Abstract
Many scholars have used experimental research methods to conduct extensive research on the impact energy release behavior of Polytetrafluoroethylene(PTFE)/Al reactive materials. However, in numerical simulation, PTFE/Al still lacks the calculation parameters of impact energy release behavior. In order to obtain the simulation parameters of PTFE/Al impact ignition, the Hill mixture law was used to calculate the material parameters of PTFE/Al (mass ratio 73.5/26.5), and according to the Hugoniot curve of PTFE/Al and the γ state equation, the JWL equation of state of a PTFE/Al unreacted substance and reaction product was fitted with a genetic algorithm. According to the PTFE/Al impact energy release experiment, the parameters of the PTFE/Al chemical kinetic equation were determined, and the parameters of the trinomial reaction rate equation were fitted. The obtained parameters were used in the simulation calculation in LS-dyna to predict the damage of the aluminum target plate under the impact of the PTFE/Al reactive fragments.
1. Introduction
A reactive material is a compound or mixture that can independently carry out a chemical reaction and release energy. A reactive material can directly react under external stimulation without an additional detonating device, which means that the entire fragment can be composed entirely of reactive materials. Compared with traditional fragments, reactive fragments can carry more energy. Reactive fragments can cause dual mechanical and chemical damage to the target, which will significantly increase the damage capability of the fragments.
Since Willis [1] discovered that PTFE/Al could react under high-speed impact conditions, reactive materials represented by PTFE/Al have gradually been extensively studied. The PTFE/Al (mass ratio 73.5/26.5) reactive material has a higher calorific value, and its calorific value is usually 2.4 times that of the TNT reaction calorific value (4.18 MJ/Kg) at the same volume [2]. PTFE/Al with a high calorific value is expected to be widely used in the military field. For example, PTFE/Al can be made into reactive fragments, which have kinetic energy damage, ignition/detonation effects on the charge structure, and high-temperature effects. The PTFE/Al reactive materials can be fabricated into the core of a PELE bomb. The PTFE/Al is excited during the penetration of the target, and the chemical energy released is higher than that of TNT, which can form a larger field of fragments behind the target.
After years of research, PTFE/Al reactive materials have been comprehensively studied regarding the preparation process, mechanical properties, constitutive relationship, reaction threshold, and energy release. Yang [3] studied the influence of the pressing and sintering process on the mechanical properties of PTFE/Al (mass ratio 73.5/26.5) reactive material. The results show that sintering for a long time will decompose a large amount of PTFE and cause the tensile strength of the material to decrease. Vasant [4] disclosed a formula and sintering molding process of PTFE/Al reactive material. Compared with the unsintered sample, the tensile strength of the sintered sample increased by four times, and the elongation at break increased by three times. Nielson [5,6] proposed a method of reactive materials prepared by mixing fluoropolymers as a matrix and adding non-oxidized metals (aluminum, zirconium, titanium, magnesium). A reactive material with good mechanical properties and material pore characteristics is prepared by cold pressing and sintering in this method. Rose [7,8] proposed a feasible method to improve the interface bonding force between the polymer and the reinforcing filler by using a coupling agent to enhance the PTFE-based energetic reactive material. Feng [9] studied the influence of different sintering temperatures on the sensitivity and structural strength of the specimen and found that when the sintering temperature was 350 °C, the sensitivity and structural strength of the specimen were highest. Raftenberg [10,11] obtained a Johnson–Cook constitutive model of pressed and sintered PTFE/Al (mass ratio 74/26) by using quasi-static and dynamic compression data with strain rates of 0.1 s−1 and 2900 s−1 at 297 K and 325 K, and Taylor impact tests at speeds of 104 m/s and 222 m/s were simulated. The comparison between the simulated boundary shape-time relationship and the actual digital image shows that the results are in good agreement under the low-speed impact. However, the deviation between them is more significant when the impact speed is higher, mainly due to the error caused by the local reaction inside the material.
Wang [12] prepared six kinds of Al-PTFE reaction material specimens with different Al particle diameters by molding and sintering. The impact sensitivity of different specimens was measured by drop-weight impact experiments on these specimens. The results show that as the Al particle size increases, the strength and sensitivity of the specimen decrease. Wang [13] used plane waves to load PTFE/Al (mass ratio 1:1) and found that the lowest reaction pressure of the PTFE/Al specimen was between 11.93 and 17.98 GPa.
Huang [14] studied the damaging effect of PTFE/Al fragments through experiments, and the results showed that the detrimental impact of reactive fragments was significantly better than that of inert fragments. Through ballistic experiments, Xu [2] found that PTFE/Al fragments hit a 3 mm steel plate at a speed of 1500 m/s, and the hole diameter can reach 14 mm. Wang [15] used Vented Chamber Calorimetry [16] to study the energy release process of PTFE/Al material collision reaction. The author believed that the reaction degree of material collision energy release and the pressure in the pressure vessel are proportional to the collision speed. Xu [17] studied the influence of PTFE content and sintering temperature on the combustion performance of PTFE/Al. The results show that with the increase of PTFE content and sintering temperature, the burning rate, burning intensity, and flame temperature of the sample all present a trend of first increasing and then decreasing. PTFE/Al (PTFE content 35%) sintered at 340 °C has the best combustion performance.
He [18] had carried out a theoretical and numerical analysis on the threshold velocity of the reactive fragment of cladding under the condition of impact initiation and established empirical formulas including fragment diameter, head shape, target material, and shell material. Jiang [19] studied the equation of state of PTFE/Al reactive materials and determined the relevant parameters of the JWL equation of state of reactive materials. AUTODYN software was used to simulate the penetration of a PTFE/Al-reinforced penetrator into a 616RHA steel target. Rosencrantz [20] calculated the state equation parameters of the ignition growth model of PTFE/Al by theory and simulated the reactive fragment impacting the multilayer target at high speed based on LS-dyna. Tang [21] established a two-dimensional model of random distribution of real Al particle size and studied the impact response and initiation behavior of PTFE/Al particle composites.
According to the literature mentioned above, PTFE/Al reactive materials have received much research in material preparation, mechanical properties, and energy release characteristics and have achieved many excellent results, which provide the basis for the study of this article. Many accurate experimental results can be obtained through reactive materials’ impact energy release experiments, but the testing process is cumbersome (material preparation, experimental site layout, etc.) and it takes much time to carry out the PTFE/Al impact energy release experiment. Numerical calculations can enable one to predict experimental results in the absence of experimental conditions rapidly. In past research, the numerical analysis of PTFE/Al mostly avoided the energy release characteristics of reactive materials, and the J-C constitutive model was chosen for simulation, which can be applied well even if the materials did not react. However, if the materials react, the numerical calculation will have significant errors. In order to obtain the impact energy release simulation parameters of PTFE/Al, the JWL equation of state of unreacted and reaction products of PTFE/Al (73.5/26.5) was obtained by theoretical calculation, and the reaction rate equation of PTFE/Al was obtained by fitting experimental data. A set of parameters for LS-dyna simulation calculations were given, which provided a reference for predicting the impact energy release behavior of PTFE/Al reactive fragments.
