The Development of Microscopy for Super-Resolution: Confocal Microscopy, and Image Scanning Microscopy
Abstract
:1. Introduction
2. Literature Review
2.1. Super-Resolution
2.1.1. Resolution Criteria and Resolution Limits
2.1.2. Information Theory
2.1.3. Classes of Super-Resolution
- Ultra-resolution: Improved spatial frequency response and two-point resolution, but the cut-off is unchanged;
- Restricted super-resolution: the cut-off is increased, but less than (coherent) or (incoherent);
- Unrestricted super-resolution: the cut-off is increased without a limit.
2.2. Imaging Geometries
2.2.1. Conventional Imaging
2.2.2. Scanned Imaging
2.3. Combining the Geometries
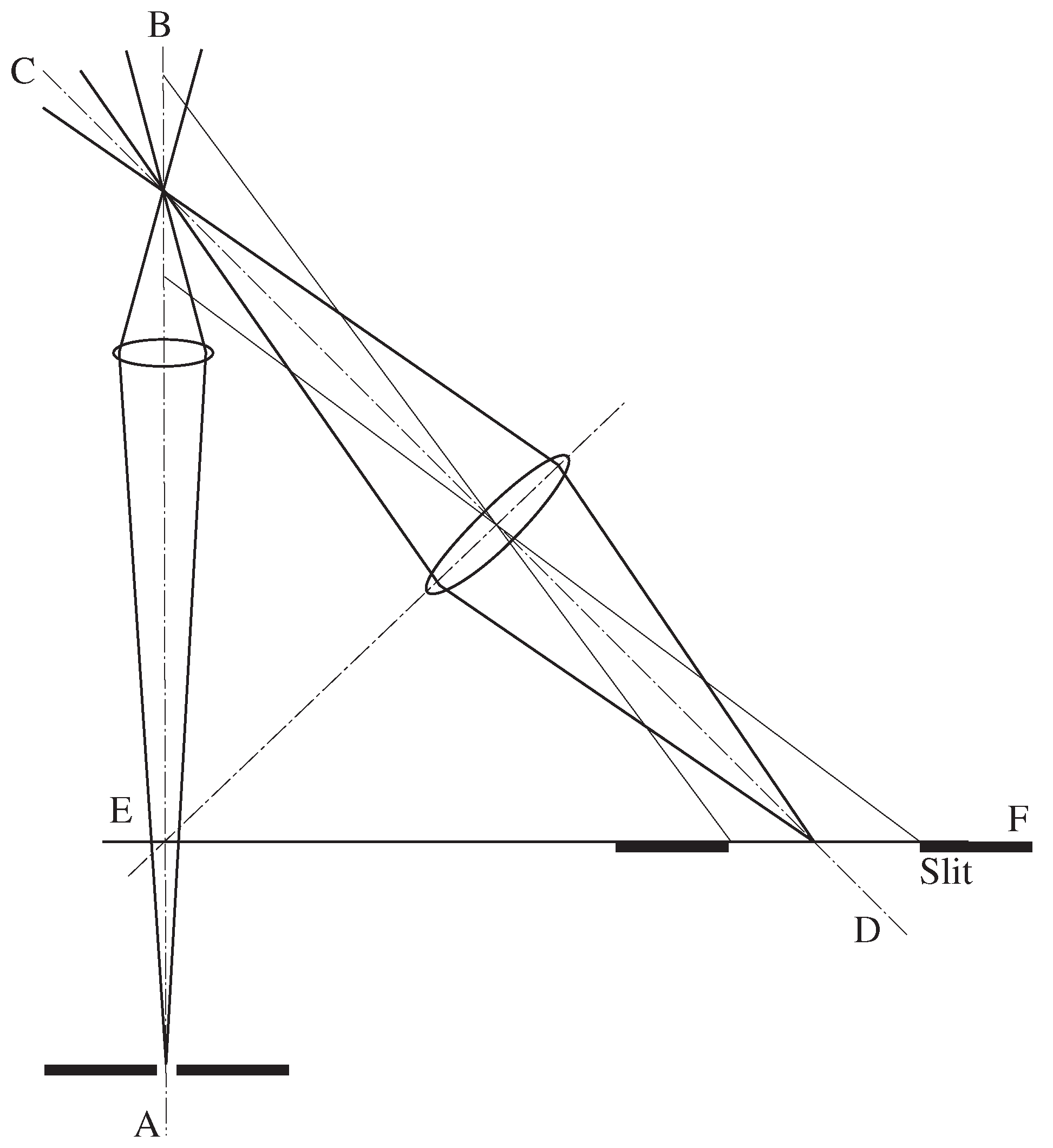
2.3.1. Confocal Microscopy
‘The analysis of photographically recorded spectra is commonly carried out by a microphotometer …A carefully adjusted, straight tungsten lamp filament is imaged by a microscope objective on the spectrum plate mounted on a mobile stage. A second, similar, microscope objective images the filament image transmitted by the spectrum plate on a slit in front of the cathode of a phototube.’
‘This high degree of selectivity afforded by tbe optical system results in a minimum of blurring, increase in signal-to-noise ratio, increase in effective resolution, and the possibility of high resolution light microscopy through unusually thick and highly-scattered specimens.’
‘The second pinhole aperture increases the optical resolution of the system by its action of squaring the intensity pattern distribution of the image diffraction. It can be shown that this results in a sharpened central diffraction zone with reduced high order zones.’
‘The specimen is imaged onto a measuring diaphragm just in front of the detector photomultiplier by a glycerol immersion quartz objective …and by a projective lens.’
