Abstract
Reactive oxygen species (ROS) are central elements of a number of physiological processes such as differentiation and intracellular signaling, as well as pathological processes, e.g., inflammation or apoptosis. ROS are involved in the growth and proliferation of stem cells, cell communication, cell aging, all types of inflammation, cancer development and proliferation, or type 2 diabetes. Elastin-derived peptides (EDPs) are detected in all these conditions and, according to the current state of knowledge, the role of the extracellular matrix (ECM) protein is crucial. It is believed that EDPs are a result of the aforementioned pathological conditions and are generated during degradation of ECM. However, as shown in the literature, the production of EDPs can be induced not only by inter alia chemical, enzymatic, and physical factors but also directly by ROS. No comprehensive study of the impact of ROS on EDPs and EDPs on ROS production has been conducted to date; therefore, the aim of this paper is to summarize the current state of knowledge of the relationship between ROS and ECM with special involvement of EDPs in the processes mentioned above. Depending on the type of cells, tissue, or organism, the relationships between ROS and ECM/EDPs may differ completely.
1. Introduction
Reactive oxygen species (ROS) are highly reactive chemical molecules formed during various biological processes [1]. ROS can be an effect of physiological and pathological processes leading to rearrangement of the cell structure, an increase or a decrease in cell metabolism, and even cell death [2]. The involvement of ROS in the development of inflammation, neurodegenerative diseases, and cancer is also well documented [3,4]. Moreover, it is currently believed that ROS are a key element in stem cell aging and autophagy [5].
The elastin protein is widely distributed in the organism [6]. However, during various physiological and pathological processes, elastin is degraded to elastin-derived peptides (EDPs) [7,8]. To date, a number of papers have reported that κ-elastin, EDPs, or peptide VGVAPG (signaling sequences from elastin) affect the ROS level in cell culture models or in vitro in organisms [9,10,11,12]. It has been described that, similar to the ROS level, the level of EDPs increases during aging and in various pathological processes [13,14]. Therefore, EDPs are recognized as hallmarks of aging and are called matrikines—matrix fragments having the ability to regulate cell physiology [8,15]. Moreover, the influence of ROS on the EDP formation, as well as the influence of EDPs on the ROS formation has now been demonstrated [9,16].
Therefore, the aim of this paper is to summarize the current state of knowledge of the interaction between ROS and EDPs levels in biological systems.
2. Role of ROS in the Origin of Elastin-Derived Peptides
Elastin occurs naturally in skin, arteries, lung, and other tissues [6]. This protein is characterized by high tolerance to mechanical damage, giving additional resistance of certain tissues. It is also a matrix for cells, especially in the nervous system [17,18]. The tropoelastin molecule is the building-block of elastin, which makes this protein nearly insoluble in water and responsible for its unique properties [19,20]. It has been observed that the half-life of elastin is over 70 years [21]. However, an intensified degradation process of this protein is observed during aging [22]. Hence, it is difficult to study the direct effects of elastin on the entire organism, especially on ROS production. However, in the 1950s, the first in vitro hydrolyzation of elastin was performed with the use of potassium hydroxide (KOH), resulting in κ-elastin production. Subsequently, the degradation of elastin by oxalic acid (C2H2O4), which yields an α-elastin molecule, was developed [23]. Thus, the next discoveries in this field were focused on characterization of the obtained molecules as products of elastin degradation called elastin-derived peptides (EDPs). It has been observed that the peptides with the Gly-x-x-Pro-Gly (GxxPG) amino sequence were repeated many times in the EDP structure, which defines the conformation of elastin [24]. The most common sequence, i.e., the Val-Gly-Val-Ala-Pro-Gly (VGVAPG) hexapeptide, has been found in the EDP group [25].