2. Modeling of PTFE/Al Impact Energy Release
2.1. Parameter Determination of JWL Equation for Unreacted PTFE/Al
Lee [22] modified the isentropic equation based on the work of Jones and Wilkins and found that the better form of the C-J isentropic equation is:
In the formula, A, B, C, R1, R2, and ω are constant coefficients, is pressure, is the relative specific volume, and is the initial specific volume. On the isentropic line, the change of internal energy E with is:
The Gruneisen equation of state can be written as [23]:
In the formula, Γ is the Gruneisen coefficient. Substitute Equations (1) and (2) into Equation (3) to get:
Equation (4) is the standard JWL equation of state. In LS-dyna, the JWL state equation adopts the expression with the temperature form:
where T is the temperature in energetic material after the shock wave and is the specific heat capacity per unit volume.
According to the mass, momentum, and energy conservation relations before and after the shock wave, the physical quantities of the reactive material after the shock wave can be calculated [24]:
In the formula, P, ρ, and u are pressure, density, and particle velocity after the shock wave, respectively; P0, ρ0, and u0 are the initial pressure, density, and particle velocity, respectively; D is the shock wave velocity; E is the total energy, including internal energy and the energy released by chemical reactions; E0 is the initial internal energy.
In a wide pressure range, the shock wave velocity D in the condensing medium has the following relationship with the post-wave particle velocity u [25]:
Assuming u0 = 0 and P0 = 0, the two conservation relations Equations (6) and (7) and the D-u relation can be used to calculate the impact compression line of the unreacted reactive material:
There are only two unknown constants, C0 and S, in Equation (10), so as long as the material parameters of PTFE/Al are obtained, the impact compression line of PTFE/Al reactive material can be obtained. The material parameters of PTFE/Al can be calculated by the Hill mixture rule, and Equation (11) is the Hill mixture rule. According to Equation (11) and material parameters of PTFE and Al (see in Table 1), the density, sound velocity, and Hugoniot parameters of the PTFE/Al(73.5/26.5) reactive material were calculated and the parameters are recorded in Table 2. According to the material parameters of PTFE/Al, the impact compression line of the PTFE/Al reactive material was obtained when PTFE/Al did not react.
In the formula, is the material parameter and is the mass fraction.

Table 1.
Material density, sound velocity, specific heat capacity, and Hugoniot coefficient of PTFE and Al.
Table 1.
Material density, sound velocity, specific heat capacity, and Hugoniot coefficient of PTFE and Al.
| Density (g/cm3) | Sound Velocity (km/s) | Specific Heat Capacity (J∙kg−1∙k−1) | S | |
|---|---|---|---|---|
| Al | 2.78 | 5.35 | 8.9 × 10−4 | 1.49 |
| PTFE | 2.15 | 1.84 | 1.05 × 10−3 | 1.71 |

Table 2.
Material density, sound velocity, specific heat capacity, and Hugoniot coefficient of PTFE and Al.
Table 2.
Material density, sound velocity, specific heat capacity, and Hugoniot coefficient of PTFE and Al.
| Density (g/cm3) | Sound Velocity (km/s) | Specific Heat Capacity (J∙kg−1∙k−1) | S | |
|---|---|---|---|---|
| PTFE/Al | 2.287 | 2.227 | 1.002 × 10−3 | 1.646 |
Six unknown constant coefficients in the JWL equation of state need to be determined. was determined by the Hill mixture law, and the other five parameters were determined according to the impact compression line of the PTFE/Al reactive material. Applying the JWL Equation of state (4) to the Hugoniot curve, Equation (12) is obtained:
When the reactive materials do not react, the initial internal energy E0 of the reactive material is 0 (regardless of chemical energy), and Equation (13) is obtained according to Equation (12):
Parameters A, B, R1, R2, and ω in Equation (13) were fitted and determined by the impact compression line of PTFE/Al, and the JWL equation of state when the reactive material does not react was obtained. A genetic algorithm was used to determine five parameters in the JWL equation of state of unreacted PTFE/Al reactive material. The specific calculation method is as follows:
- In a specific range, a group of A, B, R1, R2, and ω were chosen.
- In the range of a relative specific volume of 0.6–1, 500 values were taken at equal intervals to calculate the impact pressure of Equations (10) and (13).
- Calculate the absolute value of the difference between the impact pressure of Equations (10) and (13), and then sum 500 sets of results as the objective function.
- A genetic algorithm was used to optimize and fit five parameters continuously, and finally, the parameters of the unreacted JWL equation of state were obtained.
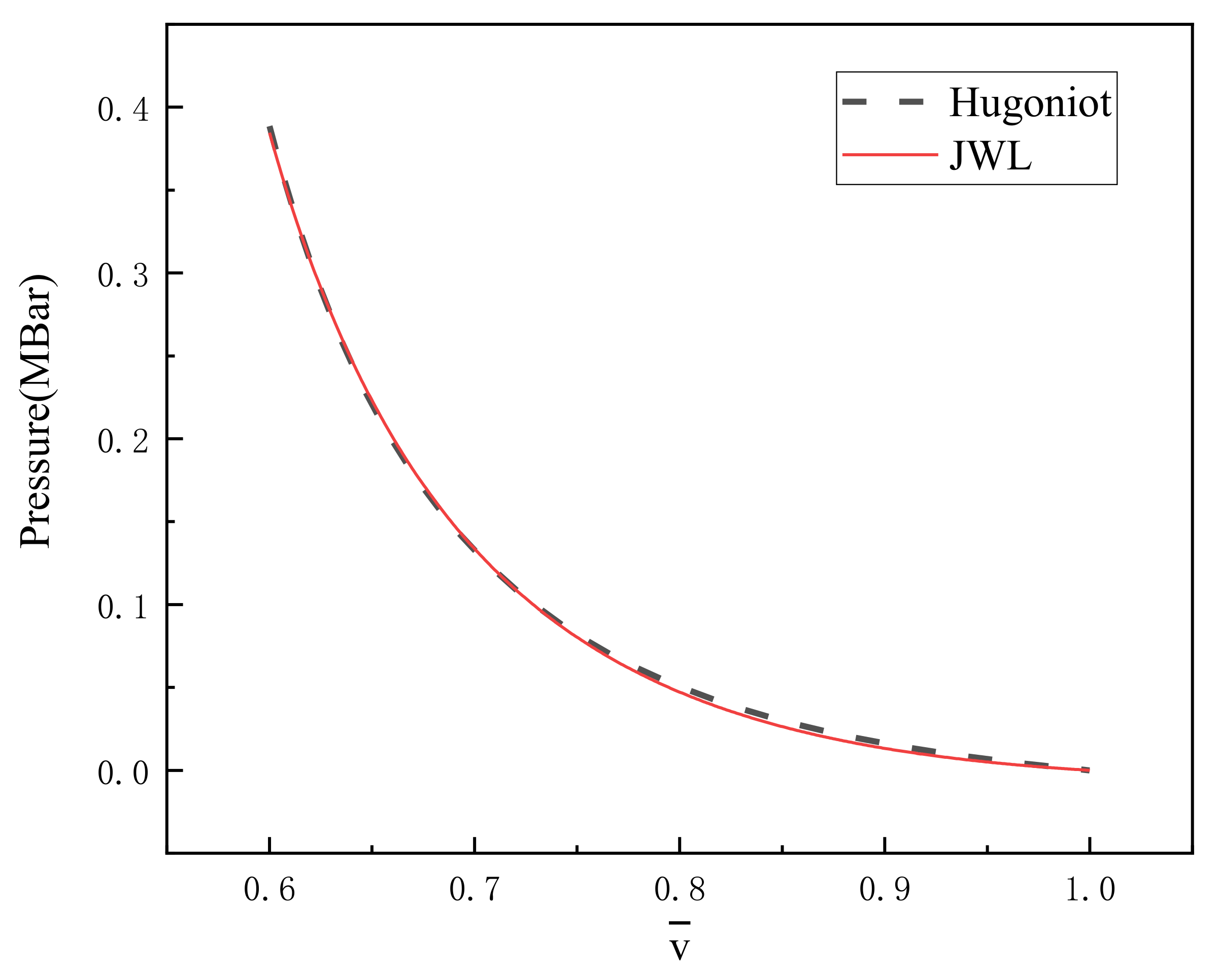
Figure 1 is the Hugoniot curve and the JWL curve obtained by fitting. It is found that there are some differences between the two curves at low-pressure conditions, but at high-pressure situations, the two curves are in good agreement. The standard deviation of the difference between the two curves under 500 relative specific volumes is 0.0019 Mbar. The five parameters obtained by fitting are recorded in Table 3.
