The Effect of Neuropathy and Diabetes Type on Multisegment Foot Kinematics: A Cohort Study on 70 Participants with Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Statistics
3. Results
3.1. Effect of Covariates on Foot Joint ROM
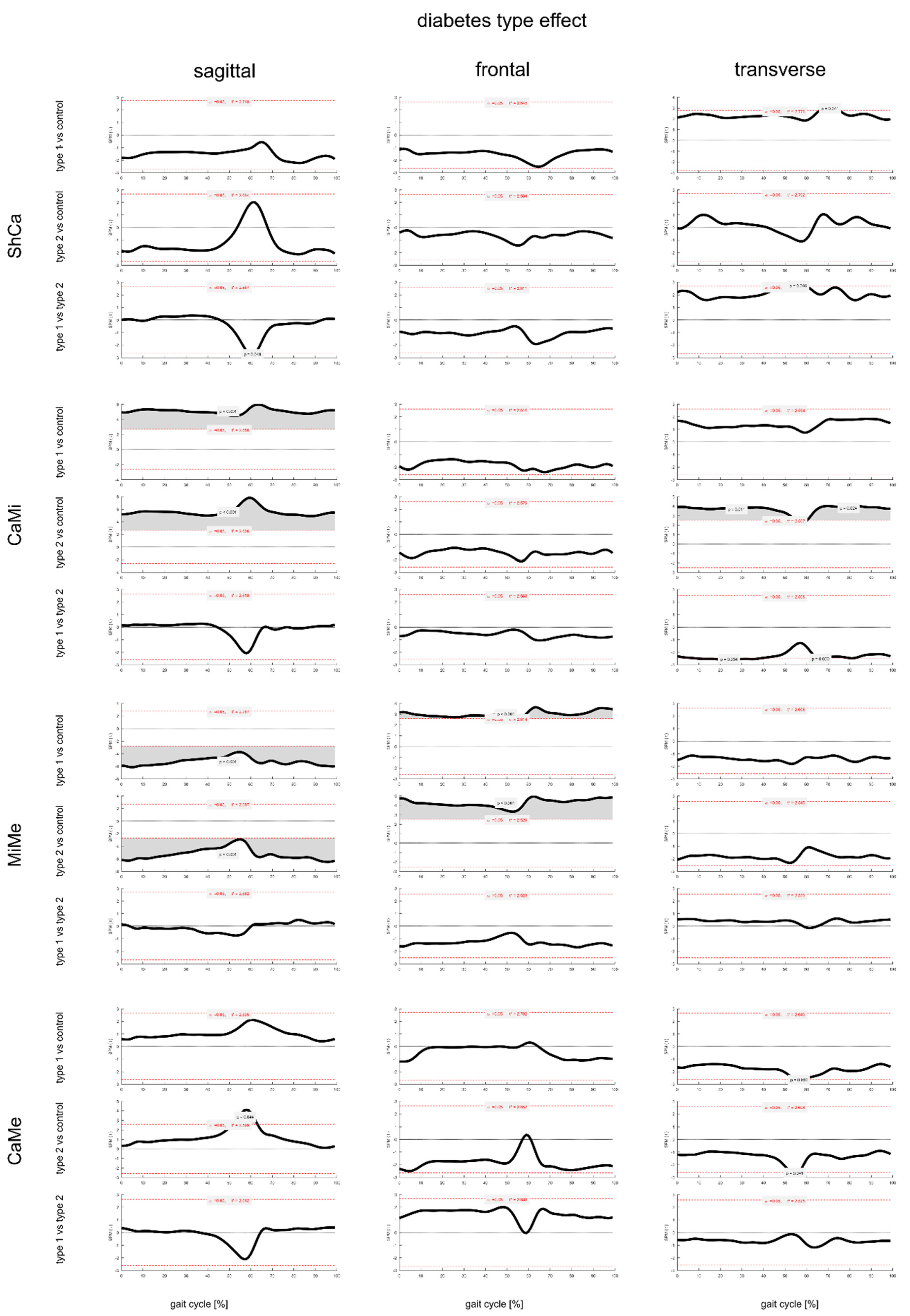
3.2. Effect of Diabetes Type on Foot Joint ROM and Gait Kinematics
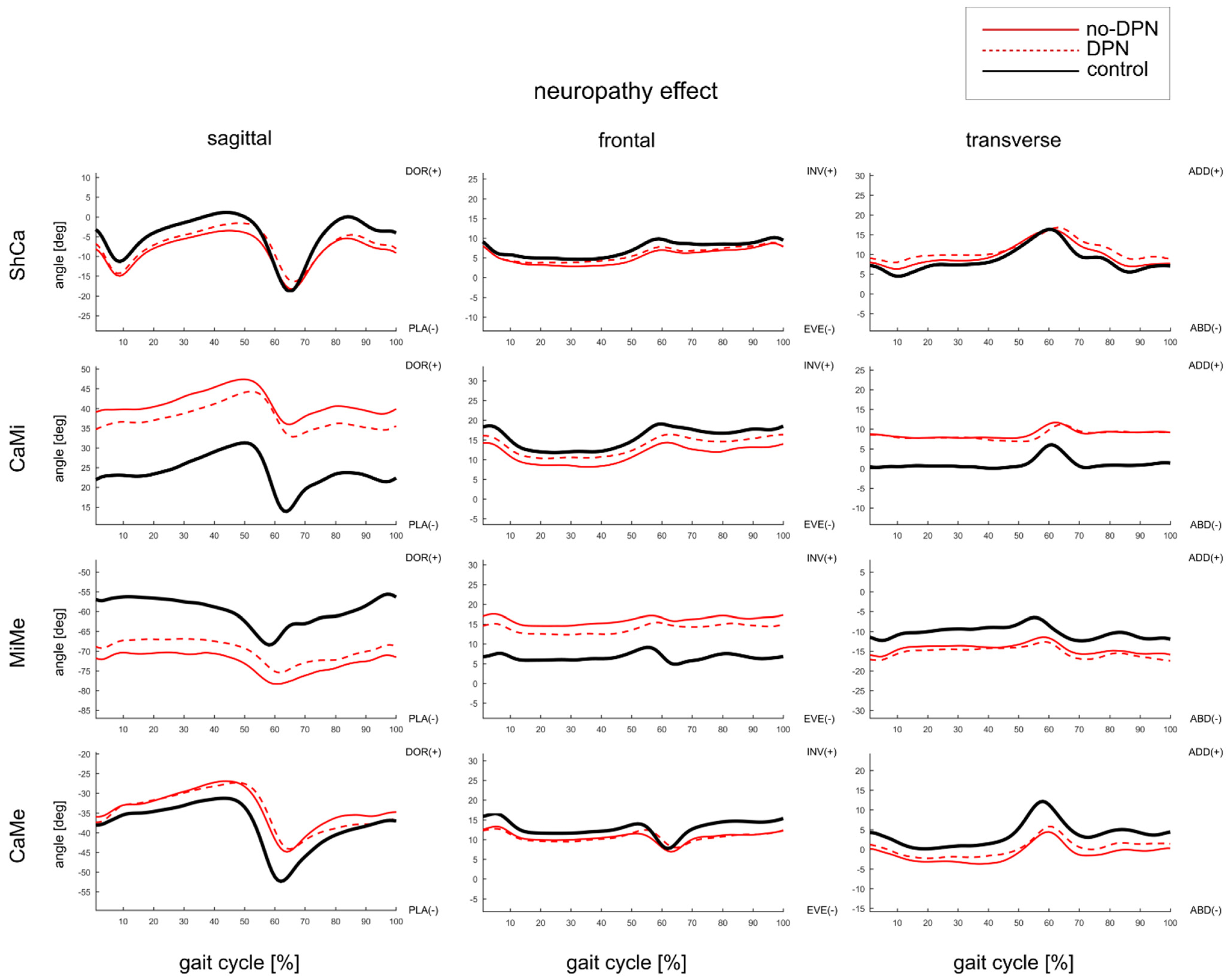
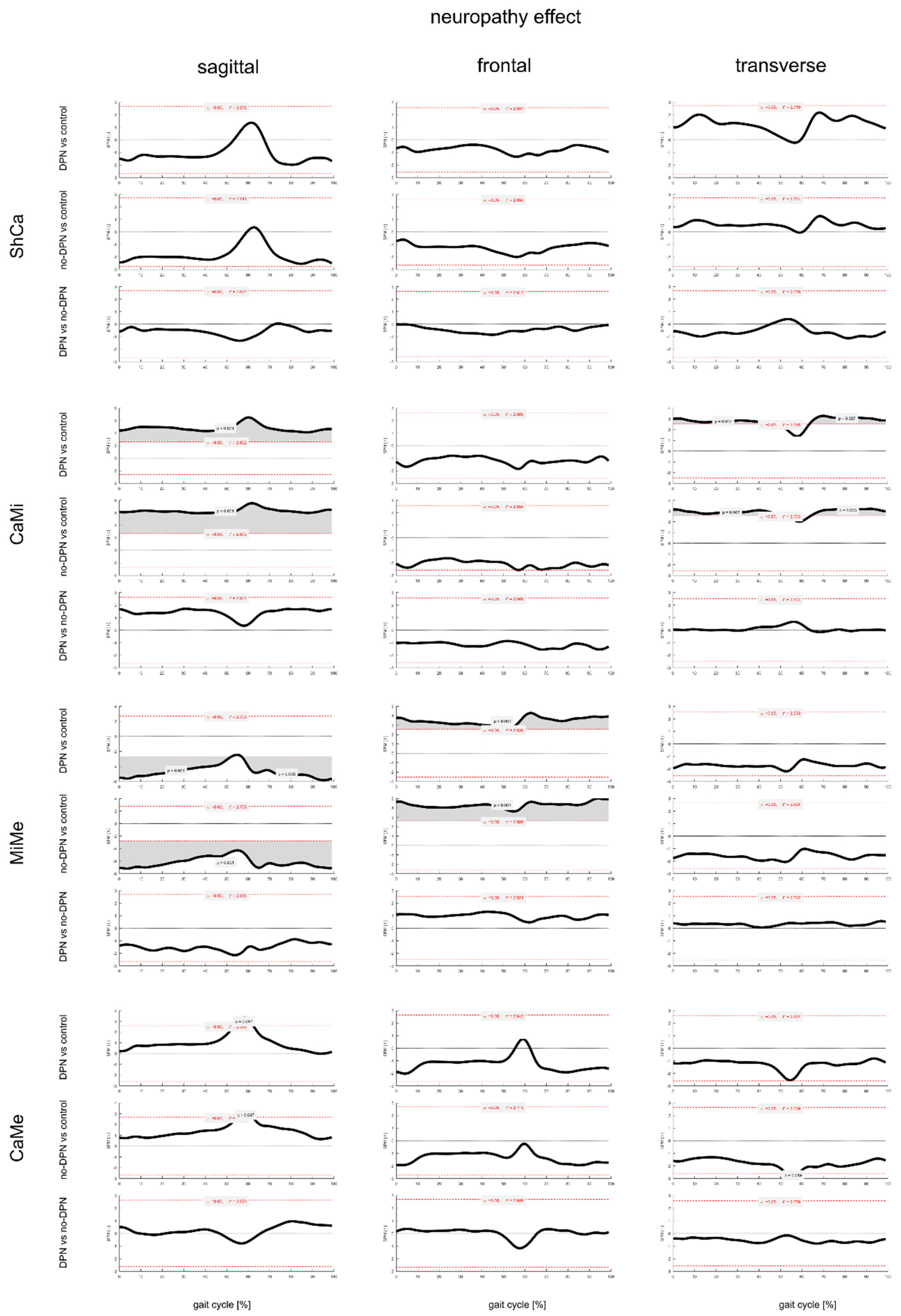
3.3. Effect of DPN on Foot Joints ROM and Gait Kinematics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lazzarini, P.A.; Pacella, R.E.; Armstrong, D.G.; Van Netten, J.J. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet. Med. J. Br. Diabet. Assoc. 2018, 35, 1297–1299. [Google Scholar] [CrossRef]
- Federation, I.D. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Monteiro-Soares, M.; Boyko, E.; Ribeiro, J.; Dinis-Ribeiro, M. Predictive factors for diabetic foot ulceration: A systematic review. Diabetes/Metabolism Res. Rev. 2012, 28, 574–600. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.M.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.; Spallone, V.; Vinik, A.; et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, H.; Gjerstad, M.D.; Jakobsen, J. Atrophy of Foot Muscles: A measure of diabetic neuropathy. Diabetes Care 2004, 27, 2382–2385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreassen, C.S.; Jakobsen, J.; Andersen, H. Muscle Weakness: A Progressive Late Complication in Diabetic Distal Symmetric Polyneuropathy. Diabetes 2006, 55, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Cheuy, V.; Hastings, M.; Commean, P.K.; Ward, S.R.; Mueller, M.J. Intrinsic foot muscle deterioration is associated with metatarsophalangeal joint angle in people with diabetes and neuropathy. Clin. Biomech. 2013, 28, 1055–1060. [Google Scholar] [CrossRef] [Green Version]
- Mahieu, R.; Coenen, M.; van Bemmel, T.; van der Zaag-Loonen, H.; Theuvenet, W. Detecting intrinsic muscle weakness of the hallux as an addition to early-stage screening of the feet in patients with diabetes. Diabetes Res. Clin. Pr. 2016, 119, 83–87. [Google Scholar] [CrossRef]