‘Spurious energy from objects at the same location as the target but which are not in the same focal plane is defocused at [the pinhole] and, therefore, greatly attenuated.’
2.3.2. Structured Illumination Microscopy
‘A new method is described for obtaining optical images with a resolution exceeding the limits set by diffraction. …A mask, or the image of a mask formed by projection is introduced in or near to the object plane. This mask has a variable transmission (for example a grating), and is movable in the object field. A second similar mask is introduced in or near to the image plane, or the plane of an intermediate image, and is moved conjugately with the object plane mask. The image obtained during the scanning by the masks is integrated in time by a receptor of suitable inertia (for example, the eye, or a photographic emulsion). There results an image of the object with enhanced resolution and contrast (the bandwidth of the transmitted spatial frequencies is increased, and the frequency response is raised). The method may be used with coherent, partially coherent or incoherent illumination.’
2.3.3. Digital Deconvolution
2.3.4. Confocal Microscopy with a Detector Array
2.3.5. Structured Illumination Microscopy versus Confocal Microscopy
2.3.6. Image Scanning Microscopy
‘It is seen that Sheppard’s method provides an improvement in resolution with respect to conventional CSLM which is smaller than the improvement provided by the method discussed in this paper.’
3. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCD | Charge Coupled Device |
| CTF | Coherent Transfer Function |
| FMM | Focal modulation microscopy |
| FWHM | Full-Width at Half-Maximum |
| ISM | Image Scanning Microscopy |
| OPRA | Optical Photon Reassignment Microscopy |
| OTF | Optical Transfer Function |
| PSF | Point Spread Function |
| Q-ISM | Quantum Image Scanning Microscopy |
| SHG | Second Harmonic Generation |
| SIM | Structured Illumination Microscopy |
| SNR | Signal-to-Noise Ratio |
| SOFI | Super-resolution Optical Fluctuation Imaging |
| SOFISM | Super-resolution Optical Fluctuation Image Scanning Microscopy |
| SPADE | Scanning Patterned Detection |
| SPIN | Scanning Patterned Illumination |
| TCC | Transmission Cross-Coefficient |
| THG | Third Harmonic Generation |
| 2D | Two-Dimensional |
| 2PF | Two-Photon Fluorescence |
| 3D | Three-Dimensional |
| 3PF | Three-Photon Fluorescence |
| 4D | Four-dimensional |
References
- Rayleigh, J.W.S. Investigations in optics, with special reference to the spectroscope. Philos. Mag. 1979, 8, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Abbe, E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Arch. Mikrosk. Anat. Entwichlungsmech. 1873, 9, 413–468. [Google Scholar] [CrossRef]
- Rayleigh, J.W.S. On the theory of optical images, with special reference to the microscope. Philos. Mag. 1896, 42, 167–195. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, H.H. On the diffraction theory of optical images. Proc. R. Soc. Lond. Ser. A 1953, 217, 737–744. [Google Scholar]
- Sheppard, C.J.R.; Choudhury, A. Image formation in the scanning microscope. Opt. Acta 1977, 24, 1051–1073. [Google Scholar] [CrossRef]
- Sheppard, C.J.R. Resolution and superresolution. Microsc. Res. Tech. 2017, 80, 590–598. [Google Scholar] [CrossRef]
- Hartley, R.V.L. Transmission of information. Bell Syst. Tech. J. 1928, 7, 535–563. [Google Scholar] [CrossRef]
- Shannon, C.E. Communications in the presence of noise. Proc. IRE 1949, 37, 10–21. [Google Scholar] [CrossRef]
- Gabor, D. Theory of communication. J. IEE 1946, 93, 429–457. [Google Scholar] [CrossRef]
- Di Francia, G.T. Super-resolution. Opt. Acta 1955, 2, 5–8. [Google Scholar] [CrossRef]
- Lukosz, W. Optical systems with resolving powers exceeding the classical limit, Part 1. J. Opt. Soc. Am. 1967, 56, 1463–1472. [Google Scholar]
- Cox, I.J.; Sheppard, C.J.R. Information capacity and resolution in an optical system. J. Opt. Soc. Am. A 1986, 3, 1152–1158. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Larkin, K. Information capacity and resolution in three-dimensional imaging. Optik 2003, 114, 548–550. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, C.J.R. Fundamentals of superresolution. Micron 2007, 38, 165–169. [Google Scholar] [CrossRef]
- Sheppard, C.J.R. Fundamentals of superresolution (Vol. 38, pg 165, 2007). Micron 2007, 38, 772. [Google Scholar] [CrossRef]
- Di Francia, G.T. Nuovo pupille superrisolvente. Atti Fond. Giorgio Ronchi 1952, 7, 366–372. [Google Scholar]
- Di Francia, G.T. Supergain antennas and optical resolving power. Nuovo Cim. Suppl. 1952, 9, 426–435. [Google Scholar] [CrossRef]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef]
- Betzig, E.; Patterson, G.; Sougrat, R.; Lindwasser, O.; Olenych, S.; Bonifacino, J.; Lippincott-Swartz, J.; Hess, H. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef] [Green Version]