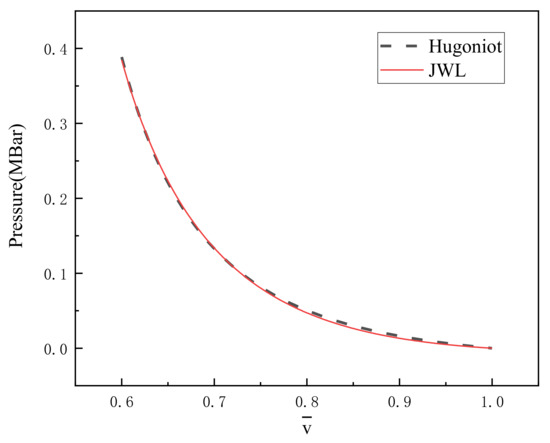
Figure 1.
Hugoniot curve and fitted JWL curve.

Table 3.
Parameters of JWL equation of state of unreacted PTFE/Al.
2.2. Parameter Determination of JWL Equation of Reaction Product
The JWL equation of state parameters of the reaction product usually needs to be subjected to a cylinder test. The obtained experimental data are calibrated to the JWL equation of state to obtain the JWL equation of state parameters. However, the detonation velocity of the PTFE/Al reactive material (mass ratio 73.5/26.5) is low, PTFE/Al is not easy to produce a stable detonation process in the experiment, and accurate experimental data cannot be obtained to fit the JWL equation of state of the reactant. Studies [26,27] have shown that, for condensed explosives with a density higher than 1 g/cm3, the γ equation of state can be used to describe the P-V relationship of the reactants, which can be used to fit the parameters of the JWL equation of state. γ law equation of state can be expressed as:
In the formula, and are constants related to the properties of explosives. The parameters on the C-J surface have the following relationship [23]:
The relational expression on the C-J surface is brought into Equation (14), and the calculation expression of the constant is obtained:
The adiabatic index is a function of the volume and temperature of the detonation product and is related to the composition and density of the material. Study [27] pointed out that the adiabatic index of detonation products of condensed explosives can be approximately determined as follows:
In the formula, is the initial density of the reactive material. The density and detonation velocity D of the explosive is needed to calculate and γ. Additionally, the γ state equation of the PTFE/Al is obtained. In the text, the density is 1.185 g/cm3 and the detonation velocity is 2.21 km/s [28].
A genetic algorithm was used to fit the JWL equation. According to the γ state equation, five parameters in Equation (4) were determined. The fitting method was the same as the fitting of the JWL equation of state of unreacted PTFE/Al. In the fitting process, the specific heat capacity of the reaction product was taken as 1.457 × 103 j/(kg·k) [20], and the internal energy E in the JWL equation of state is
In the formula, E0 is the chemical energy of the reactive material, and T is the impact temperature of the reactive material. In the process of calculating the JWL equation of state of unreacted PTFE/Al, the pressure of PTFE/Al under different relative specific volumes is obtained, and Equation (5) is rewritten to obtain Equation (22) for calculating the impact temperature:
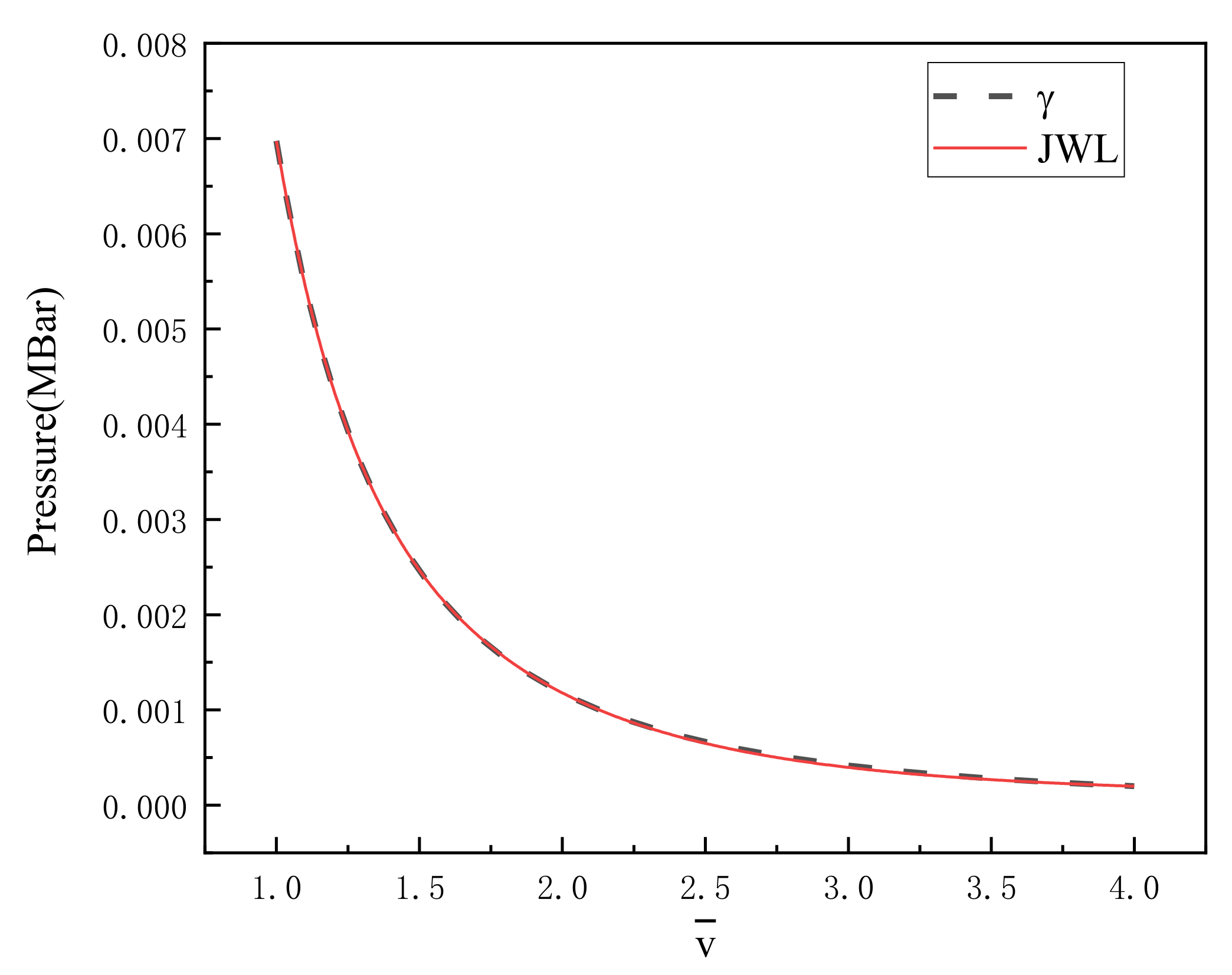
In this formula, is calculated by Equation (13). Figure 2 is the γ state equation and the fitted JWL equation of state curve. It is found that the difference between the two fitted curves is very small by comparing the two curves in the diagram. Within the fitting range, 500 values are taken at equal intervals to calculate the standard deviation of the difference between the two curves, and the calculated standard deviation is MBar. The fitted parameters of the JWL equation of state of PTFE/Al reaction products are recorded in Table 4.