- Gomes, A.A.; Onodera, A.N.; Otuzi, M.E.; Pripas, D.; Mezzarane, R.A.; Sacco, I.C. Electromyography and kinematic changes of gait cycle at different cadences in diabetic neuropathic individuals. Muscle Nerve 2011, 44, 258–268. [Google Scholar] [CrossRef]
- Sacco, I.; Hamamoto, A.; Gomes, A.; Onodera, A.; Hirata, R.; Hennig, E. Role of ankle mobility in foot rollover during gait in individuals with diabetic neuropathy. Clin. Biomech. 2009, 24, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Salsich, G.B.; Brown, M.; Mueller, M.J. Relationships between Plantar Flexor Muscle Stiffness, Strength, and Range of Motion in Subjects With Diabetes-Peripheral Neuropathy Compared to Age-Matched Controls. J. Orthop. Sports Phys. Ther. 2000, 30, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinacore, D.R.; Gutekunst, D.J.; Hastings, M.K.; Strube, M.J.; Bohnert, K.L.; Prior, F.W.; Johnson, J.E. Neuropathic midfoot deformity: Associations with ankle and subtalar joint motion. J. Foot Ankle Res. 2013, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Murphy-Lavoie, H.M.; Ramsey, A.; Nguyen, M.; Singh, S. Diabetic Foot Infections. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Giacomozzi, C.; Sartor, C.D.; Telles, R.; Uccioli, L.; Sacco, I.C.N. Ulcer-risk classification and plantar pressure distribution in patients with diabetic polyneuropathy: Exploring the factors that can lead to foot ulceration. Annali dell’Istituto Superiore di Sanità 2018, 54, 284–293. [Google Scholar] [PubMed]
- DiLiberto, F.E.; Tome, J.; Baumhauer, J.F.; Quinn, J.R.; Houck, J.; Nawoczenski, D.A. Multi-joint foot kinetics during walking in people with Diabetes Mellitus and peripheral neuropathy. J. Biomech. 2015, 48, 3679–3684. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Saltzman, C.; Yack, H.J. Segmental foot mobility in individuals with and without diabetes and neuropathy. Clin. Biomech. 