- Rust, M.; Bates, M.; Zhuang, X. ub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, S.; Girirajan, T.; Mason, M. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef] [Green Version]
- Gustafsson, M.G.L. Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimitted resolution. Proc. Natl. Acad. Sci. USA 2005, 102, 13081–13086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Synge, E.H. A suggested method for extending microscopic resolution into the ultra-microscopic region. Philos. Mag. 1928, 6, 356–362. [Google Scholar] [CrossRef]
- O’Keefe, J.A. Resolving power of visible light. J. Opt. Soc. Am. 1956, 46, 359. [Google Scholar] [CrossRef]
- Baez, A.V. Is resolving power independent of λ possible? An experiment with a sonic “macroscope”. J. Opt. Soc. Am. 1956, 46, 901. [Google Scholar] [CrossRef]
- Ash, E.A.; Nicholls, G. Super-resolution aperture scanning microscope. Nature 1972, 237, 510–512. [Google Scholar] [CrossRef]
- Massey, G.A. Microscopy and pattern generation with scanned evanescent waves. Appl. Opt. 1984, 23, 658–660. [Google Scholar] [CrossRef]
- Lewis, A.; Isaacson, M.; Harootunian, A.; Muray, A. Development of a 500 Å spatial resolution light microscope. Ultramicroscopy 1984, 13, 227–230. [Google Scholar] [CrossRef]
- Pohl, D.W.; Denk, W.; Lanz, M. Optical stethoscopy: Image recording with resolution λ/20. Appl. Phys. Lett. 1984, 44, 651–653. [Google Scholar] [CrossRef]
- Courjon, D.; Sarayeddine, K.; Spajer, M. Scanning tunneling optical microscopy. Opt. Commun. 1989, 71, 23–28. [Google Scholar] [CrossRef]
- Guerra, J.M. Photon tunneling microscope. Appl. Opt. 1990, 29, 3741–3752. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Kawata, S. Near-field scanning optical microscope with a metallic probe tip. Opt. Lett. 1994, 19, 159–161. [Google Scholar] [CrossRef]
- Zenhausen, F.; O’Boyle, M.P.; Wickramasinghe, H.K. Apertureless near-field optical microscope. Appl. Phys. Lett. 1994, 65, 1623–1625. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, C.J.R.; Kompfner, R. Resonant scanning optical microscope. Appl. Opt. 1978, 17, 2879–2882. [Google Scholar] [CrossRef]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning flourescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, C.J.R. Multiphoton microscopy: A personal historical review, with some future predictions. J. Biomed. Opt. 1981, 25, 014511. [Google Scholar] [CrossRef] [PubMed]
- Gannaway, J.N.; Sheppard, C.J.R. Second harmonic imaging in the scanning optical microscope. Opt. Quantum Elect. 1978, 10, 435–439. [Google Scholar] [CrossRef]
- Bain, A. Electric Time-pieces and Telegraphs. British Patent Application No. 9745, 27 November 1843. [Google Scholar]
- Young, J.Z.; Roberts, F. A flying-spot microscope. Nature 1951, 4241, 231. [Google Scholar] [CrossRef] [PubMed]
- Zvorykin, V.K.; Ramberg, E.G. Photoelectricity and its Applications; Wiley: New York, NY, USA, 1949. [Google Scholar]
- Sheppard, C.J.R. The generalized microscope. In Confocal and Two-Photon Microscopy: Foundations, Applications, and Advances; Diaspro, A., Ed.; Wiley-Liss: New York, NY, USA, 2002; pp. 1–17. [Google Scholar]
- Sheppard, C.J.R.; Wilson, T. Reciprocity and equivalence in scanning microscopes. J. Opt. Soc. Am. A 1986, 3, 755–756. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Gu, M. Image formation in two-photon fluorescence microscopy. Optik 1990, 86, 104–106. [Google Scholar]
- Goldmann, H. Spaltlampenphotographie und-Photometrie. Ophthalmologica 1940, 98, 257–270. [Google Scholar] [CrossRef]
- Maurice, D.M. A scanning slit optical microscope. Investig. Ophthalmol. Visual Sci. 1974, 13, 1033–1037. [Google Scholar]
- Stelzer, E.H.K.; Lindek, S. Fundamental reduction of the observation volume in far-field light microscopy by detection orthogonal to the illumination axis: Confocal theta microscopy. Opt. Commun. 1994, 111, 536–547. [Google Scholar] [CrossRef]
- Wong, L.K.; Mandella, M.J.; Kino, G.S.; Wang, T.D. Improved rejection of multiply scattered photons in confocal microscopy using dual-axes architecture. Opt. Lett. 2007, 32, 1674–1676. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Si, K.; Sheppard, C.J.R. Optimization of axial resolution in a confocal microscope with D-shaped apertures. App. Opt. 2009, 48, 3998–4002. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Si, K.; Sheppard, C.J.R. Divided-aperture technique for fluorescence confocal microscopy through scattering media. Appl. Opt. 2010, 49, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Scheimpflug, T. Improved Method and Apparatus for the Systematic Alteration or Distortion of Plane Pictures and images by Means of Lenses and Mirrors for Photography and for other Purposes. British Patent GB190401196A, 12 May 1904. [Google Scholar]