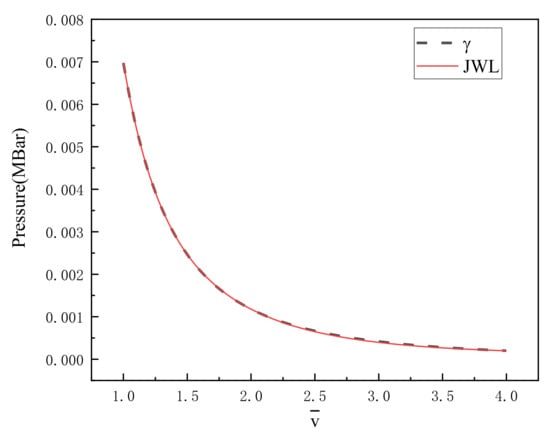
Figure 2.
γ state equation curve and JWL curve of PTFE/Al products.

Table 4.
JWL equation of state parameters for PTFE/Al reaction products.
2.3. Parameters Determination of Reaction Rate Equation
The mechanistic interpretations of solid-state kinetics are based on the concept of a single-step reaction as given by [29]:
In the formula, y is the degree of chemical reaction, t is the time, k(T) is the temperature relationship of the rate constant, and f(y) is the reaction mechanism function. Arrhenius proposed the rate constant–temperature relationship by simulating the equilibrium constant–temperature relationship:
In the formula, is the activation energy, is the pre-exponential factor, T is the temperature, and is the gas constant. In order to have a similar form to the rate equation of the trinomial ignition state equation, the Manpel reaction model was selected, and f(y) is the following formula:
Put k(T) and f(y) into Equation (23) to get Equation (25):
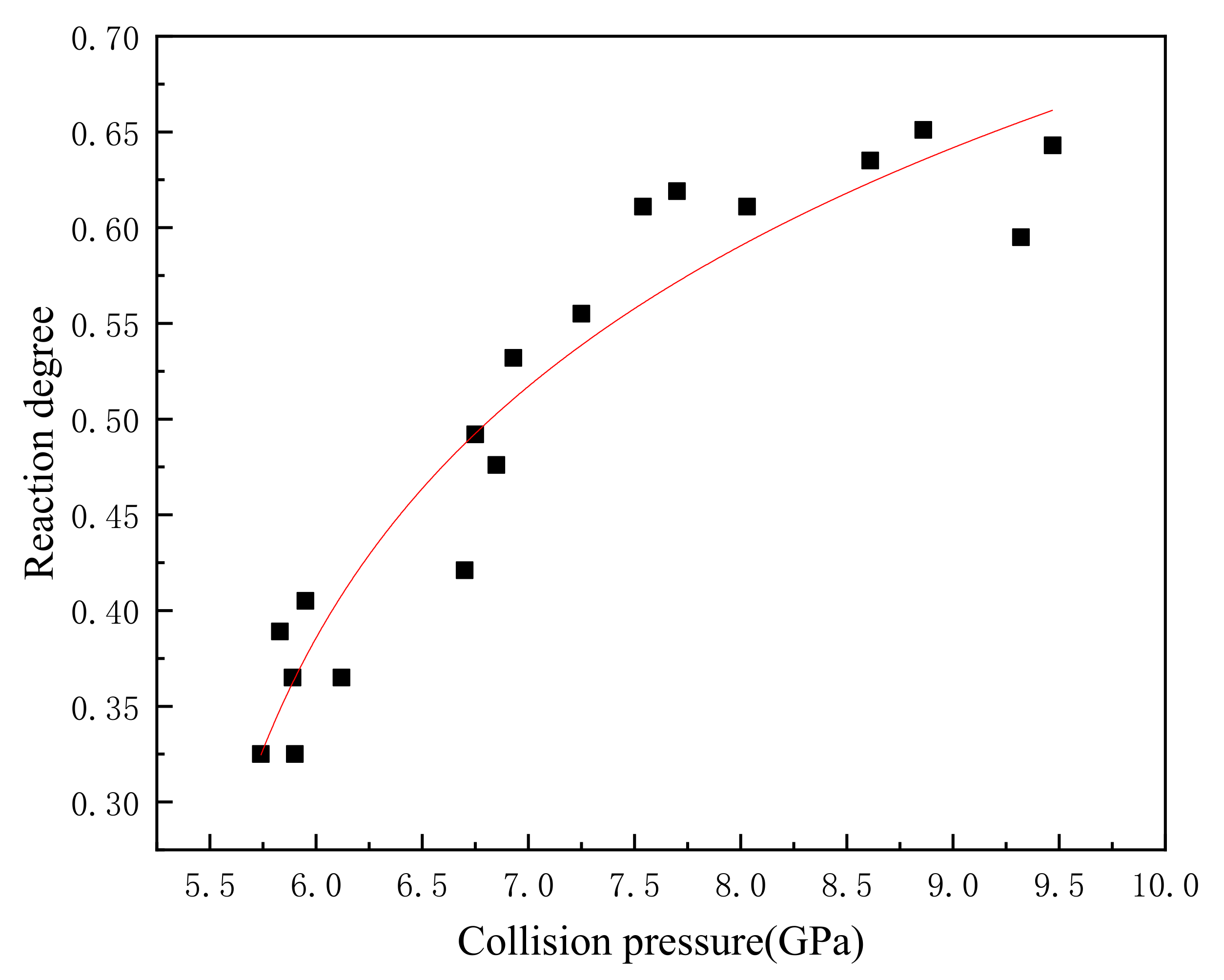
When the temperature is known, there are only two unknown constants in Equation (26). If , , and the initial temperature are known, the reaction degree of the reactive material at the end of the reaction can be calculated. Wang [15] obtained the reaction degree of the PTFE/Al reactive fragments under different impact pressures through the impact energy release experiments. Table 5 records the reaction degree and impact pressure of 20 groups of experiments, and Figure 3 shows the relationship between the reaction degree and the impact pressure.

