing Technology of Wooden Particleboards (PB) with a View to Improving Some of Their Properties. Ph.D. Thesis, Universitatea Transilvania Brasov, Brasov, Romania, 2018. [Google Scholar]
- Dukarska, D.; Czarnecki, R.; Dziurka, D.; Mirski, R. Construction particleboards made from rapeseed straw glued with hybrid pMDI/PF resin. Eur. J. Wood Prod. 2017, 75, 175–184. [Google Scholar] [CrossRef]
- European Committee for Standardization. EN 310. Wood-Based Panels. Determination of Modulus of Elasticity in Bending and of Bending Strength; European Committee for Standardization: Brussels, Belgium, 1993. [Google Scholar]
- European Committee for Standardization. EN 319. Particleboards and Fibreboards. Determination of Tensile Strength Perpendicular to the Plane of the Board; European Committee for Standardization: Brussels, Belgium, 1993. [Google Scholar]
- European Committee for Standardization. EN 312. Particleboards. Specifications; European Committee for Standardization: Brussels, Belgium, 2003. [Google Scholar]
- ISO. ISO 12460-3:2020. Wood-Based Panels—Determination of Formaldehyde Release—Part 3: Gas Analysis Method; International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- European Committee for Standardization. EN 322. Wood-Based Panels—Determination of Moisture Content; European Committee for Standardization: Brussels, Belgium, 1993. [Google Scholar]
- European Committee for Standardization. EN 323. Wood-Based Panels—Determination of Density; British Standards 1993; European Committee for Standardization: Brussels, Belgium, 1993. [Google Scholar]
- European Committee for Standardization. EN 13986: 2004 +A1:2015. Wood-Based Panels for Use in Construction—Characteristics, Evaluation of Conformity and Marking; European Committee for Standardization: Brussels, Belgium, 2015. [Google Scholar]
- Yakimenko, O.; Stepanov, A.; Patsaeva, S.; Khundzhua, D.; Osipova, O.; Gladkov, O. Formation of Humic-Like Substances during the Technological Process of Lignohumate Synthesis as a Function of Time. Separations 2021, 8, 96. [Google Scholar] [CrossRef]
- Hergert, H.L. Infrared spectra. In Lignins: Occurrence, Formation, Structure and Reactions; Sarkanen, K.V., Ludwig, C.H., Eds.; Wiley: New York, NY, USA, 1971; pp. 267–297. [Google Scholar]
- Özparpucu, M.; Rüggeberg, M.; Gierlinger, N.; Cesarin, I.; Vanholme, R.; Boerjan, W.; Burgert, I. Unravelling the impact of lignin on cell wall mechanics: A comprehensive study on young poplar trees downregulated for Cinnamyl Alcohol Dehydrogenase (CAD). Plant J. 2017, 91, 480–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, A.K.; Basu, G.; Mishra, L. Role of major constituents of coconut fibres on absorption of ionic dyes. Ind. Crops. Prod. 2018, 117, 20–27. [Google Scholar] [CrossRef]
- Lima, R.B.; Raza, R.; Qin, H.; Li, J.; Lindström, M.E.; Zhu, B. Direct lignin fuel cell for power generation. RSC Adv. 2013, 3, 5083. [Google Scholar] [CrossRef]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Frazier, C.E. Isocyanate wood binders. In Handbook of Adhesive Technology, 2nd ed.; Pizzi, A., Mittal, K.L., Eds.; Marcel Dekker: New York, NY, USA, 2003; Chapter 33; pp. 681–694. [Google Scholar]
- Wang, W.; Gardner, D.J.; Baumann, M.G.D. Factors affecting volatile organic compound emissions during hot-pressing of southern pine particleboard. For. Prod. J. 2003, 53, 65–72. [Google Scholar]
- da Costa, N.J.A. Adhesive Systems for Low Formaldehyde Emission Wood-Based Panels. Ph.D. Thesis, University of Porto, Porto, Portugal, 2013. [Google Scholar]
- Eom, Y.-G.; Kim, H.-J.; Kim, J.-S.; Kim, S.-M.; Kim, J.-A. Reduction of Formaldehyde Emission from Particleboards by Bio-Scavengers. J. Korean Wood Sci. Technol. 2006, 34, 29–41. [Google Scholar]