- Koana, Z. Problems relating to the micro-densitometer. J. Illum. Eng. Inst. Jpn. 1943, 26, 371–385. (In Japanese) [Google Scholar]
- Naora, H. Microspectrophotometry and cytochemical analysis of nucleic acids. Science 1951, 114, 279–280. [Google Scholar] [CrossRef]
- Minsky, M. Microscopy Apparatus. U.S. Patent 3013467A, 19 December 1961. [Google Scholar]
- McCutchen, C.W. Superresolution in microscopy and the Abbe resolution limit. J. Opt. Soc. Am. 1967, 57, 1190–1192. [Google Scholar] [CrossRef]
- Egger, M.D.; Petráň, M. New reflected-light microscope for viewing unstained brain and ganglion cells. Science 1967, 157, 305–307. [Google Scholar] [CrossRef]
- Petráň, M.; Hadravsky, M.; Egger, M.D.; Galambos, R. Tandem scanning reflected light microscope. J. Opt. Soc. Am. 1968, 58, 661–664. [Google Scholar] [CrossRef]
- Davidovits, P.; Egger, M.D. Scanning laser microscope. Nature 1969, 223, 831. [Google Scholar] [CrossRef]
- Wied, G.L.; Bartles, P.H.; Bahr, G.F.; Oldfield, D.G. Taxonomic intra-cellular analytic system (TICAS) for cell identification. Acta Cytol. 1968, 12, 180–204. [Google Scholar]
- Slomba, A.F.; Wasserma, D.F.; Gaufman, G.I.; Nester, J.F. A laser flying spot scanner for use in automatised fluorescence antibody instrumention. J. Assoc. Adv. Med. Instrum. 1972, 6, 230–234. [Google Scholar] [PubMed]
- Sheppard, C.J.R.; Wilson, T. Image formation in scanning microscopes with partially coherent source and detector. Opt. Acta 1978, 25, 315–325. [Google Scholar] [CrossRef]
- Brakenhoff, G.J.; Blom, P.; Barends, P. Confocal scanning light microscopy with high aperture immersion lenses. J. Microsc. 1979, 117, 219–232. [Google Scholar] [CrossRef]
- Hamilton, D.K.; Wilson, T.; Sheppard, C.J.R. Experimental observations of the depth-discrimination properties of scanning microscopes. Opt. Lett. 1981, 6, 625–626. [Google Scholar] [CrossRef]
- Cox, I.J.; Sheppard, C.J.R. Digital image processing of confocal images. Image Vis. Comp. 1983, 1, 52–56. [Google Scholar] [CrossRef]
- Cox, I.J.; Sheppard, C.J.R.; Wilson, T. Super-resolution by confocal fluorescent microscopy. Optik 1982, 60, 391–396. [Google Scholar]
- Brakenhoff, G.J. Imaging modes in confocal scanning light microscopy (CSLM). J. Microsc. 1979, 117, 233–242. [Google Scholar] [CrossRef]
- Hamilton, D.K.; Sheppard, C.J.R. A confocal interference microscope. Opt. Acta 1984, 29, 1573–1577. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Wilson, T. Gaussian-beam theory of lenses with annular aperture. IEE J. Microw. Opt. Acoust. 1978, 2, 105–112. [Google Scholar] [CrossRef]
- Sheppard, C.J.R. Scanning Microscopes. U.S. Patent 4198571, 15 April 1980. [Google Scholar]
- Sheppard, C.J.R.; Choudhury, A. Annular pupils, radial polarization, and superresolution. Appl. Opt. 2004, 43, 4322–4327. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, C.J.R.; Balla, N.K.; Rehman, S.; Yew, E.Y.S.; Teng, W.T. Bessel beams with the tightest focus. Opt. Commun. 2009, 282, 4647–4656. [Google Scholar] [CrossRef]
- Frieden, B.R. Optical transfer of the three-dimensional object. J. Opt. Soc. Am. 1967, 57, 56–66. [Google Scholar] [CrossRef]
- Gu, M.; Sheppard, C.J.R. Confocal fluorescent microscopy with a finite sized circular detector. J. Opt. Soc. Am. A 1992, 9, 151–153. [Google Scholar] [CrossRef]
- Gan, X.S.; Sheppard, C.J.R. Detectability: A new criterion for evaluation of the confocal microscope. Scanning 1993, 15, 187–192. [Google Scholar] [CrossRef]
- Lukosz, W.; Marchand, M. Optischen Abbildung unter Überschreitung der Beugungsbedingten Auflösungsgrenze. Opt. Acta 1963, 10, 241–255. [Google Scholar] [CrossRef]
- Neil, M.M.A.; Juskaitis, R.; Wilson, T. Real time 3D fluorescence microscopy by two beam interference illumination. Opt. Commun. 1998, 153, 1–4. [Google Scholar] [CrossRef]
- Hanley, Q.S.; Verveer, P.J.; Jovin, T.M. Optical sectioning spectroscopy in a programmable array microscope (PAM). Appl. Spectrosc. 1998, 52, 783–789. [Google Scholar] [CrossRef]
- Heintzmann, R.; Cremer, C. Laterally modulated excitation microscopy: Improvement of resolution by using a diffraction grating. Proc. SPIE 1999, 3568, 185–195. [Google Scholar]
- Gustafsson, M.G.L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000, 198, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafsson, M.G.L.; Shao, L.; Carlton, P.M.; Wang, C.J.R.; Golubovskaya, I.N.; Cande, W.Z.; Agard, D.A.; Sedat, J.W. Three-dimensional resolution doubling in wide-field fluorescence microscopy by structured illumination. Biophys. J. 2008, 94, 4957–4970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, X.; Kuang, C.F.; Wang, T.T.; Liu, X. Phase encoding for sharper focus of azimuthally polarized beam. Opt. Lett. 2010, 35, 3928–3930. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Rehman, S. Highly convergent focusing of light based on rotating dipole polarization. Appl. Opt. 2011, 50, 4463–4467. [Google Scholar] [CrossRef]
