Effects of Wounding Stress and Storage Temperature on the Accumulation of Chlorogenic Acid Isomers in Potatoes (Solanum tuberosum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Plant Material, Processing, and Storage Studies
2.2. Identification and Quantification of Individual Phenolic Compounds by High-Performance Liquid Chromatography–Diode Array Detection (HPLC–DAD)
2.3. Statistical Analysis
3. Results
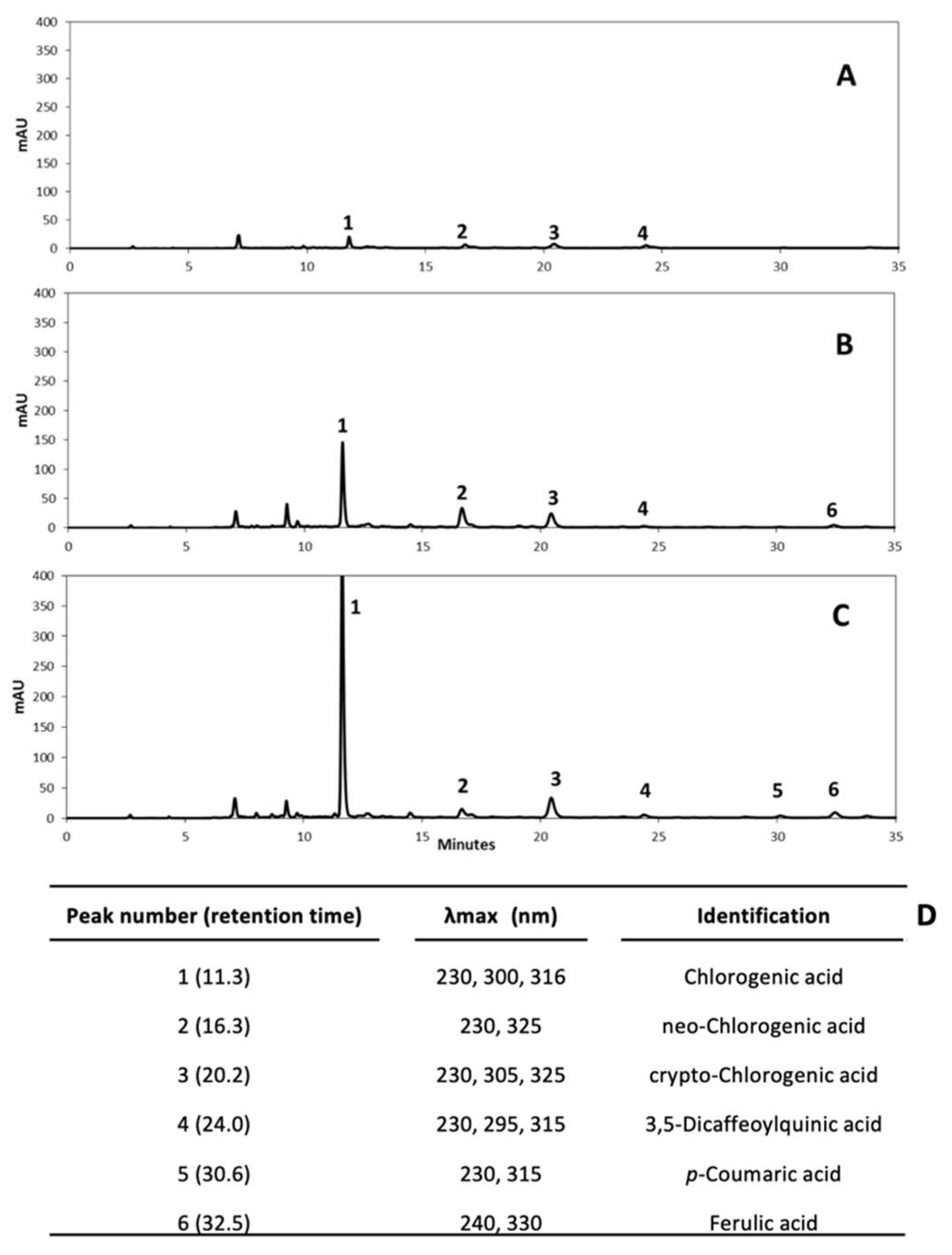
3.1. Identification of Individual Phenolic Compounds in Whole and Wounded Potatoes
3.2. Effects of Wounding Stress and Storage Temperature on the Accumulation of Individual Phenolic Compounds in Potatoes
4. Discussion
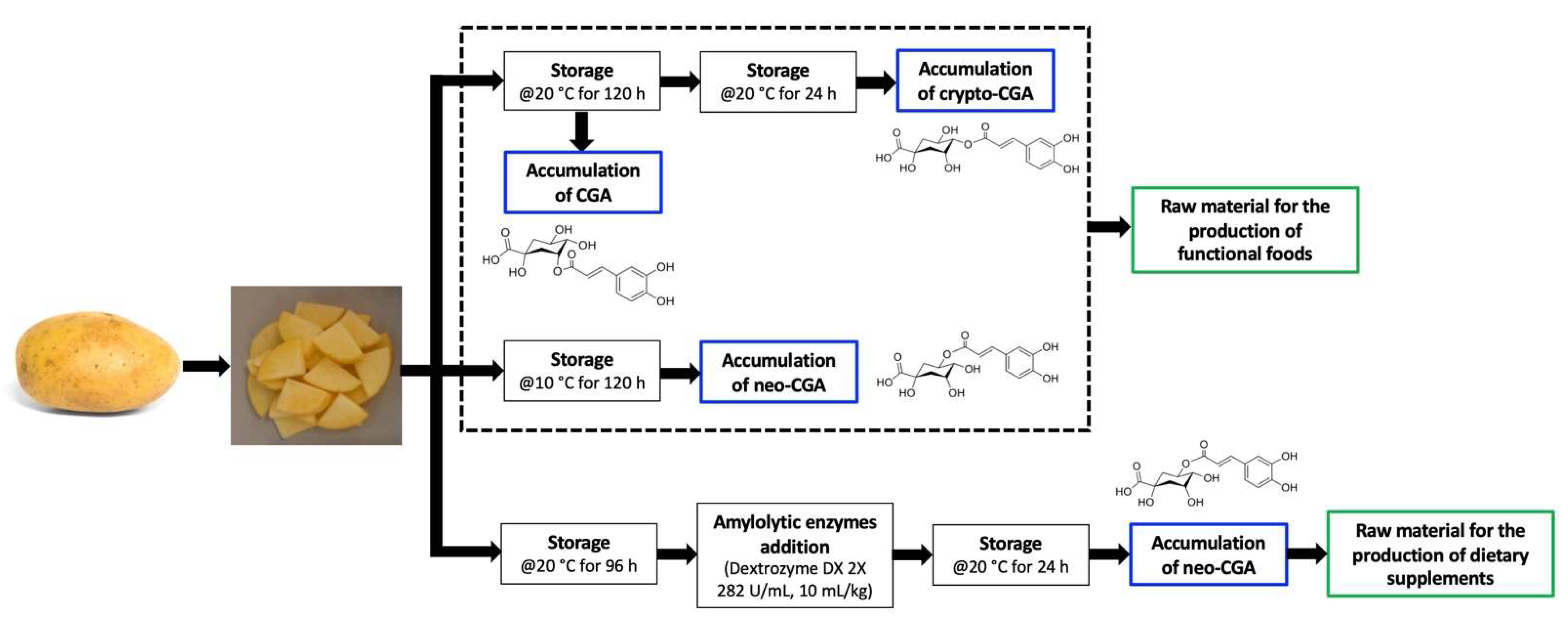
Practical Approaches for the Use of Potatoes as Biofactories of CGA Isomers and Its Potential Use as Raw Material or in the Production of Functional Foods and Ingredients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santana-Gálvez, J.; Jacobo-Velázquez, D.A. Classification of phenolic compounds. In Phenolic Compounds in Food, 1st ed.; Nollet, L.M.L., Gutierrez-Uribe, J.A., Eds.; CRC Press: New York, NY, USA, 2017; pp. 3–21. [Google Scholar]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Controlled abiotic stresses revisited: From homeostasis through hormesis to extreme stresses and the impact on nutraceuticals and quality during pre- and postharvest applications in horticultural crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- Santana-Gálvez, J.; Villela-Castrejón, J.; Serna-Saldívar, S.O.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Synergistic combinations of curcumin, sulforaphane, and dihydrocaffeic acid against human colon cancer cells. Int. J. Mol. Sci. 2020, 21, 3108. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Villela-Castrejón, J.; Serna-Saldívar, S.O.; Jacobo-Velázquez, D.A. Anticancer potential of dihydrocaffeic acid: A chlorogenic acid metabolite. CyTA-J. Food 2020, 18, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Luque-Badillo, A.C.; Hernandez-Tapia, G.; Ramirez-Castillo, D.A.; Espinoza-Serrano, D.; Cortes-Limon, A.M.; Cortes-Gallardo, J.P.; Jacobo-Velázquez, D.A.; Martinez-Fierro, M.L.; Rios-Ibarra, C.P. Gold nanoparticles enhance microRNA 31 detection in colon cancer cells after inhibition with chlorogenic acid. Oncol. Lett. 2021, 22, 742. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Stress-induced production of chlorogenic acid isomers in potato tubers as affected by wounding intensity and storage time. Ind. Crop. Prod. 2014, 62, 61–66. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Effect of exogenous amylolytic enzymes on the accumulation of chlorogenic acid isomers in wounded potato tubers. J. Agric. Food. Chem. 2014, 62, 7671–7675. [Google Scholar] [CrossRef]