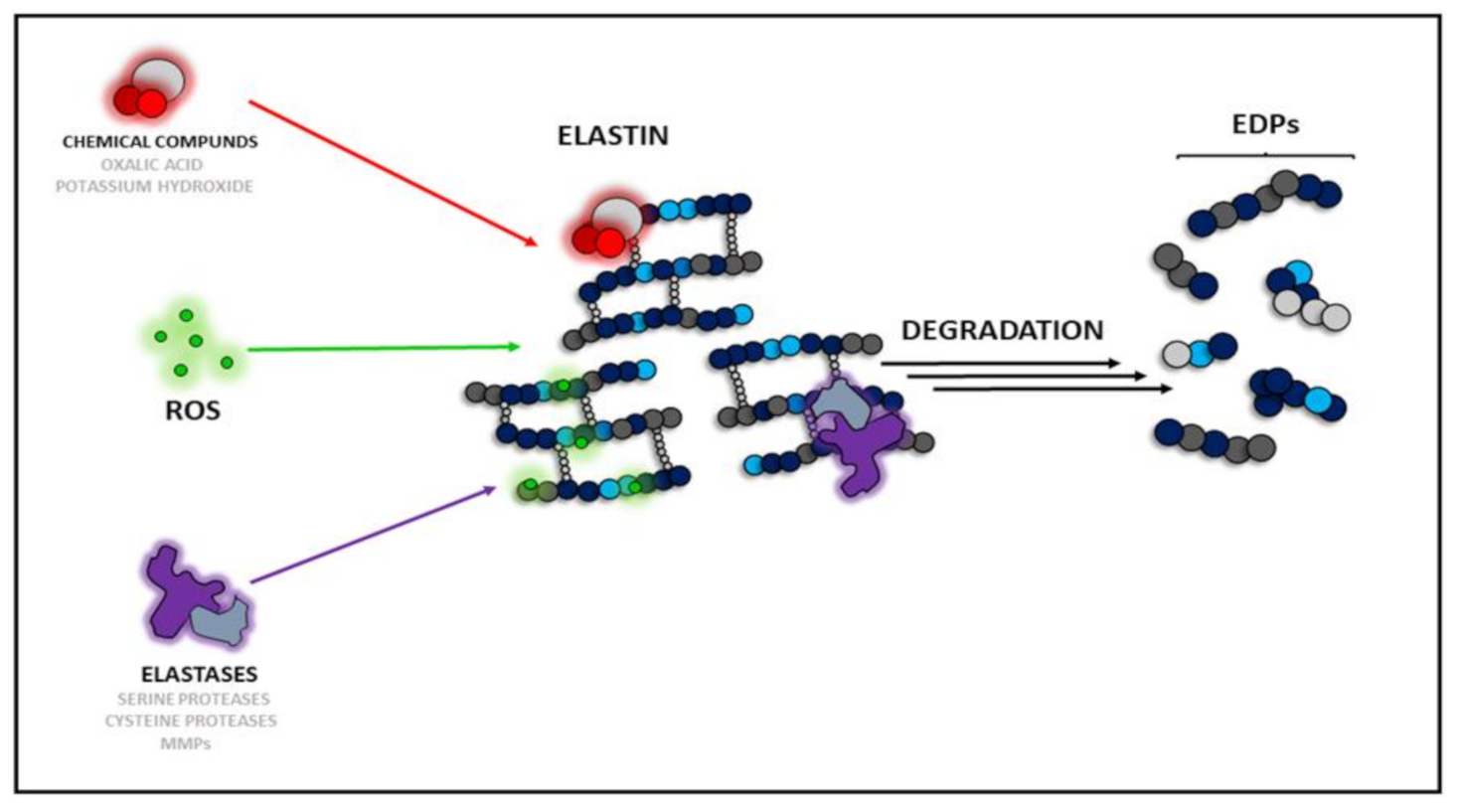
Literature highlights three main causes (types) of elastin degradation: chemical, enzymatic, and ROS-dependent (Figure 1). The first type can be achieved with the use of potassium hydroxide (KOH) or oxalic acid (C2H2O4), which are not present in the organism but are used only to prepare EDPs in vitro given their physicochemical properties. The second type of elastin degradative factors is represented by various proteinases, which act both in vitro and in vivo. To date, three groups of proteolytic enzymes acting as elastases have been described [26]. Serine proteases released from neutrophils or macrophages act as chymotrypsin-like proteins. They are able to degrade the whole elastin molecule or pre-degraded protein, e.g., proteinase 3, neutrophil elastases, and cathepsin G [27]. Another group comprises cysteine proteases such as cathepsin K (CatK), L, S, and V, which naturally occur in lysosomes [28]. The third group of proteases capable of hydrolyzing elastin are metalloproteinases (MMPs), which require metal ions for their hydrolyzing activity, e.g., MMP-2, -7, -9, and -12 [26].
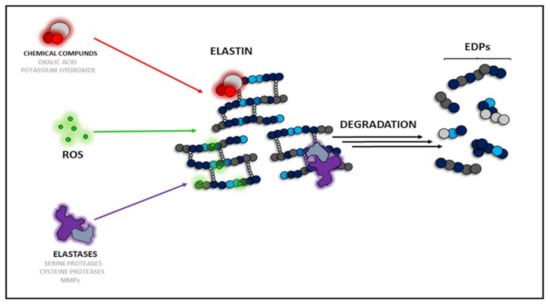
Figure 1.
Types of elastin degradation factors: chemicals, ROS-dependent, and enzyme-dependent. ROS—reactive oxygen species, MMPs—matrix metalloproteinases, EDPs—elastin derived peptides.
The last type of elastin degradation is the ROS-dependent mechanism. To date, it has been described that tropoelastin is sensitive to ROS [29]. It has been shown that ROS generated by ultraviolet A (UV-A) and hematoporphyrin rapidly degraded tropoelastin within 5 min [29]. Treatment of tropoelastin with copper sulfate/ascorbic acid resulted in degradation of tropoelastin producing fragments of molecular weight 45, 30, and 10 kDa within 30 min. The degradation of tropoelastin was partially blocked by the addition of mannitol, which is a well-described radical scavenger [29,30]. ROS generation induced by the xanthine-xanthine oxidase system caused degradation of tropoelastin within 6 h. The degradation was blocked by the antioxidant enzyme catalase (CAT), which is a ROS scavenging enzyme, proving the engagement of free radicals in the elastin degradation process. ROS generated by copper-ascorbate seems to be unique in that it cleaves relatively specific sites of the tropoelastin molecule. Thus, ROS may play a degradative role in elastin metabolism, which may cause elastolytic changes or deposition of fragmented elastic fibers in photoaged skin or age-related elastolytic disorders [29]. Moreover, a positive correlation between metal ion-induced ROS and an increased level of elastin derivatives has been showed by Umeda et al., showing that ROS can directly influence on elastin degradation [31]. However, the cited authors have also shown weakening of such an effect during solarization of elastin derivatives, suggesting the ability of ROS to degrade the whole elastin molecule, but not every derivative. Moreover, elastin is a major part of the extracellular matrix (ECM) of connective tissues, giving these tissues resistance to stretching and injuries thanks to its properties. Interestingly, as shown by Yao et al., superoxide dismutase 3 (SOD3) gene knock-out in mice contributed to an increase in ECM fragmentation after treatment with cigarette smoke, which is considered to be a source of exogenous ROS [32]. This indicates again that ECM, whose major part is elastin, can be successfully degraded by ROS. The studies cited above show that an increased ROS level in the organism, also during ischemia and inflammation, may be engaged in acceleration of elastin degradation and production of higher amounts of EDPs. This also proves that the highly reactive ROS are able to damage the highly mechanically resistant and insoluble elastin. In the further part of this study, the correlation between EDP internalization and intracellular ROS generation will be discussed.