Table 5.
The relationship between collision pressure and reaction degree of PTFE/Al [15].
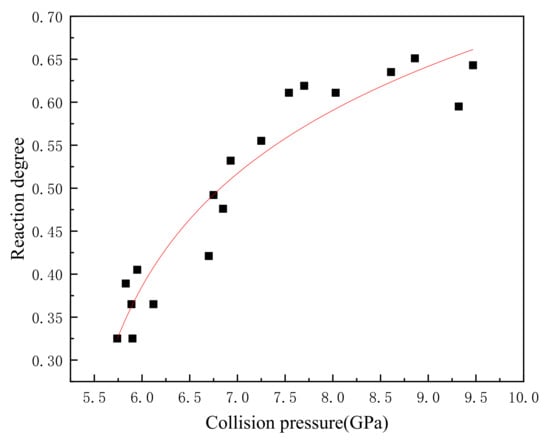
Figure 3.
The corresponding relationship between collision pressure and reaction degree.
In the process of heat transfer between PTFE/Al and air, Equation (26) is assumed to calculate the decrease in temperature of the reactive material:
In the formula, C is a constant, T is the current temperature, and T0 is the initial temperature. The relationship between the collision pressure and the reaction degree of PTFE/Al in Table 5 was used to determine the parameters of the reaction rate equation. The specific steps are as follows:
- The shock temperature corresponding to the shock pressure was calculated by Equation (21), and the relationship between impact temperature and reaction degree was obtained.
- , , and C were selected within the given range.
- The reaction rate of the PTFE/Al reactive material at the current temperature was calculated through Equation (25).
- The time step was set to 1 ms, and the reaction degree and temperature of the PTFE/Al reactive material at the next moment were calculated through Equations (27) and (28).
- In the formula, α is the reaction rate of PTFE/Al, y1 and T1 are the current reaction degree and temperature, and y2 and T2 are the reaction degree and temperature at the next moment.
- Steps 3 and 4 were repeated until the PTFE/Al reaction rate was less than 10−4, and the reaction degree of PTFE/Al at the end of the reaction was obtained.
- According to the impact temperature of the experiment, the final reaction degrees of the 18 sets of data were calculated. We subtracted the obtained reaction degree from the experimental reaction degree and took the absolute value of all the differences and summed them as the objective function.
- A0, E0, and C parameters were obtained by optimizing and fitting the objective function, and the three parameters are shown in Table 6.