- Ren, X.; Tang, T.; Xie, X.; Wang, W.; Tang, X.; Brennan, C.S.; Zhang, J.; Wang, Z. The effect of wounding intensities on vitamins and antioxidant enhancement in potato products. Int. J. Food Sci. Tech. 2021, 56, 2325–2335. [Google Scholar] [CrossRef]
- Gamborg, O.L. Aromatic metabolism in plants: V. The biosynthesis of chlorogenic acid and lignin in potato cell cultures. Can. J. Biochem. 1967, 45, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.B.; Kolattukudy, P.E. Biochemistry of suberization. Incorporation of [1-14C] oleic acid and [1-14C] acetate into the aliphatic components of suberin in potato tuber disks (Solanum tuberosum). Plant Physiol. 1977, 59, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Lulai, E.C.; Corsini, D.L. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiol. Mol. Plant. Pathol. 1998, 53, 209–222. [Google Scholar] [CrossRef]
- Bernards, M.A.; Susag, L.M.; Bedgar, D.L.; Anterola, A.M.; Lewis, N.G. Induced phenylpropanoid metabolism during suberization and lignification: A comparative analysis. J. Plant Physiol. 2000, 157, 601–607. [Google Scholar] [CrossRef]
- Bernards, M.A.; Razem, F.A. The poly(phenolic) domain of potato suberin: A non-lignin cell wall bio-polymer. Phytochemistry 2001, 57, 1115–1122. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Valiñas, M.A.; Lanteri, M.L.; Ten Have, A.; Andreu, A.B. Chlorogenic acid biosynthesis appears linked with suberin production in potato tuber (Solanum tuberosum). J. Agric. Food Chem. 2015, 63, 4902–4913. [Google Scholar] [CrossRef]
- Yang, R.; Han, Z.; Ackah, S.; Li, Z.; Bi, Y.; Yang, Q.; Prusky, D. Hot water dipping stimulated wound healing of potato tubers. Postharvest Biol. Technol. 2020, 167, 111245. [Google Scholar] [CrossRef]
- Ramamurthy, M.S.; Maiti, B.; Thomas, P.; Nair, P.M. High-performance liquid chromatography determination of phenolic acids in potato tubers (Solanum tuberosum) during wound healing. J. Agric. Food Chem. 1992, 40, 569–572. [Google Scholar] [CrossRef]
- Navarre, D.A.; Pillai, S.S.; Shakya, R.; Holden, M.J. HPLC profiling of phenolics in diverse potato genotypes. Food Chem. 2011, 127, 34–41. [Google Scholar] [CrossRef]