- Sheppard, C.J.R. Applications of scanning optical microscopy. Proc. SPIE 1982, 368, 88–95. [Google Scholar]
- Weinstein, M.; Castelman, K.R. Reconstructing 3-D specimens from 2-D specimens. Proc. SPIE 1971, 26, 131–138. [Google Scholar]
- Agard, D.A.; Sedat, J.W. Three-dimensional architecture of a polytene nucleus. Nature 1983, 302, 676–681. [Google Scholar] [CrossRef]
- Holmes, T.J. Maximum-likelihood image restoration adapted for noncoherent optical imaging. J. Opt. Soc. Am. A 1988, 5, 666–673. [Google Scholar] [CrossRef]
- Dey, N.; Blanc-Feraud, L.; Zimmer, C.; Roux, P.; Kam, Z.; Olivo-Marin, J.-C.; Zerubia, J. Richardson–Lucy algorithm With total variation regularization for 3D confocal microscope deconvolution. Microsc. Res. Tech. 2006, 69, 260–266. [Google Scholar] [CrossRef]
- Dong, B.; Shao, L.; Frangi, A.F.; Bandmann, O.; Da Costa, M. Three-dimensional deconvolution of wide field microscopy with sparse priors: Application to zebrafish imagery. In Proceedings of the 2014 22nd International Conference on Pattern Recognition, Stockholm, Sweden, 24–28 August 2014; pp. 865–870. [Google Scholar]
- Wang, H.; Rivenson, Y.; Jin, Y.; Wei, Z.; Gao, R.; Günaydin, H.; Bentolila, L.A.; Kural, C.; Ozcan, A. Deep learning enables cross-modality. Nat. Methods 2019, 16, 103–110. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Chen, L.; Tan, S. Deep learning–enhanced fluorescence microscopy via degeneration decoupling. Opt. Express 2020, 28, 14859–14873. [Google Scholar] [CrossRef]
- Bertero, M.; Pike, E.R. Resolution in diffraction-limited imaging, a singular value analysis I. The case of coherent illumination. Opt. Acta 1982, 29, 727–746. [Google Scholar] [CrossRef] [Green Version]
- Reinholz, F.; Schütt, W.; Grümmer, G.; Kuhlmann, F.; Kraeft, S.-K. A new powerful mode of laser scanning microscopy. Optik 1989, 82, 165–168. [Google Scholar]
- Sheppard, C.J.R.; Gu, M. Edge setting criterion in confocal microscopy. Optik 1992, 31, 4575–4577. [Google Scholar] [CrossRef]
- Barth, M.; Stelzer, E.H.K. Boosting the optical transfer function with a spatially resolving detector in a high numerical aperture confocal reflection microscope. Optik 1994, 96, 53–58. [Google Scholar]
- Pawley, J.; Blouke, M.; Janesick, J. The CCDiode: An optimal detector for laser confocal microscopes. Proc. SPIE 1996, 2655, 125–129. [Google Scholar]
- Liedtke, M. Pinhole for a Confocal Laser Scanning Microscope. U.S. Patent 2012/0162754 A1, 28 June 2012. [Google Scholar]
- Benedetti, P.A.; Evangelista, V.; Guidarini, D.; Vestri, S. Electronic multi-confocal-points microscopy. Proc. SPIE 1995, 2412, 56–62. [Google Scholar]
- Heintzmann, R.; Benedetti, P.A. High-resolution image reconstruction in fluorescence microscopy with patterned excitation. Appl. Opt. 2006, 45, 5037–5045. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, P.A. Spread Analysis in Video-Confocal Microscopy Resolves Better, Faster and Cheaper; Focus on Microscopy: Bordeaux, France, 2017; Available online: http://www.focusonmicroscopy.org/2017/index.html (accessed on 1 September 2021).
- Chen, N.G.; Howe, W.C.; Sheppard, C.J.R. Focal modulation microscopy. Opt. Express 2008, 16, 18764–18769. [Google Scholar] [CrossRef]
- Gong, W.; Si, K.; Chen, N.G.; Sheppard, C.J.R. Improved spatial resolution in fluorescence focal modulation microscopy. Opt. Lett. 2009, 34, 3508–3510. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Min, W.; Conchello, J.-A.; Xie, X.S.; Lichtman, J.W. Super-Resolution Laser Scanning Microscopy through Spatiotemporal Modulation. Nano Lett. 2009, 9, 3883–3889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.W.; Wang, B.-W.; Zhang, Q.-X.; Yao, X.-C. Super-resolution scanning laser microscopy through virtually structured detection. Biomed. Opt. Express 2013, 4, 1673–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laporte, G.P.J.; Stasio, N.; Sheppard, C.J.R.; Psaltis, D. Resolution enhancement in nonlinear scanning microscopy through post-detection digital computation. Optica 2014, 1, 455–460. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Cogswell, C.J. Confocal microscopy with detector arrays. J. Mod. Opt. 1990, 37, 267–279. [Google Scholar] [CrossRef]
- Gauderon, R.; Sheppard, C.J.R. Improvement in imaging in confocal fluorescent microscopes using multiple detectors. Bioimaging 1999, 6, 126–129. [Google Scholar] [CrossRef]
- Heintzmann, R.; Sarafis, V.; Munroe, P.; Nailon, J.; Hanley, Q.S.; Jovin, T.M. Resolution enhancement by subtraction of confocal signals taken at different pinhole sizes. Micron 2003, 34, 293–300. [Google Scholar] [CrossRef]
- Sanchez-Ortiga, E.; Sheppard, C.J.R.; Saavedra, G.; Martinez-Corral, M.; Doblas, A.; Calatayud, A. Subtractive imaging in confocal scanning microscopy using a CCD camera as a detector. Opt. Lett. 2012, 37, 1280–1282. [Google Scholar] [CrossRef] [Green Version]