 Table 6. PTFE/Al chemical kinetic equation parameters.
Table 6. PTFE/Al chemical kinetic equation parameters.
In order to conveniently use these parameters in LS-dyna, the PTFE/Al chemical kinetic equation is used to calibrate the ignition model parameters of LS-dyna. In LS-dyna, the reaction rate equation is a trinomial ignition growth model:
The values of fmxig, fmxgr, and fmngr were taken as 0. Under different initial reaction degrees, different pressures, and reaction degrees, the reaction rate equation parameters were fitted by Equation (25). The calculated parameters of the PTFE/Al ignition state equation are shown in Table 7.

Table 7.
Parameters of ignition growth model for PTFE/Al.
3. Numerical Simulation of Impact Energy Release of PTFE/Al
The impact ignition simulation parameters of PTFE/Al were used to predict the damage caused by the impact of the reactive fragments on the target plate. At present, the commonly used finite element calculation methods include the Lagrange method, the fluid–structure coupling method, and the smooth particle method. Because PTFE/Al reactive fragments will generate many gaseous products after impact ignition, the fluid–structure coupling method was used to simulate and calculate the simulation process.
3.1. Numerical Simulation Model
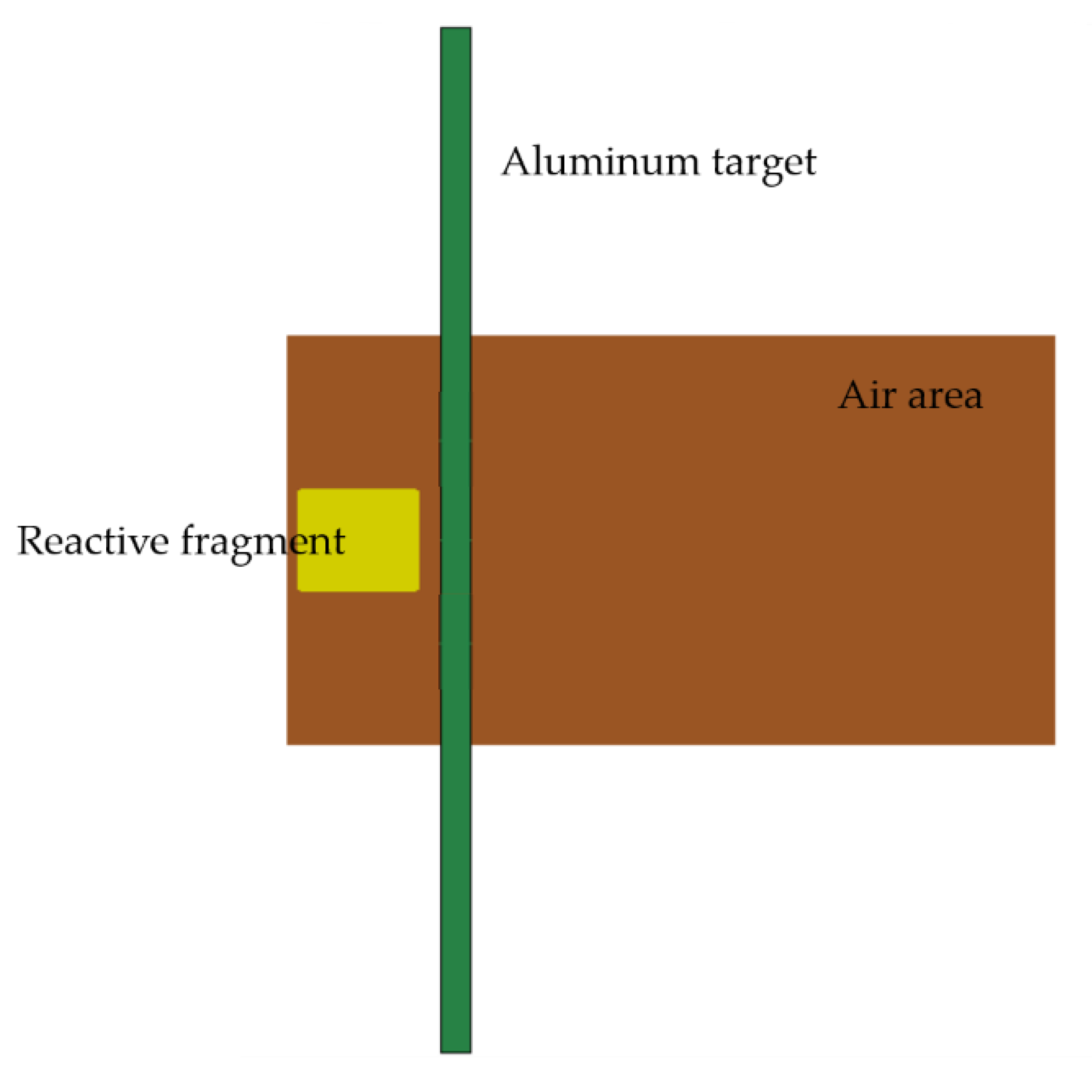
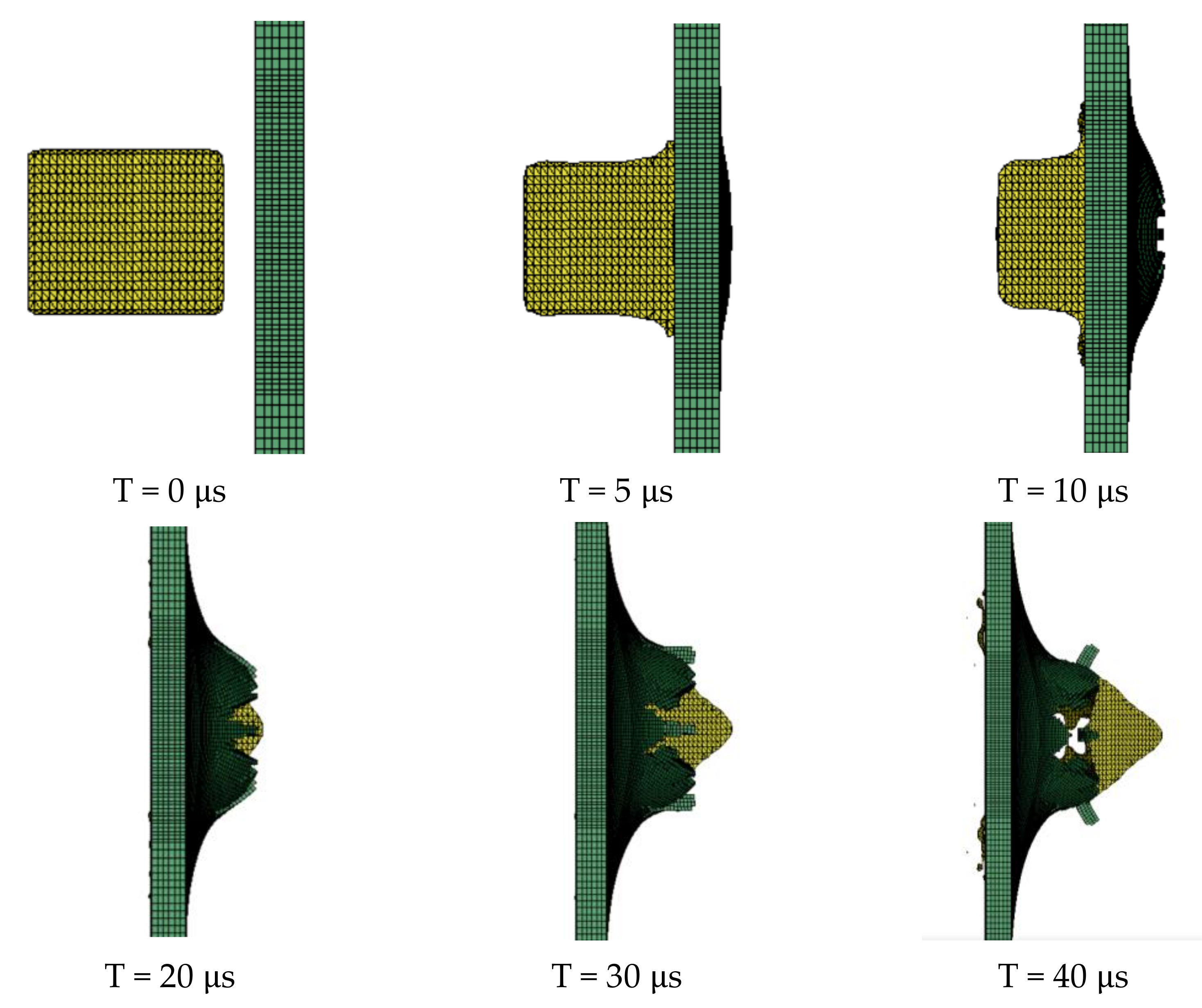
The target plate is an aluminum target plate of mm, and the four sides of the target plate are restrained by total displacement. The air zone of mm is set in the center of the target plate along the axial direction, and the boundary of the air domain is set as a non-reflective boundary. The PTFE/Al reactive fragment is filled 2 mm in front of the target plate, and its size is mm. The initial velocity of the fragment is 798 m/s. Figure 4 is a schematic diagram of the simulation model.
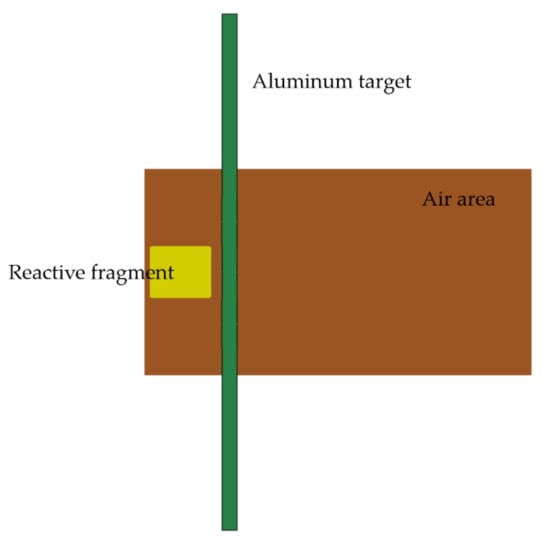
Figure 4.
Simulation model of impact energy release of PTFE/Al reactive fragment.