- Reyes, L.F.; Cisneros-Zevallos, L. Wounding stress increases the phenolic content and antioxidant capacity of purple-flesh potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2003, 51, 5296–5300. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Boudet, A.M. Lignins and lignification: Selected issues. Plant Physiol. Bioch. 2000, 38, 81–96. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front. Plant Sci. 2015, 6, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.M.; Bolwell, G.P.; Smith, C. Wound-induced phenylalanine ammonia-lyase in potato (Solanum tuberosum) tuber discs. Significance of glycosylation and immunolocalization of enzyme subunits. Biochem. J. 1990, 267, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantos, E.; Tudela, J.A.; Gil, M.I.; Espín, J.C. Phenolic compounds and related enzymes are not rate-limiting in browning development of fresh-cut potatoes. J. Agric. Food Chem. 2002, 50, 3015–3023. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Xu, C.; Li, X.; Ferguson, I.; Chen, K. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]
- Payyavula, R.S.; Shakya, R.; Sengoda, V.G.; Munyaneza, J.E.; Swamy, P.; Navarre, D.A. Synthesis and regulation of chlorogenic acid in potato: Rerouting phenylpropanoid flux in HQT-silenced lines. Plant Biotechnol. J. 2015, 13, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Koshiro, Y.; Jackson, M.C.; Katahira, R.; Wang, M.; Nagai, C.; Ashihara, H. Biosynthesis of chlorogenic acids in growing and ripening fruits of Coffea arabica and Coffea canephora plants. Z. Naturforsch. C 2007, 62, 731. [Google Scholar] [CrossRef]
- Van der Rest, B.; Danoun, S.; Boudet, A.M.; Rochange, S.F. Down-regulation of cinnamoyl-CoA reductase in tomato (Solanum lycopersicum L.) induces dramatic changes in soluble phenolic pools. J. Exp. Bot. 2006, 57, 1399–1411. [Google Scholar] [CrossRef] [Green Version]
- Chabannes, M.; Barakate, A.; Lapierre, C.; Marita, J.M.; Ralph, J.; Pean, M.; Danoun, S.; Halpin, C.; Grima-Pettenati, J.; Boudet, A.M. Strong decrease in lignin content without significant alteration of plant development is induced by simultaneous down-regulation of cinnamoyl CoA reductase (CCR) and cinnamyl alcohol dehydrogenase (CAD) in tobacco plants. Plant J. 2001, 28, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Graça, J. Hydroxycinnamates in suberin formation. Phytochem. Rev. 2010, 9, 85–91. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Santana-Gálvez, J.; Cisneros-Zevallos, L. Designing next-generation functional food and beverages: Combining nonthermal processing technologies and postharvest abiotic stresses. Food Eng. Rev. 2020, in press. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Pérez-Carrillo, E.; Velázquez-Reyes, H.H.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Application of wounding stress to produce a nutraceutical-rich carrot powder ingredient and its incorporation to nixtamalized corn flour tortillas. J. Funct. Foods 2016, 27, 655–666. [Google Scholar] [CrossRef]
- Alvarado-Ramírez, M.; Santana-Gálvez, J.; Santacruz, A.; Carranza-Montealvo, L.D.; Ortega-Hernández, E.; Tirado-Escobosa, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Using a functional carrot powder ingredient to produce sausages with high levels of nutraceuticals. J. Food Sci. 2018, 83, 2351–2361. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Postharvest wounding stress in horticultural crops as a tool for designing novel functional foods and beverages with enhanced nutraceutical content: Carrot juice as a case study. J. Food Sci. 2019, 84, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, J.M.; Santana-Gálvez, J.; Aguilera-González, C.; Santacruz, A.; Amaya-Guerra, C.A.; Jacobo-Velázquez, D.A. Effects of carrot puree with enhanced levels of chlorogenic acid on rat cognitive abilities and neural development. CyTA-J. Food 2020, 18, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Viacava, F.; Santana-Gálvez, J.; Heredia-Olea, E.; Pérez-Carrillo, E.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Sequential application of postharvest wounding stress and extrusion as an innovative tool to increase the concentration of free and bound phenolics in carrots. Food Chem. 2020, 307, 125551. [Google Scholar] [CrossRef]
- Santos, D.I.; Faria, D.L.; Lourenço, S.C.; Alves, V.D.; Saraiva, J.A.; Vicente, A.A.; Moldão-Martins, M. Heat treatment and wounding as abiotic stresses to enhance the bioactive composition of pineapple by-products. Appl. Sci. 2021, 11, 4313. [Google Scholar] [CrossRef]
| Temperature (°C) | Sample | Concentration (mg/kg) 1,2 | ||||
|---|---|---|---|---|---|---|
| Storage Time (h) | CGA | neo-CGA | crypto-CGA | 3,5-diCQA | ||
| Control 3 | 0 | 8.14 ± 0.6 f | 7.99 ± 0.3 e | 12.19 ± 0.9 f,g | 8.11 ± 0.4 d,e | |
| 10 | Wholes | 96 | 9.24 ± 0.4 e,f | 7.75 ± 0.2 e | 15.59 ± 0.5 c,d,e,f | 7.55 ± 0.1 e,f |