Interestingly, solar elastosis of skin elastin is a complex process combining UV, ROS, and enzymatic degradation [33,34]. To date, UV radiation has been described to reduce desmosine cross-links in elastin [35]. Besides direct destruction of the elastin structure, UV irradiation also causes the formation of large amounts of ROS [36]. Moreover, data suggest a role of intracellular elastin degradation by catK in the formation of solar elastosis [33]. Induction of catK expression in fibroblasts was observed both in vitro and in vivo after exposure to longwave UVA. Moreover, UV irradiation has been shown to induce the expression of MMPs. Irradiation of skin was found to produce an 11.9-fold increase in the expression of human macrophage elastase (MMP-12) mRNA within 16 h of UV exposure [34]. Research reports also show that the increase in ROS generation during inflammation and bacterial and viral infections can accelerate elastin degradation [37].
3. Impact of EDPs on ROS Production
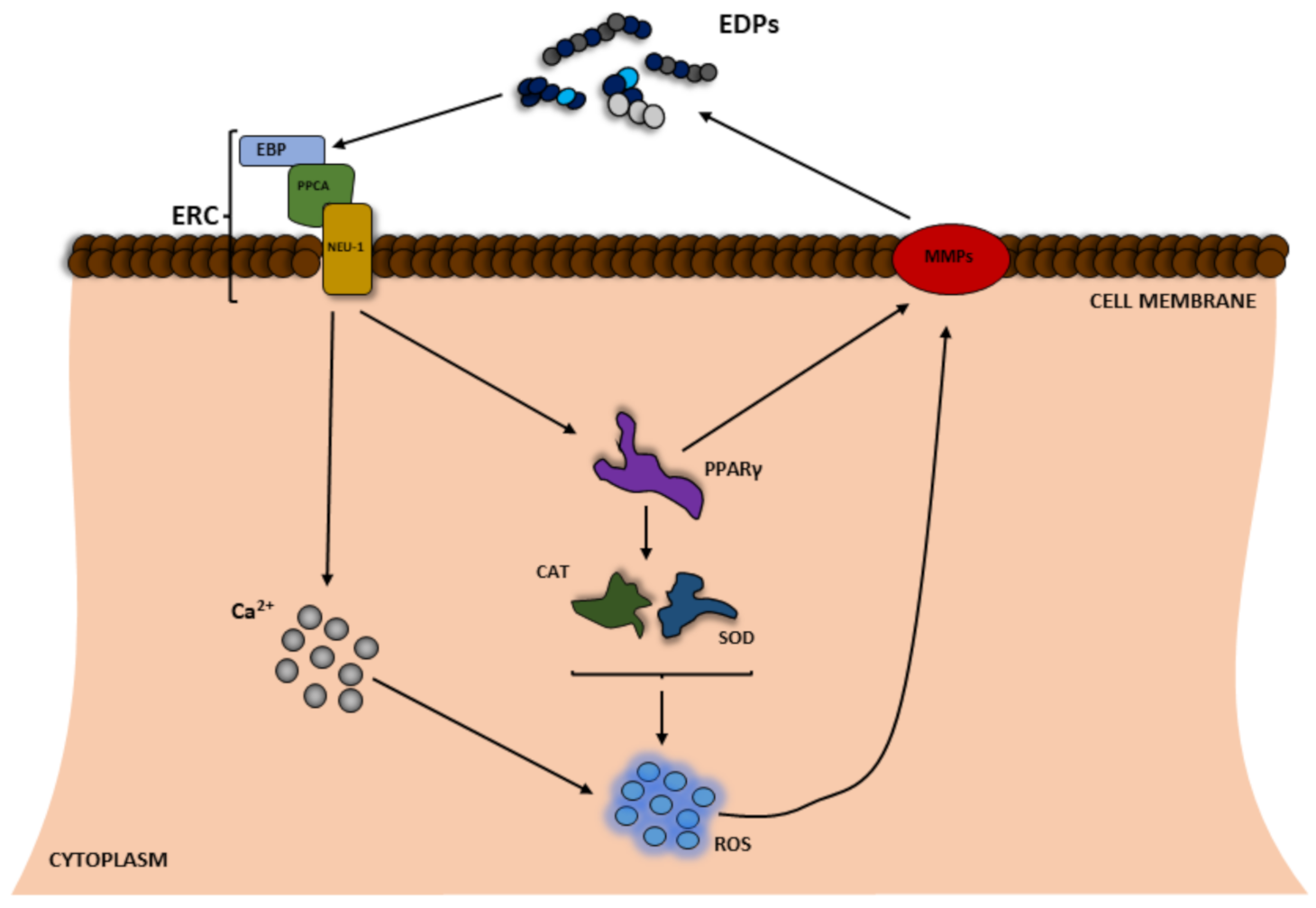
As described above, ROS can take part in elastin degradation directly, resulting in increased amounts of EDP protein in the organism. To date, EDPs have been well-described to cause biological effects mainly through activation of the cell receptor present naturally in the cell membrane [18,38]. EDP binds to 67-kDa elastin-binding protein (EBP), which is a catalytically inactive form of beta-galactosidase produced by alternative splicing of the GLB1 gene (Figure 2) [39,40]. The presence of EDPs in the extracellular matrix is able to have an impact on many metabolic parameters in cells, e.g., proliferation, metabolic activity, expression of such proteolytic enzymes as MMPs, and even cell death [41,42,43]. Moreover, the ability of these peptides to induce ROS production has been also described in the literature [9,44,45].