- Korobchevskaya, K.; Peres, C.; Li, Z.; Antipov, A.; Sheppard, C.J.R.; Diaspro, A.; Bianchini, P. Intensity weighted subtraction microscopy approach for image contrast and resolution enhancement. Sci. Rep. 2016, 36, 25816. [Google Scholar] [CrossRef] [Green Version]
- Sheppard, C.J.R. Structured illumination microscopy (SIM) and image scanning microscopy (ISM): A review and comparison of imaging properties. Philos. Trans. R. Soc. A 2021, 379, 20200154. [Google Scholar] [CrossRef]
- Mueller, C.B.; Enderlein, J. Image scanning microscopy. Phys. Rev. Lett. 2010, 104, 198101. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, C.J.R. Super-resolution in confocal imaging. Optik 1988, 80, 53–54. [Google Scholar]
- Cox, I.J.; Sheppard, C.J.R.; Wilson, T. Improvement in resolution by nearly confocal microscopy. Appl. Opt. 1982, 21, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, C.J.R.; Cox, I.J.; Hamilton, D.K. Edge detection in micrometrology with nearly confocal microscopy. Appl. Opt. 1984, 23, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.; Carlini, A.R. Effect of detector displacement in confocal imaging systems. Appl. Opt. 1988, 27, 3791–3799. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Wilson, T. The theory of the direct-view confocal microscope. J. Microsc. 1978, 124, 107–117. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Mao, X.Q. Confocal microscopes with slit apertures. J. Mod. Opt. 1988, 35, 1169–1185. [Google Scholar] [CrossRef]
- Conchello, J.-A.; Lichtman, J.W. Theoretical analysis of a rotating-disk partially confocal scanning microscope. Appl. Opt. 1994, 33, 585–596. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Castello, M.; Tortarolo, G.; Vicidomini, G.; Diaspro, A. Image formation in image scanning microscopy, including the case of two-photon excitation. J. Opt. Soc. Am. A 2017, 34, 1339–1350. [Google Scholar] [CrossRef]
- Bertero, M.; Boccacci, P.; Defrise, M.; De Mol, C.; Pike, E.R. Super-resolution in confocal scanning microscopy: II. The incoherent case. Inverse Probl. 1989, 5, 441–461. [Google Scholar] [CrossRef]
- Defrise, M.; De Mol, C. Super-resolution in confocal scanning microscopy: Generalized inversion formulae. Inverse Probl. 1992, 8, 175–185. [Google Scholar] [CrossRef]
- York, A.G.; Parekh, S.H.; Nogare, D.D.; Fischer, R.S.; Temprine, K.; Mione, M.; Chitnis, A.B.; Combes, C.A.; Shroff, H. Resolution doubling in live, multicellular organisms via multifocal structured illumination microscopy. Nat. Methods 2012, 9, 749–754. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Chandris, P.; Nogare, D.D.; Head, J.; Wawrzusin, P.; Fischer, R.S.; Chitnis, A.B.; Combs, C.A.; Shroff, H. Instant super-resolution imaging in live cells and embryos via analog image processing. Nat. Methods 2013, 10, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.M.R.; Breedijk, R.M.P.; Brandt, R.A.J.; Zeelenberg, C.H.C.; de Jong, B.E.; Timmermans, W.; Nahidi Azar, L.; Hoebe, R.A.; Stallinga, S.; Manders, E.M. Re-scan confocal microscopy: Scanning twice for better resolution. Biomed. Opt. Express 2013, 4, 2644–2656. [Google Scholar] [CrossRef] [PubMed]
- Brakenhoff, G.J.; Visscher, K. Confocal imaging with bilateral scanning and array detectors. J. Microsc. 1992, 165, 139–146. [Google Scholar] [CrossRef]
- Schulz, O.; Pieper, C.; Clever, M.; Pfaff, J.; Ruhlandt, A.; Kehlenbach, R.H.; Wouters, F.; Grosshans, J.; Bunt, G.; Enderlein, J. Resolution doubling in fuorescence microscopy with confocal spinning-disk image scanning microscopy. Proc. Natl. Acad. Sci. USA 2013, 110, 21000–21005. [Google Scholar] [CrossRef] [Green Version]
- Azuma, T.; Kei, T. Super-resolution spinning-disk confocal microscopy using optical photon reassignment. Opt. Express 2015, 23, 15003–15011. [Google Scholar] [CrossRef]
- Roth, S.; Sheppard, C.J.R.; Wicker, K.; Heintzmann, R. Optical photon reassignment microscopy (OPRA). Opt. Nanoscopy 2013, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.; Heintzmann, R. Optical photon reassignment with increased axial resolution by structured illumination. Meth. Appl. Fluoresc. 2016, 4, 045005. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Mehta, S.B.; Heintzmann, R. Superresolution by image scanning microscopy using pixel reassignment. Opt. Lett. 2013, 38, 2889–2892. [Google Scholar] [CrossRef]
- Castello, M.; Sheppard, C.J.R.; Diaspro, A.; Vicidomini, G. Image scanning microscopy with a quadrant detector. Opt. Lett. 2015, 40, 5355–5358. [Google Scholar] [CrossRef]