3.2. Numerical Simulation Results
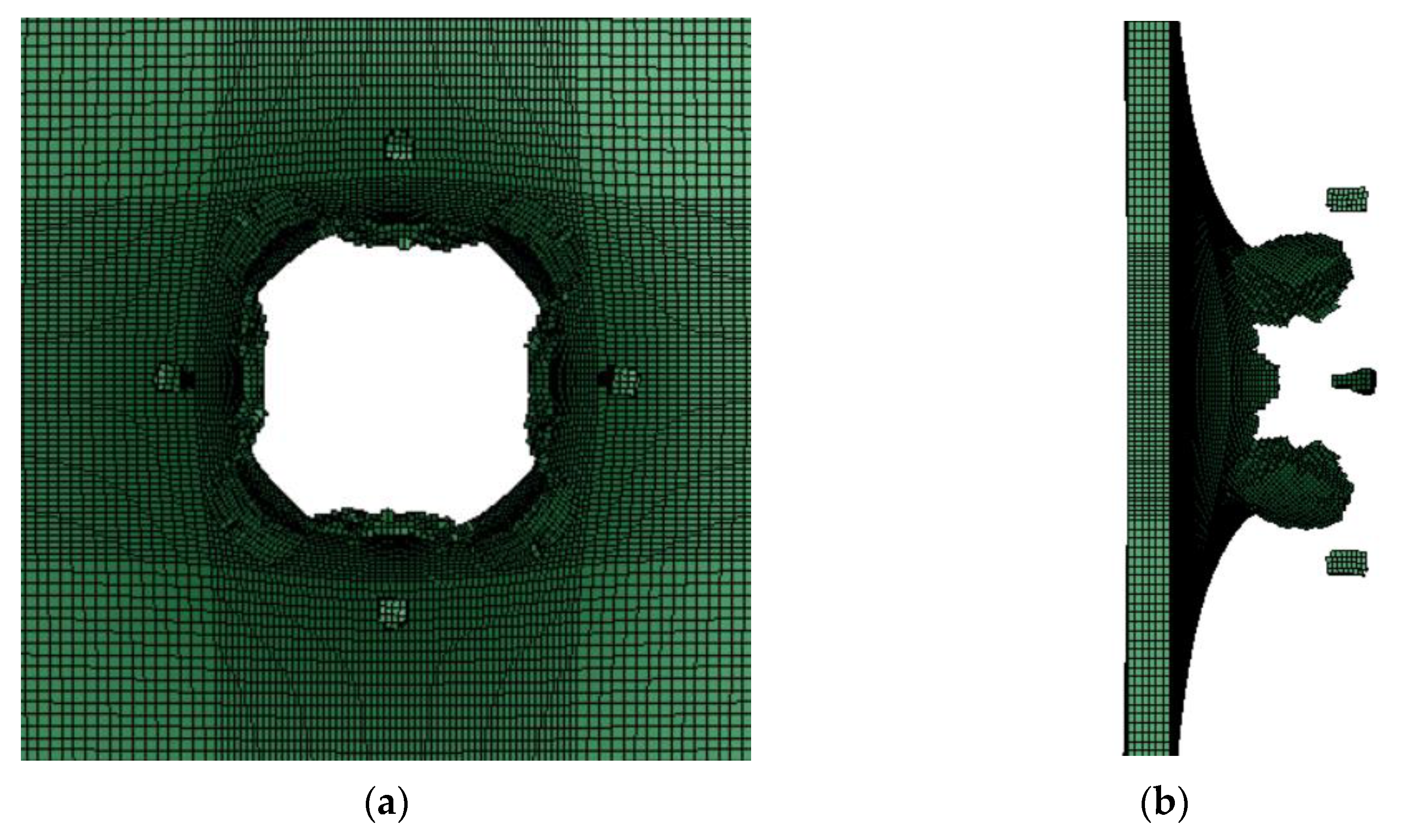
The obtained PTFE/Al simulation parameters are used to simulate the impact of the PTFE/Al reactive fragments on the aluminum target plate. Numerical calculation results show that mm PTFE/Al reactive fragments can penetrate the target plate at a speed of 798 m/s. Figure 5 is the process of projectile-target deformation. During the penetration process, the damage shape of the target plate is approximately square, and the average destruction diameter of the target plate is 19.75 mm, shown in Figure 6. Zhang [30] experimentally measured that the failure diameter of a 3 mm aluminum target plate under the impact of a 5 mm PTFE/Al reactive fragment at a speed of 798m/s was 25 mm. Compared with the experimental results, the error of the simulation calculation results is 26.58%.
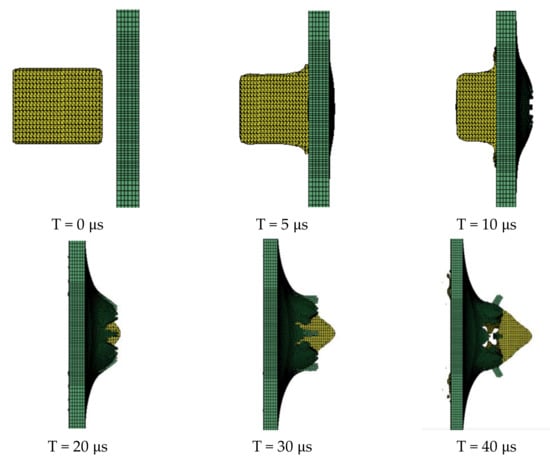
Figure 5.
The process of projectile-target deformation.
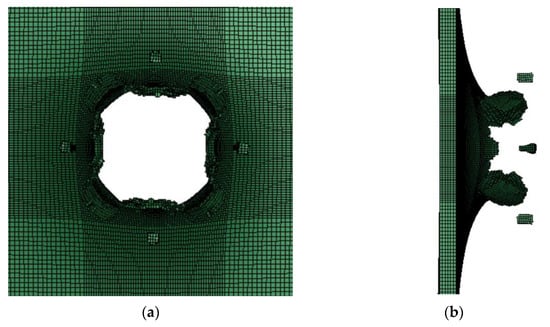
Figure 6.
The destruction of the target board in the simulation: (a) Back view of target plate; (b) side view of the target plate.
4. Conclusions
This article introduces the method of determining the parameters of the JWL equation of state of unreacted explosives and the JWL equation of state of detonation products in the process of impact initiation of PTFE/Al, and how to determine the parameters of the explosive reaction rate equation based on the experimental data of impact initiation.
According to the conservation relationship between the front and back of the shock wave array and the D-u relationship, the Hugoniot curve of PTFE/Al is obtained, and the parameters of the JWL equation of state of unreacted PTFE/Al are determined using a genetic algorithm.
According to the γ-law equation of state, the genetic algorithm was used to determine the parameters of the JWL equation of state for the PTFE/Al reaction product.
According to the PTFE/Al impact energy release experiment, the chemical kinetic equation parameters of PTFE/Al were determined, and the parameters of the trinomial ignition growth model were fitted.
The simulation calculation was performed in LS-dyna to simulate the process of a mm PTFE/Al reactive fragment hitting a 3 mm aluminum target at a speed of 798 m/s. The simulation results show that the failure diameter of the aluminum plate is about 19.75 mm. In the simulation, the failure diameter of the aluminum plate is about 19.75 mm, which can be used to predict the experimental results.