2007, 22, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Saltzman, C.L.; Yack, H.J. Relationships between segmental foot mobility and plantar loading in individuals with and without diabetes and neuropathy. Gait Posture 2010, 31, 251–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacco, I.C.; Picon, A.P.; Macedo, D.O.; Butugan, M.K.; Watari, R.; Sartor, C. Alterations in the Lower Limb Joint Moments Precede the Peripheral Neuropathy Diagnosis in Diabetes Patients. Diabetes Technol. Ther. 2015, 17, 405–412. [Google Scholar] [CrossRef]
- Watari, R.; Sartor, C.D.; Picon, A.P.; Butugan, M.K.; Amorim, C.F.; Ortega, N.R.S.; Sacco, I.C.N. Effect of diabetic neuropathy severity classified by a fuzzy model in muscle dynamics during gait. J. Neuroeng. Rehabil. 2014, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, K.; Matricali, G.; Roosen, P.; Nobels, F.; Tits, J.; Desloovere, K.; Bruyninckx, H.; Flour, M.; Deleu, P.-A.; Verhoeven, W.; et al. Comparison of foot segmental mobility and coupling during gait between patients with diabetes mellitus with and without neuropathy and adults without diabetes. Clin. Biomech. 2013, 28, 813–819. [Google Scholar] [CrossRef]
- Sawacha, Z.; Cristoferi, G.; Guarneri, G.; Corazza, S.; Donà, G.; Denti, P.; Facchinetti, A.; Avogaro, A.; Cobelli, C. Characterizing multisegment foot kinematics during gait in diabetic foot patients. J. Neuroeng. Rehabil. 2009, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Sawacha, Z.; Gabriella, G.; Cristoferi, G.; Guiotto, A.; Avogaro, A.; Cobelli, C. Diabetic gait and posture abnormalities: A biomechanical investigation through three dimensional gait analysis. Clin. Biomech. 2009, 24, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.J.; Masson, E.A.; Veves, A.; Boulton, A.J. Relationship of Limited Joint Mobility to Abnormal Foot Pressures and Diabetic Foot Ulceration. Diabetes Care 1991, 14, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.-Y.; Minor, S.D.; Maluf, K.S.; Mueller, M.J. Comparison of muscle activity during walking in subjects with and without diabetic neuropathy. Gait Posture 2003, 18, 105–113. [Google Scholar] [CrossRef]
