Abstract
Ultrasound (US) still represents the mainstay of scrotal imaging. However, contrast-enhanced ultrasound (CEUS) is a relatively novel, but increasingly utilized diagnostic modality. In consequence, we performed a systematic review (SR) and pooled meta-analysis to investigate the diagnostic performance of CEUS in the evaluation of testicular masses (TM). A SR up to June 2021 was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The diagnostic performance of CEUS was evaluated basing on two different endpoints: neoplastic vs. non-neoplastic and malignant vs. benign TM. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) from eligible studies were pooled and summary receiver operating characteristic (SROC) curves were constructed for each endpoint. Overall, six qualified studies were deemed suitable for this meta-analysis. Diagnostic performance of CEUS showed an accuracy of 0.96 in detecting neoplastic masses (sensitivity of 0.89, PPV of 0.85, specificity of 0.62, and NPV of 0.69) and an accuracy of 0.96 in detecting malignant masses (sensitivity of 0.86, PPV of 0.73, specificity of 0.87, and NPV of 0.91). Taken together, CEUS may represent a promising minimally invasive diagnostic tool for characterization of TM, since it allows clinicians to identify neoplastic lesions and exclude malignant tumor.
1. Introduction
Identification and differential diagnosis of testicular masses (TM) in daily practice is often challenging. According to EAU guidelines, ultrasound (US) still represents the mainstay of scrotal imaging [1]. However, magnetic resonance imaging (MRI) of the scrotum provides higher sensitivity and specificity than US in the diagnosis of TM [2,3]. Nonetheless, the cost of MRI is still too high to justify its routine use [4]. Hence, there is an urgent need for new, minimally invasive, cost-effective, and highly reproducible imaging techniques for real-time evaluation of TM.
Since the introduction of US contrast agents, contrast-enhanced ultrasound (CEUS) has been applied for morphological characterization and local staging of different malignancies [5]. CEUS is always performed after conventional B-mode US, therefore it must be regarded as a combination of both methods. Over the past decades, some studies described the role of CEUS, as a promising approach in differential diagnosis of testicular masses [6,7]. Here, the use of qualitative and quantitative parameters, such as evaluating wash-in, washout, and the time-intensity curves, may help distinguish between malignant and benign neoplastic TM [8]. Meanwhile, fewer studies evaluated the role of CEUS in non-neoplastic testicular lesions, such as segmental ischemia, abscess, and orchiepididymitis, with overlapping results [7,9,10].
In consequence, to assess the real value of this promising imaging technique and its potential applications in clinical practice, we performed a systematic review and meta-analysis of the literature. Notably, our aim was to evaluate the diagnostic accuracy of CEUS in differentiating both neoplastic vs. non-neoplastic and malignant vs. benign TM.
2. Materials and Methods
2.1. Search Strategy
We searched Pubmed, Embase, and Web of Science databases with no restriction of language from inception up to June 2021, following the PRISMA (“Preferred Reporting Items for Systematic Reviews and Meta-Analyses”) statement [11]. Medical subject heading (MeSH, Pubmed controlled vocabulary) and free text keywords used in the search strategy are provided in supplementary materials (Supplementary Figure S1). Reference lists of included papers were also screened for additional relevant studies.
2.2. Inclusion and Exclusion Criteria
The population, intervention, comparison, outcome, and study design principle (PICOS) were adopted to define study eligibility.
- Population—patients with US diagnosis of TM;
- Intervention—CEUS;
- Comparator—histopathological examination and/or follow-up/clinical surveillance;
- Outcome—diagnostic accuracy of CEUS (sensitivity, specificity, accuracy, positive predictive value, and negative predictive value); and
- Study design—prospective and retrospective cohort studies.
Abstracts, case reports. and studies with overlapping patients were excluded. Two authors (A.T. and R.S.F.) explored. Two investigators (A.T. and L.A.) independently reviewed and extracted the data, and any potential conflict was resolved by discussion or by a third investigator (C.L.).
2.3. Methodological Quality Assessment
The quality of each study was evaluated by the Quality Assessment of Diagnostic Accuracy Studies–2 (QUADAS–2) [12]. The QUADAS–2 format includes four domains: (1) patient selection, (2) index test, (3) reference standard, and (4) flow and timing. For each domain, the risk of bias and concerns about applicability were analyzed and rated as low, high, or unclear risk (Supplementary Table S1a,b). The results of quality assessment were used for descriptive purposes to provide an evaluation of the overall quality of the included studies and to investigate potential sources of heterogeneity.