- Kim, S.; Kim, H.-J.; Kim, H.-S.; Lee, H.H. Effect of bio-scavengers on the curing behavior and bonding properties of melamine-formaldehyde resins. Macromol. Mater. Eng. 2006, 291, 1027–1034. [Google Scholar] [CrossRef]
- Hansen, E.L.; Naerum, L.; Nissen, P. Method of Reducing the Formaldehyde Emission of a Mineral Fibre Product, and Mineral Fibre Product with Reduced Formaldehyde Emission. Patent BR112013013409A2, 14 June 2012. [Google Scholar]
- Salem, M.Z.M.; Böhm, M. Understanding of formaldehyde emissions from solid wood: An overview. BioResources 2013, 8, 4775–4790. [Google Scholar] [CrossRef]





| Sieve Size mm × mm | Particles Sizes (Range of Values), in mm | ||
|---|---|---|---|
| Length | Width | Thickness | |
| 4.00 × 4.00 | 7.6–25.8 | 4.1–10.6 | 0.2–4.1 |
| 3.15 × 3.15 | 6.1–18.0 | 4.1–5.7 | 0.4–3.1 |
| 2.00 × 2.00 | 4.2–34.1 | 1.1–5.2 | 0.2–1.8 |
| 1.25 × 1.25 | 3.7–25.6 | 0.9–3.4 | 0.1–1.6 |
| 1.00 × 1.00 | 2.4–19.5 | 0.5–1.7 | 0.2–0.9 |
| 0.8 × 0.8 | 2.0–5.0 | 0.2–0.7 | 0.2–0.5 |
| Characteristic | Description/Value |
|---|---|
| pH Value | 5.5 ± 1% |
| Dry matter mass | 93 ± 2% |
| Insolubility in water | 1% max |
| Magnesium | 6 ± 1% min |
| Bulk density | 400 kg/m3 |
| Moisture content | 7% max |
| Material | Values |
|---|---|
| Solid content | 58.4% |
| pH | 9 |
| Viscosity (flow time through the viscosmetric STAS cup, Φ 6 mm, 20 °C) | 16 s |
| Adhesive reactivity at 160 °C | 3 min 15 s |
| Adhesive Composition for the Manufactured Panels | Panels’ Codes |
|---|---|
| Magnesium lignosulfonate powder | L20 |
| Oxidized magnesium lignosulfonate | LO 20 |
| Oxidized magnesium lignosulfonate + 1% PMDI | LO 20 P1 |
| Oxidized magnesium lignosulfonate + 2% PMDI | LO 20 P2 |
| Oxidized magnesium lignosulfonate + 3% PMDI | LO 20 P3 |
| Oxidized magnesium lignosulfonate + 1% PMDI + glucose | LO 20 P1G |
| Oxidized magnesium lignosulfonate + 2% PMDI + glucose | LO 20 P2G |
| Panel Type | MOE 1 (N/mm2) | MOR 1 (N/mm2) | IB 1 (N/mm2) |
|---|---|---|---|
| L 20 | 1847 (133) | 8.1 (0.94) | 0.10 (0.03) |
| LO 20 | 2783 (319) | 10.6 (0.76) | 0.12 (0.03) |
| LO 20 P1 | 2526 (185) | 11.0 (0.34) | 0.22 (0.03) |
| LO 20 P2 | 2778 (239) | 13.0 (0.47) | 0.31 (0.05) |
| LO 20 P3 | 2931(89) | 15.3 (0.73) | 0.41 (0.04) |
| LO 20 P1G | 3123 (152) | 13.3 (0.74) | 0.38 (0.06) |
| LO 20 P2G | 3320 (112) | 14.3 (0.58) | 0.48 (0.07) |
| Type of the Receipt | Values |
|---|---|
| LP 20 (LIGNEX MG F powder) | 0.789 |
| LO 20 (oxidized LIGNEX MG F) | 0.616 |
| LO 20 P1 (oxidized LIGNEX MG F + 1% PMDI) | 0.553 |
| LO 20 P2 (oxidized LIGNEX MG F + 2% PMDI) | 0.477 |
| LO 20 P3 (oxidized LIGNEX MG F + 3% PMDI) | 0.401 |
| LO 20 P1G (oxidized LIGNEX MG F + 1% PMDI + glucose) | 0.386 |
| LO 20 P2G (oxidized LIGNEX MG F + 2% PMDI + glucose) | 0.347 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balea Paul, G.; Timar, M.C.; Zeleniuc, O.; Lunguleasa, A.; Coșereanu, C. Mechanical Properties and Formaldehyde Release of Particleboard Made with Lignin-Based Adhesives. Appl. Sci. 2021, 11, 8720. https://doi.org/10.3390/app11188720
Balea Paul G, Timar MC, Zeleniuc O, Lunguleasa A, Coșereanu C. Mechanical Properties and Formaldehyde Release of Particleboard Made with Lignin-Based Adhesives. Applied Sciences. 2021; 11(18):8720. https://doi.org/10.3390/app11188720
Chicago/Turabian StyleBalea Paul, Gabriela, Maria Cristina Timar, Octavia Zeleniuc, Aurel Lunguleasa, and Camelia Coșereanu. 2021. "Mechanical Properties and Formaldehyde Release of Particleboard Made with Lignin-Based Adhesives" Applied Sciences 11, no. 18: 8720. https://doi.org/10.3390/app11188720
APA StyleBalea Paul, G., Timar, M. C., Zeleniuc, O., Lunguleasa, A., & Coșereanu, C. (2021). Mechanical Properties and Formaldehyde Release of Particleboard Made with Lignin-Based Adhesives. Applied Sciences, 11(18), 8720. https://doi.org/10.3390/app11188720