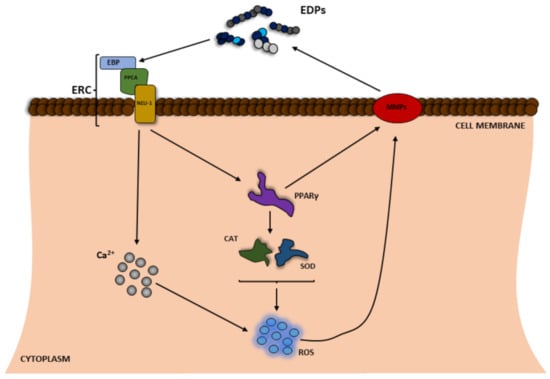
Figure 2.
Scheme of the positive feedback of reactive oxygen species (ROS) and elastin degradation yielding elastin-derived peptides (EDPs), which induce ROS production. EBP—elastin-binding protein; Neu1—neuraminidase; PPARγ—peroxisome proliferator-activated receptor gamma; PPCA—protective protein/cathepsin A; ERC—elastin receptor complex; CAT—catalase; SOD—superoxide dismutase.
The increase in the level of ROS in the cell is believed to be caused by at least two main processes, i.e., Ca2+ influx and disruption of the expression and/or activity of antioxidant enzymes mediated through the peroxisome proliferator-activated receptor gamma (PPARγ) pathway (Figure 2) [9]. A number of studies have reported that tropoelastin, κ-elastin, EDPs, and the VGVAPG peptide increase Ca2+ influx in human monocytes, fibroblasts, human umbilical venous endothelial cells (HUVEC), different glioma cell lines (C6, CB74, CB109, and CB191), and smooth muscle cells from pig aorta or mouse astrocytes [10,46,47,48,49]. It is well known that different Ca2+ signaling pathways can increase the level of cell ROS [50]. To date, depending on the model, EDPs have been shown to decrease or increase the cell ROS level [51]. However, the vast majority of studies show that EDPs induce ROS production in human fibroblasts and neuroblastoma (SH-SY5Y) as well as and murine monocytes and astrocytes [9,12,44,45,52]. In addition to the impact on ROS, various authors have shown that EDPs affect the expression and/or activity of antioxidant enzymes. As reported by Gmiński et al., EDPs enhance the activities of superoxide dismutase (SOD), CAT, or glutathione peroxidase (GPx) and increase lipid peroxidation in human fibroblasts [53]. Similarly, Szychowski et al. have described that the VGVAPG peptide increases GPx activity and slightly (but not statistically significantly) influenced the activity of SOD and CAT [9]. Szychowski et al., have also described PPARγ as a key receptor involved in the VGVAPG peptide mechanism of action in mouse astrocytes and the human SH-SY5Y neuroblastoma cell line [9,43]. Furthermore, it is generally accepted that PPARγ together with ROS are crucial in the inflammation process [54,55], which is in line with data on the proinflammatory mechanism of action of EDPs. To date, EDPs have been reported to increase inflammatory markers in various cell types such as human malignant melanoma (M3Da), human monocytes, and human ligamentum flavum cells [56,57,58]. Moreover, EDPs have also been shown to be chemotactic agents for monocytes, which are responsible for development of inflammation [58,59,60]. Interestingly, the VGVAPG peptide does not activate the inflammatory process in mouse astrocytes, probably due to the special role of the nervous system [61].
PPARγ is also involved in the control of the expression of MMPs [62]. To date, it has been well described that EDPs increase the expression of various MMPs and degradation of ECM [63,64,65]. It has been shown that the VGVAPG peptide in human endothelial cells upregulates the expression of mRNA of membrane-type matrix metalloprotease-1 (MT1-MMP) and MMP-2 [63]. Similarly, Ntayi et al. (2004) described that cell culture plates coated with the VGVAPG peptide were characterized by increased expression and activation of MMP-2 and MT1-MMP in two melanoma (M1Dor and M3Da) cell lines [66]. Furthermore, the VGVAPG peptide added to the culture medium upregulated the MMP-2, MT1-MMP, and TIMP-2 mRNA expression and activity in the human fibrosarcoma (HT-1080) cell line [64,65]. Furthermore, in human glioblastoma multiforme cell lines CB74, CB109, and CB191 and the rat astrocytoma cell line C6 exposed to the (VGVAPG)3 peptide, mRNA expression of MMP-2 increased with very low stimulation of MMP-9 [49]. The authors correlate the high expression of MMP-2 mRNA with increasing degradation of ECM and production of EDPs.
All these mechanisms, i.e., the increase in ROS production, Ca2+ influx, PPARγ signaling pathway activation, and the increase in the expression and/or activity of MMPs lead to accelerated elastin degradation in ECM and production of increased numbers of EDPs (Figure 2).