- Sacco, I.; Amadio, A. Influence of the diabetic neuropathy on the behavior of electromyographic and sensorial responses in treadmill gait. Clin. Biomech. 2003, 18, 426–434. [Google Scholar] [CrossRef]
- Bus, S.A.; Yang, Q.X.; Wang, J.H.; Smith, M.B.; Wunderlich, R.; Cavanagh, P.R. Intrinsic Muscle Atrophy and Toe Deformity in the Diabetic Neuropathic Foot: A magnetic resonance imaging study. Diabetes Care 2002, 25, 1444–1450. [Google Scholar] [CrossRef] [Green Version]
- Akashi, P.M.; Sacco, I.C.; Watari, R.; Hennig, E. The effect of diabetic neuropathy and previous foot ulceration in EMG and ground reaction forces during gait. Clin. Biomech. 2008, 23, 584–592. [Google Scholar] [CrossRef]
- Caravaggi, P.; Leardini, A.; Crompton, R. Kinematic correlates of walking cadence in the foot. J. Biomech. 2010, 43, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Caravaggi, P.; Pataky, T.; Günther, M.; Savage, R.; Crompton, R. Dynamics of longitudinal arch support in relation to walking speed: Contribution of the plantar aponeurosis. J. Anat. 2010, 217, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.; Stevens, M.J.; Thomas, P.K.; Brown, M.B.; Canal, N.; Greene, D.A. A Practical Two-Step Quantitative Clinical and Electrophysiological Assessment for the Diagnosis and Staging of Diabetic Neuropathy. Diabetes Care 1994, 17, 1281–1289. [Google Scholar] [CrossRef]
- Portinaro, N.; Leardini, A.; Panou, A.; Monzani, V.; Caravaggi, P. Modifying the Rizzoli foot model to improve the diagnosis of pes-planus: Application to kinematics of feet in teenagers. J. Foot Ankle Res. 2014, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leardini, A.; Benedetti, M.G.; Berti, L.; Bettinelli, D.; Nativo, R.; Giannini, S. Rear-foot, mid-foot and fore-foot motion during the stance phase of gait. Gait Posture 2007, 25, 453–462. [Google Scholar] [CrossRef]