2.4. Extractable Data
The diagnostic performance was evaluated based on two different endpoints: (1) neoplastic vs. non-neoplastic; and (2) malignant vs. benign. According to different endpoints, true positive (TP), false positive (FP), true negative (TN), and false negative (FN) values were extracted, whenever possible, from each study and reported in 2 × 2 contingency tables (Supplementary Table S2). Subsequently, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for each endpoint. Other descriptive variables were extracted: study design, time, number of patients, median age, tumor size, histology, dose of contrast injected, and number/experience of readers.
2.5. Statistical Analysis
To address diagnostic performance of CEUS according to different endpoints, sensitivity, specificity, PPV, and NPV from different studies were pooled and summary receiver operating characteristic (SROC) curves were constructed by using the Moses–Shapiro–Littenberg method [13]. We applied a random effect model (DerSimonian–Laird method), accounting for both heterogeneity of data and small sample size of the eligible studies. Meta Discv.1.4 (Unit of clinical Biostatistics, Madrid, Spain) was used to perform this meta-analysis. Statistical analysis was two-sided and statistical significance was set at p < 0.05 [14].
3. Results
3.1. Characteristics of Included Studies
The initial literature search yielded a total of 263 articles. After removing duplicate records, 128 articles were included for title and abstract screening. Subsequently, 68 studies were identified for further full-text evaluation. Finally, six qualified studies were deemed suitable for this meta-analysis (Figure 1).
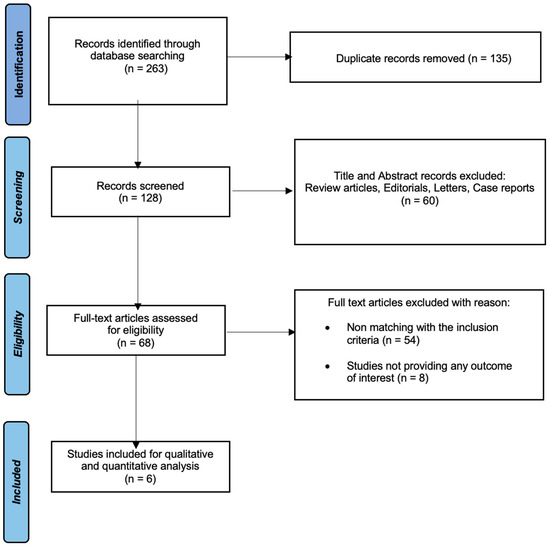
Figure 1.
Study flow chart.
Characteristics of eligible studies were summarized in Table 1a,b. Of those, three were prospective and three were retrospective. Studies were conducted in Germany (n = 3), UK (n = 1), Italy (n = 1), or Austria (n = 1) and published between 2011 and 2020.

Table 1.
(a,b) Summary of published research: Baseline characteristics among eligible studies testing the diagnostic performance of preoperative CEUS.
A total of 419 patients for a total of 428 testicular masses were analyzed in this meta-analysis. The mean patient age ranged from 33 to 42 years. Median tumor size was reported in 4/6 studies, ranging from 6 to 13 mm. The most common malignant, benign, and non-neoplastic histopathological specimens were represented by seminoma, Leydig cell tumor, and segmental testis infarction, respectively.
All studies reported doses of contrast agent used, ranging from 2.4 mL to 4.8 mL. Moreover, three studies relied on four or more radiologist readers, one had a single reader and one study did not provide any information. Finally, inter-observer agreement ranged from 0.65 to 0.98, when reported.
3.2. Diagnostic Accuracy of CEUS in Distinguishing Neoplastic from Non-Neoplastic Lesions
Four studies [15,16,17,18] evaluated the accuracy of CEUS in detecting neoplastic lesion, including both benign and malignant masses. Here, pooled sensitivity and specificity were 0.89 (95% CI: 0.84–0.92) and 0.62 (95% CI: 0.52–0.72), respectively. Additionally, pooled PPV and NPV were 0.85 (95% CI: 0.80–0.89) and 0.69 (95% CI: 0.58–0.79), respectively. SROC curve depicted diagnostic accuracy of 96% (Figure 2).