4. Conclusions and Perspectives
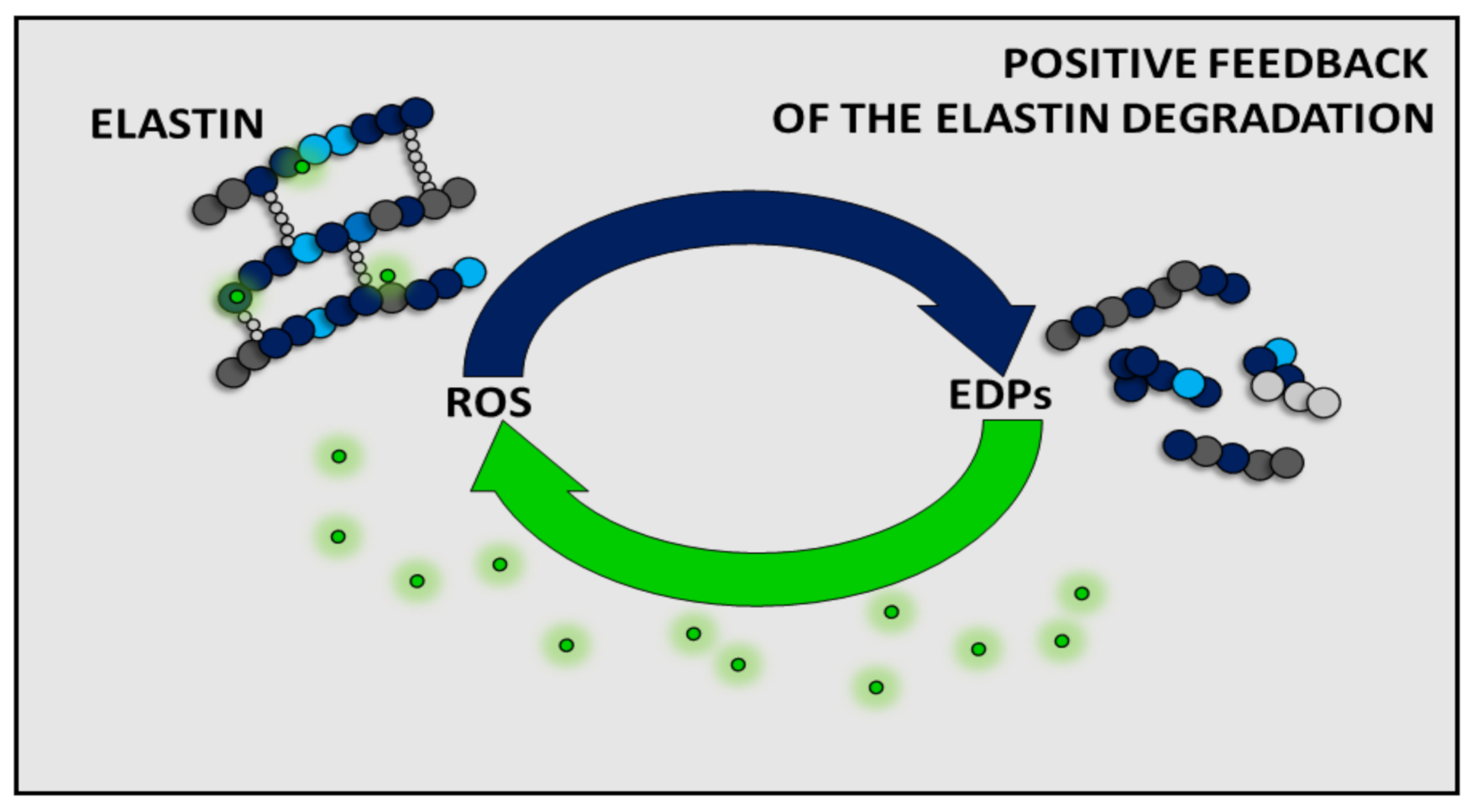
To date, the effect of EDPs has been shown to be dependent on the type of cells or model organisms. However, it is generally accepted that EDPs can indirectly (by affecting the expression and/or activity of antioxidant enzymes such as CAT or SOD) and/or directly affect the level of ROS. Moreover, increased EDP levels have also been detected in various pathological conditions associated with a high level of ROS (e.g., cancers, arteriosclerosis, obesity). Given the literature data presented here, it can be assumed that there is some positive feedback between ROS and EDPs, and the presence of one component increases the amount of the other one (Figure 3). This phenomenon fits into the free radical theory of aging. Moreover, due to the inseparability of EDPs and ROS, the cellular aging can only be slowed down by reduction of the amount of ROS in the organism. A healthy lifestyle and an antioxidant-rich diet or avoidance of high sun exposure may be helpful to slow down aging. Unfortunately, we have no influence on the spontaneous elastin degradation process during aging. Therefore, future medicine should focus on the antiaging therapies on inhibition of elastin degradation, removal of EDPs from the organism, and acceleration of the reconstruction of elastin in tissues.
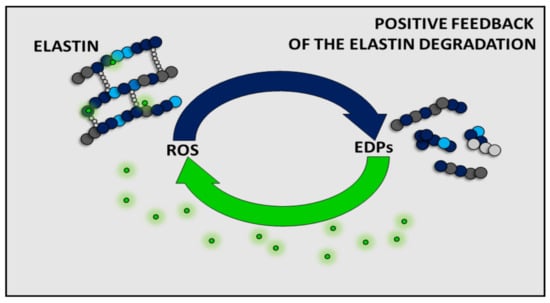
Figure 3.
Summarizing scheme of the positive feedback of reactive oxygen species (ROS) and elastin degradation yielding elastin-derived peptides (EDPs).