- Grood, E.S.; Suntay, W.J. A Joint Coordinate System for the Clinical Description of Three-Dimensional Motions: Application to the Knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Vector field statistical analysis of kinematic and force trajectories. J. Biomech. 2013, 46, 2394–2401. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Belvedere, C.; Giacomozzi, C.; Carrara, C.; Lullini, G.; Caravaggi, P.; Berti, L.; Marchesini, G.; Baccolini, L.; Durante, S.; Leardini, A. Correlations between weight-bearing 3D bone architecture and dynamic plantar pressure measurements in the diabetic foot. J. Foot Ankle Res. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Arnold, J.B.; Mackintosh, S.; Jones, S.; Thewlis, D. Differences in foot kinematics between young and older adults during walking. Gait Posture 2014, 39, 689–694. [Google Scholar] [CrossRef] [PubMed]
- A Ranger, T.; Wong, A.M.Y.; Cook, J.L.; Gaida, J.E. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br. J. Sports Med. 2015, 50, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Giacomozzi, C.; D’Ambrogi, E.; Uccioli, L.; Macellari, V. Does the thickening of Achilles tendon and plantar fascia contribute to the alteration of diabetic foot loading? Clin. Biomech. 2005, 20, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Ledoux, W.R. The compressive mechanical properties of diabetic and non-diabetic plantar soft tissue. J. Biomech. 2010, 43, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.C.; Frykberg, R.G.; Armstrong, D.G.; Boulton, A.J.M.; Edmonds, M.; Van, G.H.; Hartemann, A.; Game, F.; Jeffcoate, W.; Jirkovska, A.; et al. The Charcot foot in diabetes. J. Am. Podiatr. Med Assoc. 2011, 101, 437–446. [Google Scholar] [CrossRef]
- D’Ambrogi, E.; Giacomozzi, C.; Macellari, V.; Uccioli, L. Abnormal foot function in diabetic patients: The altered onset of Windlass mechanism. Diabet. Med. 2005, 22, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.B.; Caravaggi, P.; Leardini, A.; Taddei, U.T.; Ortolani, M.; Sacco, I. Repeatability of skin-markers based kinematic measures from a multi-segment foot model in walking and running. J. Biomech. 2020, 110, 109983. [Google Scholar] [CrossRef]