- Ingaramo, M.; York, A.G.; Hoogendoorn, E.; Potsma, M.; Shroff, H.; Patterson, G.H. Richardson-Lucy deconvolution as a general tool for combining images with complementary strengths. Chem. Phys. Chem. 2014, 15, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Ingaramo, M.; York, A.G.; Wawrzusin, P.; Milberg, O.; Hong, A.; Weigert, R.; Shroff, H.; Patterson, G.H. Two-photon excitation improves multifocal structured illumination microscopy in thick scattering tissue. Proc. Natl. Acad. Sci. USA 2014, 111, 5254–5259. [Google Scholar] [CrossRef] [Green Version]
- Winter, P.W.; York, A.G.; Nogare, M.; Ingaramo, M.; Christensen, R.; Chitnis, A.; Patterson, G.H.; Shroff, H. Two-photon instant structured illumination microscopy improves the depth penetration of super-resolution imaging in thick scattering samples. Optica 2014, 1, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Huff, J. The Airyscan detector from ZEISS: Confocal imaging with improved signal-to-noise ratio and super-resolution. Nat. Meth. 2015, 12, i–ii. [Google Scholar] [CrossRef]
- Korobchevskaya, K.; Lagerholm, B.; Colin-York, H.; Fritzsche, M. Exploring the potential of Airyscan microscopy for live cell imaging. Photonics 2017, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Sivaguru, M.; Urban, M.A.; Fried, G.; Wesseln, C.J.; Mander, L.; Punyasena, S.W. Comparative performance of Airyscan and structured illumination superresolution microscopy in the study of the surface texture and 3D shape of pollen. Microsc. Res. Tech. 2016, 81, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Scipioni, L.; Lanzano, L.; Diaspro, A.; Gratton, E. Comprehensive correlation analysis for super-resolution dynamic fingerprinting of cellular compartments using the Zeiss Airyscan detector. Nat. Commun. 2018, 9, 5120. [Google Scholar] [CrossRef] [Green Version]
- Roth, S.; Sheppard, C.J.R.; Heintzmann, R. Superconcentration of light - Circumventing the classical limit to achievable irradiance. Opt. Lett. 2016, 41, 2109–2112. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, C.J.R.; Roth, S.; Heintzmann, R.; Castello, M.; Vicidomini, G.; Chen, R.; Chen, X.; Diaspro, A. Interpretation of the optical transfer function: Significance for image scanning microscopy. Optics Express 2016, 24, 27280–27287. [Google Scholar] [CrossRef] [PubMed]
- Gregor, I.; Spieker, M.; Petrovsky, R.; Grosshans, J.; Ros, R.; Enderlein, J. Rapid nonlinear image scanning microscopy. Nat. Methods 2017, 14, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Castello, M.; Tortarolo, G.; Buttafava, M.; Deguchi, T.; Villa, F.; Koho, S.; Pesce, L.; Oneto, M.; Pelicci, S.; Lanzano, L.; et al. A robust and versatile platform for image scanning microscopy enabling super-resolution FLIM. Nat. Methods 2019, 16, 175–178. [Google Scholar] [CrossRef]
- Roider, C.; Piestun, R.; Jesacher, A. High-resolution confocal Raman microscopy using pixel reassignment. Opt. Lett. 2016, 41, 3825–3828. [Google Scholar] [CrossRef] [PubMed]
- Roider, C.; Piestun, R.; Jesacher, A. 3D image scanning microscopy with engineered excitation and detection. Optica 2017, 4, 1373–1381. [Google Scholar] [CrossRef]
- Tzang, O.; Feldkhun, D.; Agrawal, A.; Jesacher, A.; Piestun, R. Two-photon PSF-engineered Image Scanning Microscopy. Opt. Lett. 2019, 44, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Breedijk, R.M.P.; Wen, J.; Krishnaswami, V.; Bernas, T.; Manders, E.M.M.; Setlow, P.; Vischer, N.O.E.; Brul, S. A live-cell super-resolution technique demonstrated by imaging germinosomes in wild-type bacterial spores. Sci. Rep. 2020, 10, 5312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koho, S.V.; Slenders, E.; Tortarolo, G.; Castello, M.; Buttafava, M.; Villa, F.; Tcarenkova, E.; Ameloot, M.; Bianchini, P.; Sheppard, C.J.R.; et al. Two-photon image scanning microscopy with SPAD array and blind image reconstruction. Biomed. Opt. Express 2020, 11, 2905–2924. [Google Scholar] [CrossRef]
- Ma, Y.; Kuang, C.; Fang, Y.; Ge, B.; Li, D.; Liu, X. Virtual fluorescence emission difference microscopy based on photon reassignment. Opt. Lett. 2015, 40, 4627–4630. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Kuang, C.; Liu, X. Method of super-resolution based on array detection and maximum-likelihood estimation. Appl. Opt. 2016, 55, 9925–9931. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, S.; Zhu, D.; Kuang, C.; Liu, X. Parallel detecting super-resolution microscopy using correlation based image restoration. Opt. Commun. 2017, 404, 139–146. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Sun, S.; Kuang, C.; Ding, Z.; Liu, X. Image scanning fluorescence emission difference microscopy based on a detector array. J. Microsc. 2017, 266, 288–297. [Google Scholar] [CrossRef]
- Sun, S.; Liu, S.; Wang, W.; Zhang, Z.; Kuang, C.; Liu, X. Improving the resolution of two-photon microscopy using pixel reassignment. Appl. Opt. 2018, 57, 6181–6187. [Google Scholar] [CrossRef]
- Ye, X.; McCluskey, M.D. Modular Scanning Confocal Microscope with Digital Image Processing. PLoS ONE 2016, 11, e0166212. [Google Scholar] [CrossRef] [Green Version]