Author Contributions
Conceptualization, K.A.S.; software, B.S.; writing—original draft preparation, K.A.S. and B.S; writing—review and editing, K.A.S. and B.S.; visualization, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by statutory funds of the University of Information Technology and Management in Rzeszow, Poland (DS 503-07-01-27).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Anna Wesołowska-Zoń, a proofreading and copyediting expert, for improving the language in the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Wanjala, G.W.; Onyango, A.; Onyango, C.; Makayoto, M. Reactive oxygen species (ROS) generation, impacts on tissue oxidation and dietary management of non-communicable diseases: A review. Afr. J. Biochem. Res. 2017, 11, 79–90. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Tuveson, D.A. ROS in Cancer: The Burning Question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Guan, J.L.; Simon, A.K.; Prescott, M.; Menendez, J.A.; Liu, F.; Wang, F.; Wang, C.; Wolvetang, E.; Vazquez-Martin, A.; Zhang, J. Autophagy in stem cells. Autophagy 2013, 9, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Clifford, P.S.; Ella, S.R.; Stupica, A.J.; Nourian, Z.; Li, M.; Martinez-Lemus, L.A.; Dora, K.A.; Yang, Y.; Davis, M.J.; Pohl, U.; et al. Spatial Distribution and Mechanical Function of Elastin in Resistance Arteries. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2889–2896. [Google Scholar] [CrossRef]
- Hinek, A.; Wrenn, D.S.; Mecham, R.P.; Barondes, S.H. The elastin receptor: A galactoside-binding protein. Science 1988, 239, 1539–1541. [Google Scholar] [CrossRef]
- Duca, L.; Floquet, N.; Alix, A.J.P.; Haye, B.; Debelle, L. Elastin as a matrikine. Crit. Rev. Oncol. Hematol. 2004, 49, 235–244. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Rombel-Bryzek, A.; Dołhańczuk-Śródka, A.; Gmiński, J. Antiproliferative Effect of Elastin-Derived Peptide VGVAPG on SH-SY5Y Neuroblastoma Cells. Neurotox. Res. 2019, 36, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Gmiński, J. Specific role of N-methyl-D-aspartate (NMDA) receptor in elastin-derived VGVAPG peptide-dependent calcium homeostasis in mouse cortical astrocytes in vitro. Sci. Rep. 2019, 9, 20165. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Kim, H.J.; Wang, Y.; Wang, A.; Mitts, T.F. Sodium l-ascorbate enhances elastic fibers deposition by fibroblasts from normal and pathologic human skin. J. Dermatol. Sci. 2014, 75, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Gayral, S.; Garnotel, R.; Castaing-Berthou, A.; Blaise, S.; Fougerat, A.; Berge, E.; Montheil, A.; Malet, N.; Wymann, M.P.; Maurice, P.; et al. Elastin-derived peptides potentiate atherosclerosis through the immune Neu1-PI3K pathway. Cardiovasc. Res. 2014, 102, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Parfrey, N.A.; Doyle, C.T. Elastosis in benign and malignant breast disease. Hum. Pathol. 1985, 16, 674–676. [Google Scholar] [CrossRef]
- Duca, L.; Blaise, S.; Romier, B.; Laffargue, M.; Gayral, S.; El Btaouri, H.; Kawecki, C.; Guillot, A.; Martiny, L.; Debelle, L.; et al. Matrix ageing and vascular impacts: Focus on elastin fragmentation. Cardiovasc. Res. 2016, 110, 298–308. [Google Scholar] [CrossRef]
- Salesse, S.; Odoul, L.; Chazée, L.; Garbar, C.; Duca, L.; Martiny, L.; Mahmoudi, R.; Debelle, L. Elastin molecular aging promotes MDA-MB-231 breast cancer cell invasiveness. FEBS Open Bio 2018, 8, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Parasaram, V.; Nosoudi, N.; Chowdhury, A.; Vyavahare, N. Pentagalloyl glucose increases elastin deposition, decreases reactive oxygen species and matrix metalloproteinase activity in pulmonary fibroblasts under inflammatory conditions. Biochem. Biophys. Res. Commun. 2018, 499, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin Biomaterials in Dermal Repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef]
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Elastin—Derived Peptides in the Central Nervous System: Friend or Foe. Cell. Mol. Neurobiol. 2021. [Google Scholar] [CrossRef]
- Ozsvar, J.; Yang, C.; Cain, S.A.; Baldock, C.; Tarakanova, A.; Weiss, A.S. Tropoelastin and Elastin Assembly. Front. Bioeng. Biotechnol. 2021, 9, 138. [Google Scholar] [CrossRef]
- Wise, S.G.; Yeo, G.C.; Hiob, M.A.; Rnjak-Kovacina, J.; Kaplan, D.L.; Ng, M.K.C.; Weiss, A.S. Tropoelastin: A versatile, bioactive assembly module. Acta Biomater. 2014, 10, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.J.; Bolton, C.E.; Miller, B.E.; Tal-Singer, R.; Rabinovich, R.A.; Palmer, C.N.A.; Thomson, N.C.; MacNee, W. Age-dependent elastin degradation is enhanced in chronic obstructive pulmonary disease. Eur. Respir. J. 2016, 48, 1215–1218. [Google Scholar] [CrossRef]
- Adair, G.S.; Davis, H.F.; Partridge, S.M. A Soluble protein derived from elastin. Nature 1951, 167, 605. [Google Scholar] [CrossRef] [PubMed]