- Caravaggi, P.; Benedetti, M.G.; Berti, L.; Leardini, A. Repeatability of a multi-segment foot protocol in adult subjects. Gait Posture 2011, 33, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Van Netten, J.J.; Hinchliffe, R.J.; Apelqvist, J.; Lipsky, B.A.; Schaper, N.C. IWGDF Editorial Board Standards for the Development and Methodology of the 2019 International Working Group on the Diabetic Foot Guidelines. Diabetes/Metabolism Res. Rev. 2020, 36. [Google Scholar] [CrossRef] [PubMed]

| Type 1 | Type 2 | DPN | Non-DPN | Control | Type 1 vs. Type 2 vs. Control | DPN vs. Non-DPN vs. Control | |
|---|---|---|---|---|---|---|---|
| group size [#] | 25 | 45 | 40 | 30 | 27 | NA | NA |
| gender (#M/#F) | 13/12 | 25/20 | 22/18 | 15/15 | 11/16 | NA | NA |
| age [yrs] | 50 ± 15 ^ | 62 ± 8 *^ | 60 ± 11 * | 55 ± 13 | 53 ± 9 | p < 0.001 | p < 0.05 |
| BMI [kg/m2] | 24 ± 3 ^ | 31 ± 6 *^ | 29 ± 6 * | 28 ± 7 * | 24 ± 4 | p < 0.001 | p < 0.01 |
| diabetes duration [yrs] | 29 ± 14 ^ | 14 ± 8 ^ | 21 ± 13 | 17 ± 12 | NA | NA | NA |
| normalized walking speed [s−1] | 0.73 ± 0.14 ^ | 0.60 ± 0.11 *^ | 0.62 ± 0.14 * | 0.68 ± 0.13 * | 0.75 ± 0.10 | p < 0.001 | p < 0.001 |
| Type 1 | Type 2 | DPN | Non-DPN | Control | Type 1 vs. Type 2 vs. Control | DPN vs. Non-DPN vs. Control | |
|---|---|---|---|---|---|---|---|
| cadence [step/min] | 55 ± 5 ^ | 52 ± 5 *^ | 52 ± 6 * | 55 ± 5 | 56 ± 5 | p < 0.01 | p < 0.05 |
| gait cycle time [s] | 1.09 ± 0.10 ^ | 1.17 ± 0.15 *^ | 1.17 ± 0.16* | 1.11 ± 0.10 | 1.08 ± 0.08 | p < 0.01 | p < 0.05 |
| stance time [% gait cycle] | 60.5 ± 1.4 ^ | 62.7 ± 2.2 *^ | 62.3 ± 2.4 * | 61.4 ± 2.4 | 60.5 ± 1.3 | p < 0.001 | p < 0.001 |
| swing time [% gait cycle] | 39.7 ± 1.9 ^ | 37.1 ± 2.2 *^ | 37.7 ± 2.4 ^* | 38.6 ± 2.4 ^* | 39.5 ± 1.28 | p < 0.001 | p < 0.01 |
| stride nomalized [% height] | 78.8 ± 11.7 * | 68.8 ± 8.8 * | 71.2 ± 11.3 * | 74.0 ± 10.5 * | 79.8 ± 0.2 | p < 0.001 | p < 0.001 |