- Mozafarri, S.; Jaedicke, V.; Larocca, F.; Tiruveedhula, P.; Roorda, A. Versatile multi-detector scheme for adaptive optics scanning laser ophthalmoscopy. Biomed. Opt. Express 2018, 9, 5477–5488. [Google Scholar] [CrossRef]
- Strasse, F.; Offterdinger, M.; Piestun, R.; Jesacher, A. Spectral image scanning microscopy. Biomed. Opt. Express 2019, 10, 2513–2527. [Google Scholar] [CrossRef]
- Mandracchia, B.; Son, J.; Jia, S. Super-resolution optofluidic scanning microscopy. Lab Chip 2020, 21, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, B.; Zhang, B.; Zhang, Z.; Li, X.; Zheng, S.; Fan, Z.; Tan, J. Second harmonic generation microscopy using pixel reassignment. J. Microsc. 2021, 281, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Dan, D.; Wang, Z.; Zhou, X.; Lei, M.; Zhao, T.; Qian, J.; Yu, X.; Yan, S.; Min, J.; Bianco, P.R. Rapid image reconstruction of structured illumination microscopy directly in the spatial domain. IEEE Photonics J. 2021, 13, 3900411. [Google Scholar] [CrossRef] [PubMed]
- McGregor, J.E.; Mitchell, C.A.; Hartell, N.A. Post-processing strategies in image scanning microscopy. Methods 2015, 88, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, C.J.R.; Castello, M.; Tortarolo, G.; Deguchi, T.; Koho, S.V.; Vicidomini, G.; Diaspro, A. Pixel reassignment in image scanning microscopy: A re-evaluation. J. Opt. Soc. Am. A 2020, 37, 154–162. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Castello, M.; Tortarolo, G.; Deguchi, T.; Koho, S.V.; Vicidomini, G.; Diaspro, A. Image scanning microscopy with multiphoton excitation or Bessel beam illumination. J. Opt. Soc. Am. A 2020, 37, 1639–1649. [Google Scholar] [CrossRef]
- Sheppard, C.J.R.; Castello, M.; Tortarolo, G.; Slenders, E.; Deguchi, T.; Koho, S.V.; Bianchini, P.; Vicidomini, G.; Diaspro., A. Pixel reassignment in image scanning microscopy with a doughnut beam: Example of maximum likelihood restoration. J. Opt. Soc. Am. A 2021, 38, 1075–1084. [Google Scholar] [CrossRef]
- Yu, J.-Y.; Becker, S.R.; Folberth, J.; Wallin, B.F.; Chen, S.; Cogswell, C.J. Achieving superresolution with illumination-enhanced sparsity. Opt. Express 2018, 26, 9850–9865. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Chen, S.; Becker, S.; Yu, J.-Y.; Cogswell, C.J. l1-regularized maximum likelihood estimation with focused-spot illumination quadruples the diffraction-limited resolution in fluorescence microscopy. Opt. Express 2020, 28, 39413–39429. [Google Scholar] [CrossRef]
- Dertinger, T.; Colyer, R.; Iyer, G.; Weiss, S.; Enderlein, J. Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI). Proc. Natl. Acad. Sci. USA 2009, 106, 22287–22292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geissbuehler, S.; Dellagiacoma, C.; Lasser, T. Comparison between SOFI and STORM. Biomed. Opt. Express 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenne, R.; Rossman, U.; Rephael, B.; Israel, Y.; Krupinski-Ptaszek, A.; Lapkiewicz, R.; Silberberg, Y.; Oron, D. Super-resolution enhancement by quantum image scanning microscopy. Nat. Photon. 2019, 13, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Sroda, A.; Makowski, A.; Tenne, R.; Rossman, U.; Lubin, G.; Oron, D.; Lapkiewicz, R. SOFISM: Super-resolution optical fluctuation image scanning microscopy. Optica 2020, 7, 1308–1316. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Song, W. Super-resolution confocal microscopy through pixel reassignment. In Microscopy and Analysis; Stanciu, S.G., Ed.; IntechOpen: London, UK, 2016; pp. 81–98. [Google Scholar]
- Ward, E.N.; Pal, R. Image scanning microscopy: An overview. J. Microsc. 2017, 266, 221–228. [Google Scholar] [CrossRef] [Green Version]
| System | Resolution Improvement Factor |
|---|---|
| Conventional | 1 |
| 2-beam (a) SIM | 1.40 |
| 2-beam (b) SIM | 2.26 |
| 3-beam SIM | 1.71 |
| Confocal, ideal | 1.39 |
| Confocal, ideal, with Bessel beam | 1.72 |
| ISM, 2 AU array | 1.53 |
| ISM 0.836 AU array, with Bessel beam | 1.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheppard, C.J.R. The Development of Microscopy for Super-Resolution: Confocal Microscopy, and Image Scanning Microscopy. Appl. Sci. 2021, 11, 8981. https://doi.org/10.3390/app11198981
Sheppard CJR. The Development of Microscopy for Super-Resolution: Confocal Microscopy, and Image Scanning Microscopy. Applied Sciences. 2021; 11(19):8981. https://doi.org/10.3390/app11198981
Chicago/Turabian StyleSheppard, Colin J. R. 2021. "The Development of Microscopy for Super-Resolution: Confocal Microscopy, and Image Scanning Microscopy" Applied Sciences 11, no. 19: 8981. https://doi.org/10.3390/app11198981
APA StyleSheppard, C. J. R. (2021). The Development of Microscopy for Super-Resolution: Confocal Microscopy, and Image Scanning Microscopy. Applied Sciences, 11(19), 8981. https://doi.org/10.3390/app11198981