- Senior, R.M.; Griffin, G.L.; Mecham, R.P. Chemotactic activity of elastin-derived peptides. J. Clin. Investig. 1980, 66, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Senior, R.M.; Griffin, G.L.; Mecham, R.P.; Wrenn, D.S.; Prasad, K.U.; Urry, D.W. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J. Cell Biol. 1984, 99, 870–874. [Google Scholar] [CrossRef]
- Le Page, A.; Khalil, A.; Vermette, P.; Frost, E.H.; Larbi, A.; Witkowski, J.M.; Fulop, T. The role of elastin-derived peptides in human physiology and diseases. Matrix Biol. 2019, 84, 81–96. [Google Scholar] [CrossRef]
- Gudmann, N.S.; Manon-Jensen, T.; Sand, J.M.B.; Diefenbach, C.; Sun, S.; Danielsen, A.; Karsdal, M.A.; Leeming, D.J. Lung tissue destruction by proteinase 3 and cathepsin G mediated elastin degradation is elevated in chronic obstructive pulmonary disease. Biochem. Biophys. Res. Commun. 2018, 503, 1284–1290. [Google Scholar] [CrossRef]
- Novinec, M.; Grass, R.N.; Stark, W.J.; Turk, V.; Baici, A.; Lenarčič, B. Interaction between Human Cathepsins K, L, and S and Elastins. J. Biol. Chem. 2007, 282, 7893–7902. [Google Scholar] [CrossRef]
- Hayashi, A.; Ryu, A.; Suzuki, T.; Kawada, A.; Tajima, S. In vitro degradation of tropoelastin by reactive oxygen species. Arch. Dermatol. Res. 1998, 290, 497–500. [Google Scholar] [CrossRef]
- André, P.; Villain, F. Free radical scavenging properties of mannitol and its role as a constituent of hyaluronic acid fillers: A literature review. Int. J. Cosmet. Sci. 2017, 39, 355–360. [Google Scholar] [CrossRef]
- Umeda, H.; Nakamura, F.; Suyama, K. Oxodesmosine and Isooxodesmosine, Candidates of Oxidative Metabolic Intermediates of Pyridinium Cross-Links in Elastin. Arch. Biochem. Biophys. 2001, 385, 209–219. [Google Scholar] [CrossRef]
- Yao, H.; Arunachalam, G.; Hwang, J.-W.; Chung, S.; Sundar, I.K.; Kinnula, V.L.; Crapo, J.D.; Rahman, I. Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM. Proc. Natl. Acad. Sci. USA 2010, 107, 15571–15576. [Google Scholar] [CrossRef] [PubMed]
- Codriansky, K.A.; Quintanilla-Dieck, M.J.; Gan, S.; Keady, M.; Bhawan, J.; Rünger, T.M. Intracellular Degradation of Elastin by Cathepsin K in Skin Fibroblasts—A Possible Role in Photoaging. Photochem. Photobiol. 2009, 85, 1356–1363. [Google Scholar] [CrossRef]
- Jin, H.C.; Jin, Y.S.; Mi, K.L.; Hee, C.E.; Joo, H.L.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. J. Investig. Dermatol. 2002, 119, 507–512. [Google Scholar]
- Dhital, B.; Durlik, P.; Rathod, P.; Gul-E-Noor, F.; Wang, Z.; Sun, C.; Chang, E.J.; Itin, B.; Boutis, G.S. Ultraviolet radiation reduces desmosine cross-links in elastin. Biochem. Biophys. Rep. 2017, 10, 172–177. [Google Scholar] [CrossRef]
- Weihermann, A.C.; Lorencini, M.; Brohem, C.A.; de Carvalho, C.M. Elastin structure and its involvement in skin photoageing. Int. J. Cosmet. Sci. 2017, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- de Castro Brás, L.E.; Frangogiannis, N.G. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef]
- Blood, C.H.; Sasse, J.; Brodt, P.; Zetter, B.R. Identification of a tumor cell receptor for VGVAPG, an elastin-derived chemotactic peptide. J. Cell Biol. 1988, 107, 1987–1993. [Google Scholar] [CrossRef]
- Skeie, J.M.; Hernandez, J.; Hinek, A.; Mullins, R.F. Molecular responses of choroidal endothelial cells to elastin derived peptides through the elastin-binding protein (GLB1). Matrix Biol. 2012, 31, 113–119. [Google Scholar] [CrossRef]
- Péterszegi, G.; Robert, L. Cell death induced in lymphocytes expressing the elastin-laminin receptor by excess agonists: Necrosis and apoptosis. Biomed. Pharmacother. 1998, 52, 369–377. [Google Scholar] [CrossRef]
- Devy, J.; Duca, L.; Cantarelli, B.; Joseph-Pietras, D.; Scandolera, A.; Rusciani, A.; Parent, L.; Thevenard, J.; Pasco, S.B.; Tarpin, M.; et al. Elastin-derived peptides enhance melanoma growth in vivo by upregulating the activation of Mcol-A (MMP-1) collagenase. Br. J. Cancer 2010, 103, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Gmiński, J. Impact of elastin-derived VGVAPG peptide on bidirectional interaction between peroxisome proliferator-activated receptor gamma (Pparγ) and beta-galactosidase (β-Gal) expression in mouse cortical astrocytes in vitro. Naunyn Schmiedeberg’s Arch. Pharmacol. 2019, 392, 405–413. [Google Scholar] [CrossRef]
- Robert, L.; Jacob, M.P.; Frances, C.; Godeau, G.; Hornebeck, W. Interaction between elastin and elastases and its role in the aging of the arterial wall, skin and other connective tissues. A review. Mech. Ageing Dev. 1984, 28, 155–166. [Google Scholar] [CrossRef]
- Scandolera, A.; Rabenoelina, F.; Chaintreuil, C.; Rusciani, A.; Maurice, P.; Blaise, S.; Romier-Crouzet, B.; El Btaouri, H.; Martiny, L.; Debelle, L.; et al. Uncoupling of Elastin Complex Receptor during In Vitro Aging Is Related to Modifications in Its Intrinsic Sialidase Activity and the Subsequent Lactosylceramide Production. PLoS ONE 2015, 10, e0129994. [Google Scholar] [CrossRef]