| walking speed [m/s] | 1.21 ± 0.23 ^ | 1.02 ± 0.21 ^* | 1.07 ± 0.25 * | 1.10 ± 0.21 * | 1.28 ± 0.18 | p < 0.001 | p < 0.001 |
| Type 1 | Type 2 | Control | ANCOVA (p < 0.05) | |||||
|---|---|---|---|---|---|---|---|---|
| Joint | Plane | Mean | STD | Mean | STD | Mean | STD | |
| ShFo | Sagittal | 22.6 | 4.2 | 21.2 | 3.5 | 25.2 | 3.4 | Control > type1 Control > type2 |
| Frontal | 12.0 | 3.0 | 12.5 | 3.2 | 11.9 | 2.3 | ||
| Transverse | 16.0 | 3.9 | 13.9 | 4.1 | 18.4 | 3.8 | Control > type2 | |
| ShCa | Sagittal | 16.2 | 2.9 | 14.8 | 2.9 | 16.6 | 3.7 | |
| Frontal | 7.0 | 2.0 | 6.8 | 1.8 | 6.8 | 1.5 | ||
| Transverse | 12.3 | 3.1 | 11.2 | 3.8 | 13.6 | 3.3 | ||
| CaMi | Sagittal | 13.2 | 4.3 | 14.3 | 4.2 | 17.0 | 4.7 | Control > type1 Control > type2 |
| Frontal | 7.9 | 1.8 | 8.6 | 2.4 | 9.5 | 2.0 | Control > type1 | |
| Transverse | 7.8 | 2.5 | 7.2 | 2.3 | 8.6 | 2.5 | ||
| MiMe | Sagittal | 11.6 | 2.9 | 12.6 | 4.1 | 15.5 | 3.5 | Control > type1 Control > type2 |
| Frontal | 6.1 | 1.2 | 6.0 | 1.6 | 6.0 | 1.5 | ||
| Transverse | 7.4 | 1.7 | 8.9 | 2.9 | 8.5 | 2.9 | ||
| CaMe | Sagittal | 19.6 | 4.4 | 19.4 | 5.6 | 23.1 | 4.7 | Control > type1 Control > type2 |
| Frontal | 9.1 | 2.4 | 8.7 | 3.1 | 10.6 | 2.7 | ||
| Transverse | 11.1 | 3.1 | 11.4 | 3.7 | 15.1 | 3.9 | ||
| 1st MTPj | Sagittal | 44.2 | 7.3 | 38.9 | 8.2 | 41.0 | 8.1 | type1 > type2 |
| DPN | Non-DPN | Control | DPN vs. Non-DPN vs. Control | |||||
|---|---|---|---|---|---|---|---|---|
| Joint | Plane | Mean | STD | Mean | STD | Mean | STD | |
| ShFo | Sagittal | 21.4 | 4.0 | 22.2 | 3.5 | 25.2 | 3.4 | Control > DPN Control > non-DPN |
| Frontal | 12.8 | 3.0 | 11.7 | 3.2 | 11.9 | 2.3 | ||
| Transverse | 14.0 | 4.1 | 15.5 | 4.2 | 18.4 | 3.8 | Control > DPN Control > non-DPN | |
| ShCa | Sagittal | 15.4 | 3.3 | 15.2 | 2.6 | 16.6 | 3.7 | |
| Frontal | 6.8 | 1.8 | 7.0 | 2.0 | 6.8 | 1.5 | ||
| Transverse | 11.1 | 3.0 | 12.2 | 4.2 | 13.6 | 3.3 | ||
| CaMi | Sagittal | 13.9 | 4.2 | 13.8 | 4.2 | 17.0 | 4.7 | Control > DPN Control > non-DPN |