Author Contributions
Conceptualization, X.Z., J.Z., and X.R.; methodology, W.T. and X.R.; validation, X.Z. and J.Z.; formal analysis, Y.W. and X.Z.; resources, P.C. and H.W.; data curation, J.Z. and X.Z.; writing—original draft preparation, Y.W. and X.Z.; writing—review and editing, X.R. and Y.W.; supervision, P.C., H.W., and W.T.; and project administration, P.C. and H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mock, W.; Drotar, J.T. Effect of aluminum particle size on the impact initiation of pressed PTFE/Al compositerods. AIP Conf. Proc. 2007, 955, 971–974. [Google Scholar]
- Xu, S. Study on the Mechanical Performance of Polytetrafluorethyleoe/Al Energetic Reactive Materials. Ph.D. Thesis, National University of Defense Technology, Changsha, China, 2010. [Google Scholar]
- Yang, S.; Xu, S.; Zhang, T. Preparation and performance of PTFE/Al reactive materials. J. Natl. Univ. Def. Technol. 2008, 30, 39–42. [Google Scholar]
- Vasant, S.J.; Waldorf, M.D. Process for Making Polytetrafluoroethylene-Aluminum Composite and Product Made. U.S. Patent 6,547,993, 15 April 2003. [Google Scholar]
- Nielson, D.B. Manufacture of Sintered Reactive Material for Warheads, Comprises Blending Polymer Matrix Comprising Fluoropolymer and Fuel Particles Comprising Specidic Metals, Drying, Pressing, and Sintering Obtained Reactive Mixture. U.S. Patent 2,003,096,897, 22 May 2005. [Google Scholar]
- Nielson, D.B.; Tanner, R.L.; Lund, G.K. High Strength Reactive Materials. U.S. Patent US6593410, 15 July 2003. [Google Scholar]
- Rose, M.T.; Nielson, D.B.; Gary, K.L. High Strength Reactive Materials and Methods of Making. U.S. Patent 659,341,017, 17 June 2004. [Google Scholar]
- Rose, M.T.; Doll, D.W.; Hodgson, J.R. Reactive material enhanced projectiles and related methods. U.S. Patent 20060011086, 20 October 2004. [Google Scholar]
- Feng, B.; Qiu, C.L.; Zhang, T.H. Sensitivity of Al-PTFE upon Low-Speed Impact. Propellants Explos. Pyrotech. 2019, 44, 630–636. [Google Scholar] [CrossRef]
- Raftenberg, M.N.; Willis, M.J.; Kirby, G.C. Modeling the impact deformation of rods of a pressed PTFE-Al composite mixture. Int. J. Impact Eng. 2008, 35, 1735–1744. [Google Scholar] [CrossRef]
- Raftenberg, M.N.; Scheidler, M.J.; Casem, D.A. A Yield Strength Model and Thoughts on an Ignition Criterion for a Reactive PTFE-Aluminum Composite. In Proceedings of the 55th JANNAF Conference, Newton, MA, USA, 12–16 May 2008. [Google Scholar]
- Wang, J.; Li, Y.; Fang, X.; Wang, H.; Feng, B.; Wu, Z. Effect of Al particle size on quasi-static pressure reaction and drop hammer impact sensitivity of Al-PTFE. Energetic Mater. 2018, 26, 524–529. [Google Scholar]
- Wang, Z.; Li, Z.; Jiang, C. Explosion shock compression characteristics and reaction behavior of PTFE-based materials with high Al content. Trans. Beijing Inst. Technol. 2021, 41, 356–363. [Google Scholar]
- Huang, H.; Huang, H.; Yang, S. Preliminary Research on Damage Enhanced Fragment. J. Natl. Univ. Def. Technol. 2007, 15, 566–569. [Google Scholar]
- Wang, H.; Zheng, Y.; Yu, Q. Impact-induced initiation and energy release behavior of reactive materials. J. Appl. Phys. 2011, 110, 1–6. [Google Scholar]
- Ames, R. Vented Chamber Calorimetry for Impact-Initiated Energetic Materials. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 10–13 January 2005; pp. 279–283. [Google Scholar]
- Xu, W.; Hang, S.; Li, Y.; Han, Z.; Wang, B. Effects of PTFE content and sintering temperature on morphology and combustion performance of Al/PTFE composite particles. Energetic Mater. 2020, 181, 1061–1067. [Google Scholar]
- He, Y.; He, Y.; Zhang, X. Research on Critical Initiation Conditions of Energetic Fragment Impact to Initiation. J. Ballist. 2010, 22, 45–49. [Google Scholar]
- Jiang, J.W.; Wang, S.Y.; Mou, Z. Modeling and simulation of JWL equation of state for reactive Al/PTFE mixture. J. Beijing Inst. Technol. 2012, 21, 150–156. [Google Scholar]
- Rosencrantz, S.D. CCharacterization and Modeling Methodology of Polytetrafluoroethylene Based Reactive Materials for the Development of Parametric Models. Master’s Thesis, Wright State University, Dayton, OH, USA, 2007. [Google Scholar]
- Tang, L.; Wang, H.; Lu, G. Mesoscale study on the shock response and initiation behavior of Al-PTFE granular composites. Mater. Des. 2021, 200, 109446. [Google Scholar]
- Lee, E.L.; Hornig, H.C.; Kury, J.W. Adiabatic Expansion of High. Explosive Detonation Products; Lawerence Radiation Laboratory Report: UCRL-50422; University of California Radiation Laboratory at Livermore: Livermore, CA, USA, 1968. [Google Scholar]
- Tang, W.; Zhang, R. Introduction to the Theory and Calculation of the Equation of State; Higher Education Press: Beijing, China, 2008; pp. 199–200. [Google Scholar]
- Tang, W. Shock wave in gas. In Shockwave Physics Tutorial, 1st ed.; National University of Defense Technology Press: Changsha, China, 2016; pp. 139–143. [Google Scholar]
- Tang, W. Shock waves in solids. In Shockwave Physics Tutorial, 1st ed.; National University of Defense Technology Press: Changsha, China, 2016; pp. 229–230. [Google Scholar]
- Wang, C.; Xu, W.; Guo, Y. Calculation of JWL Equation of State Parameters Based on Genetic Algorithm and γ Equation of State. Acta Armamentarii 2017, 38, 167–173. [Google Scholar]
- Zhao, Z.; Tao, G.; Du, C. Application Research on JWL Equation of State of Detonation Products. J. High. Press. Phys. 2009, 23, 277–282. [Google Scholar]
- Li, L. Research on Detonation Properties of Metal-Fluoride Reactive Materials. Master’s Thesis, North University of China, Taiyuan, China, 2015. [Google Scholar]
- Vyazovkin, S. Kinetic concepts of thermally stimulated reactions in solids: A view from a historical perspective. Int. Rev. Phys. Chem. 2000, 19, 45–60. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Yu, Q. Perforation of Double-Spaced Aluminum Plates by Reactive Projectiles with Different Densities. Materials 2021, 14, 1229. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).