- Jacob, M.P.; Fülöp, T.; Foris, G.; Robert, L. Effect of elastin peptides on ion fluxes in mononuclear cells, fibroblasts, and smooth muscle cells. Proc. Natl. Acad. Sci. USA 1987, 84, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Faury, G.; Usson, Y.; Robert-Nicoud, M.; Robert, L.; Verdetti, J. Nuclear and cytoplasmic free calcium level changes induced by elastin peptides in human endothelial cells. Proc. Natl. Acad. Sci. USA 1998, 95, 2967–2972. [Google Scholar] [CrossRef]
- Faury, G.; Garnier, S.; Weiss, A.S.; Wallach, J.; Fülöp, T.; Jacob, M.P.; Mecham, R.P.; Robert, L.; Verdetti, J. Action of tropoelastin and synthetic elastin sequences on vascular tone and on free Ca2+ level in human vascular endothelial cells. Circ. Res. 1998, 82, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Coquerel, B.; Poyer, F.; Torossian, F.; Dulong, V.; Bellon, G.; Dubus, I.; Reber, A.; Vannier, J.P. Elastin-derived peptides: Matrikines critical for glioblastoma cell aggressiveness in a 3-D system. Glia 2009, 57, 1716–1726. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Dupont, A.; Dury, S.; Gafa, V.; Lebargy, F.; Deslée, G.; Guenounou, M.; Antonicelli, F.; Le Naour, R. Impairment of neutrophil reactivity to elastin peptides in COPD. Thorax 2013, 68, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Gmiński, J. The VGVAPG Peptide Regulates the Production of Nitric Oxide Synthases and Reactive Oxygen Species in Mouse Astrocyte Cells In Vitro. Neurochem. Res. 2019, 44, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Gmiński, J.; Wȩglarz, L.; Dróżdż, M.; Goss, M. Pharmacological modulation of the antioxidant enzymes activities and the concentration of peroxidation products in fibroblasts stimulated with elastin peptides. Gen. Pharmacol. Vasc. Syst. 1991, 22, 495–497. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Selvakumar, G.P.; Zaheer, S.; Ahmed, M.E.; Raikwar, S.P.; Zahoor, H.; Saeed, D.; Natteru, P.A.; Iyer, S.; et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front. Cell. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Martin, H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2010, 690, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Baranek, T.; Debret, R.; Antonicelli, F.; Lamkhioued, B.; Belaaouaj, A.; Hornebeck, W.; Bernard, P.; Guenounou, M.; Le Naour, R. Elastin Receptor (Spliced Galactosidase) Occupancy by Elastin Peptides Counteracts Proinflammatory Cytokine Expression in Lipopolysaccharide-Stimulated Human Monocytes through NF- B Down-Regulation. J. Immunol. 2007, 179, 6184–6192. [Google Scholar] [CrossRef]
- Debret, R.; Le Naour, R.R.; Sallenave, J.-M.; Deshorgue, A.; Hornebeck, W.G.; Guenounou, M.; Bernard, P.; Antonicelli, F.D. Elastin fragments induce IL-1beta upregulation via NF-kappaB pathway in melanoma cells. J. Investig. Dermatol. 2006, 126, 1860–1868. [Google Scholar] [CrossRef]
- Chao, Y.H.; Yang, H.S.; Sun, M.G.; Sun, J.S.; Chen, M.H. Elastin-derived peptides induce inflammatory responses through the activation of NF-κB in human ligamentum flavum cells. Connect. Tissue Res. 2012, 53, 407–414. [Google Scholar] [CrossRef]
- Satta, J.; Laurila, A.; Pääkkö, P.; Haukipuro, K.; Sormunen, R.; Parkkila, S.; Juvonen, T. Chronic inflammation and elastin degradation in abdominal aortic aneurysm disease: An immunohistochemical and electron microscopic study. Eur. J. Vasc. Endovasc. Surg. 1998, 15, 313–319. [Google Scholar] [CrossRef]
- Kobayashi, K.; Jokaji, R.; Miyazawa-Hira, M.; Takatsuka, S.; Tanaka, A.; Ooi, K.; Nakamura, H.; Kawashiri, S. Elastin-derived peptides are involved in the processes of human temporomandibular disorder by inducing inflammatory responses in synovial cells. Mol. Med. Rep. 2017, 16, 3147–3154. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Gmiński, J. The Elastin-Derived Peptide VGVAPG Does Not Activate the Inflammatory Process in Mouse Cortical Astrocytes In Vitro. Neurotox. Res. 2020, 37, 136–145. [Google Scholar] [CrossRef]
- Luo, X.; Wu, J.; Wu, G. PPARγ activation suppresses the expression of MMP9 by downregulating NF-κB post intracerebral hemorrhage. Neurosci. Lett. 2021, 752, 135770. [Google Scholar] [CrossRef] [PubMed]
- Robinet, A. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J. Cell Sci. 2005, 118, 343–356. [Google Scholar] [CrossRef]
- Brassart, B.; Randoux, A.; Hornebeck, W.; Emonard, H. Regulation of matrix metalloproteinase-2 (gelatinase A, MMP-2), membrane-type matrix metalloproteinase-1 (MT1-MMP) and tissue inhibitor of metalloproteinases-2 (TIMP-2) expression by elastin-derived peptides in human HT-1080 fibrosarcoma cell line. Clin. Exp. Metastasis 1998, 16, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Donet, M.; Brassart-Pasco, S.; Salesse, S.; Maquart, F.-X.; Brassart, B. Elastin peptides regulate HT-1080 fibrosarcoma cell migration and invasion through an Hsp90-dependent mechanism. Br. J. Cancer 2014, 111, 139–148. [Google Scholar] [CrossRef]
- Ntayi, C.; Labrousse, A.L.; Debret, R.; Birembaut, P.; Bellon, G.; Antonicelli, F.; Hornebeck, W.; Bernard, P. Elastin-Derived Peptides Upregulate Matrix Metalloproteinase-2-ediated Melanoma Cell Invasion Through Elastin-Binding Protein. J. Investig. Dermatol. 2004, 122, 256–265. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).