| Frontal | 8.5 | 2.5 | 8.1 | 1.7 | 9.5 | 2.0 | Control > non-DPN | |
| Transverse | 7.3 | 2.1 | 7.6 | 2.7 | 8.6 | 2.5 | ||
| MiMe | Sagittal | 12.2 | 4.0 | 12.3 | 3.5 | 15.5 | 3.5 | Control > DPN Control > non-DPN |
| Frontal | 5.9 | 1.5 | 6.1 | 1.4 | 6.0 | 1.5 | ||
| Transverse | 8.3 | 2.7 | 8.4 | 2.6 | 8.5 | 2.9 | ||
| CaMe | Sagittal | 19.0 | 5.5 | 20.0 | 4.7 | 23.1 | 4.7 | Control > DPN Control > non-DPN (p = 0.06) |
| Frontal | 8.6 | 2.8 | 9.1 | 2.9 | 10.6 | 2.7 | ||
| Transverse | 11.3 | 4.1 | 11.2 | 2.4 | 15.1 | 3.9 | Control > DPN Control > non-DPN | |
| 1st MTPj | Sagittal | 40.1 | 8.8 | 41.7 | 7.5 | 41.0 | 8.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caravaggi, P.; Giacomozzi, C.; Lullini, G.; Marchesini, G.; Baccolini, L.; Ortolani, M.; Sacco, I.C.N.; Berti, L.; Leardini, A. The Effect of Neuropathy and Diabetes Type on Multisegment Foot Kinematics: A Cohort Study on 70 Participants with Diabetes. Appl. Sci. 2021, 11, 8848. https://doi.org/10.3390/app11198848
Caravaggi P, Giacomozzi C, Lullini G, Marchesini G, Baccolini L, Ortolani M, Sacco ICN, Berti L, Leardini A. The Effect of Neuropathy and Diabetes Type on Multisegment Foot Kinematics: A Cohort Study on 70 Participants with Diabetes. Applied Sciences. 2021; 11(19):8848. https://doi.org/10.3390/app11198848
Chicago/Turabian StyleCaravaggi, Paolo, Claudia Giacomozzi, Giada Lullini, Giulio Marchesini, Luca Baccolini, Maurizio Ortolani, Isabel C. N. Sacco, Lisa Berti, and Alberto Leardini. 2021. "The Effect of Neuropathy and Diabetes Type on Multisegment Foot Kinematics: A Cohort Study on 70 Participants with Diabetes" Applied Sciences 11, no. 19: 8848. https://doi.org/10.3390/app11198848
APA StyleCaravaggi, P., Giacomozzi, C., Lullini, G., Marchesini, G., Baccolini, L., Ortolani, M., Sacco, I. C. N., Berti, L., & Leardini, A. (2021). The Effect of Neuropathy and Diabetes Type on Multisegment Foot Kinematics: A Cohort Study on 70 Participants with Diabetes. Applied Sciences, 11(19), 8848. https://doi.org/10.3390/app11198848