| 120 | 12.15 ± 0.4 e,f | 22.51 ± 0.9 c,d | 11.43 ± 0.6 g | 7.58 ± 0.1 e,f | ||
| 144 | 11.26 ± 0.4 e,f | 18.37 ± 0.4 d | 11.00 ± 0.3 g | 9.29 ± 0.3 b,c | ||
| 168 | 10.69 ± 0.3 e,f | 22.53 ± 1.2 c,d | 11.66 ± 0.7 g | 9.04 ± 0.6 c | ||
| Pie-cuts | 96 | 49.81 ± 3.4 d | 21.12 ± 0.8 d | 18.12 ± 1.4 b,c,d | 6.92 ± 0.1 f | |
| 120 | 134.0 ± 9.7 b,c | 64.90 ± 5.7 a | 15.64 ± 0.4 c,d,e,f | 7.45 ± 0.2 e,f | ||
| 144 | 133.14 ± 10.8 b,c | 47.95 ± 4.3 b | 18.18 ± 4.0 b,c,d | 7.40 ± 0.2 e,f | ||
| 168 | 139.12 ± 6.0 b | 61.63 ± 6.6 a | 16.62 ± 1.4 c,d,e | 7.20 ± 0.0 f | ||
| 20 | Wholes | 96 | 10.42 ± 0.4 e,f | 8.92 ± 0.2 e | 14.53 ± 1.1 d,e,f,g | 9.00 ± 0.1 c |
| 120 | 21.44 ± 1.3 e | 24.39 ± 0.5 c,d | 13.59 ± 0.3 e,f,g | 10.08 ± 0.4 a,b | ||
| 144 | 13.12 ± 1.0 e,f | 23.56 ± 4.0 c,d | 22.55 ± 1.1 a | 10.77 ± 0.6 a | ||
| 168 | 12.19 ± 0.4 e,f | 29.22 ± 1.5 c | 13.34 ± 0.9 e,f,g | 9.89 ± 0.3 b | ||
| Pie-cuts | 96 | 121.83 ± 4.1 c | 17.68 ± 3.2 d | 25.41 ± 2.5 a | 8.07 ± 0.3 d,e | |
| 120 | 164.68 ± 8.2 a | 18.83 ± 0.6 d | 21.27 ± 0.9 b | 8.52 ± 0.4 c,d | ||
| 144 | 126.87 ± 9.3 b,c | 22.50 ± 2.3 c,d | 18.47 ± 1.4 b,c | 7.20 ± 0.1 e,f | ||
| 168 | 121.9 ± 1.7 c | 23.71 ± 2.0 c,d | 20.89 ± 1.9 b | 9.04 ± 0.4 c | ||
| Significance 4 | ||||||
| Wounding stress | *** | *** | *** | *** | ||
| Temperature | *** | *** | *** | *** | ||
| Storage time | *** | *** | *** | *** | ||
| Wounding stress × temperature | ** | *** | N.S. | N.S. | ||
| Wounding stress × storage time | *** | *** | *** | *** | ||
| Temperature × storage time | *** | *** | ** | ** | ||
| Wounding stress × temperature × storage time | *** | *** | *** | * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Contreras, A.M.; Jacobo-Velázquez, D.A. Effects of Wounding Stress and Storage Temperature on the Accumulation of Chlorogenic Acid Isomers in Potatoes (Solanum tuberosum). Appl. Sci. 2021, 11, 8891. https://doi.org/10.3390/app11198891
Torres-Contreras AM, Jacobo-Velázquez DA. Effects of Wounding Stress and Storage Temperature on the Accumulation of Chlorogenic Acid Isomers in Potatoes (Solanum tuberosum). Applied Sciences. 2021; 11(19):8891. https://doi.org/10.3390/app11198891
Chicago/Turabian StyleTorres-Contreras, Ana M., and Daniel A. Jacobo-Velázquez. 2021. "Effects of Wounding Stress and Storage Temperature on the Accumulation of Chlorogenic Acid Isomers in Potatoes (Solanum tuberosum)" Applied Sciences 11, no. 19: 8891. https://doi.org/10.3390/app11198891