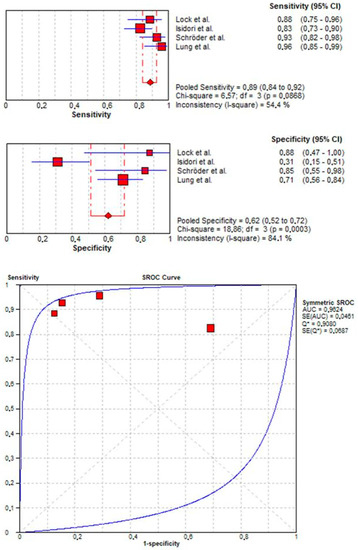
Figure 2.
Pooled sensitivity, specificity, and SROC curve of CEUS in detecting neoplastic lesions.
3.3. Diagnostic Accuracy of CEUS in Distinguishing Malignant from Benign Lesions
Three studies [16,19,20] evaluated the accuracy of CEUS in detecting malignant lesions. Here, pooled sensitivity and specificity were 0.86 (95% CI: 0.75–0.93) and 0.87 (95% CI: 0.80–0.92), respectively. Additionally, pooled PPV and NPV were 0.73 (95% CI: 0.62–0.83) and 0.91 (95% CI: 0.85–0.95), respectively. SROC curve depicted diagnostic accuracy of 96% (Figure 3).
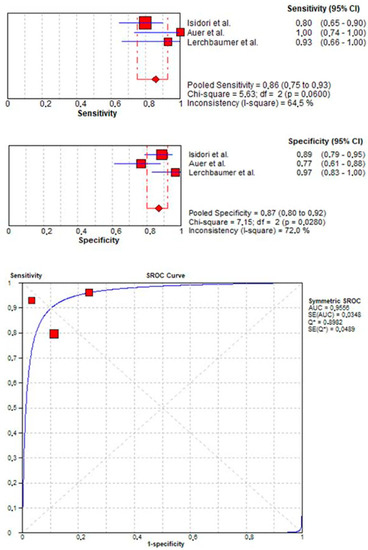
Figure 3.
Pooled sensitivity, specificity, and SROC curve of CEUS in detecting malignant lesions.
4. Discussion
Based on contemporary literature, CEUS appears to be a useful tool for characterization of testicular masses. To assess the real value of this imaging technique and its possible applications in everyday clinical practice, we performed a systematic review and meta-analysis of the literature. Our analyses resulted in several noteworthy observations.
First, we identified 419 patients for a total of 428 testicular masses. All studies were conducted in Europe between 2011 and 2020. Of those, three were retrospective and three were prospective. These results show a growing interest for CEUS especially in Europe, where public healthcare systems are in search of cost-effective strategy to support clinician in differential diagnosis [21].
Second, pooled sensitivity, specificity, and accuracy of CEUS in the diagnosis of neoplastic vs. non-neoplastic testicular masses were 0.89, 0.62, and 0.96, respectively. The low value of specificity was mainly attributable to Isidori et al., who reported the lowest specificity (0.31), probably due to the high heterogeneity of the histological findings in the non-neoplastic group. Moreover, we observed a high pooled PPV (0.85) in detecting neoplastic lesions.
Third, pooled sensitivity, specificity, and diagnostic accuracy of CEUS in the diagnosis of malignant vs. benign testicular masses were 0.86, 0.87, and 0.96, respectively. Here, the three eligible studies analyzed for this endpoint reported overlapping diagnostic performance, with sample size of 115, 55, and 45 patients in Isidori et al., Auer et al., and Lerchbaumer et al., respectively. Moreover, we observed a high NPV (0.91) in excluding benign testicular masses.
Taken together, our findings strongly suggest that CEUS is a high-accuracy and non-invasive tool in differential diagnosis of TM. Specifically, in the daily clinical practice, CEUS may support the clinician in the identification of neoplastic TM, thanks to the high PPV. Moreover, when a neoplastic testicular mass is identified, CEUS may provide a further guidance in excluding a malignant tumor, thanks to the high NPV.
To the best of our knowledge, we are the first to systematically review and meta-analyze the diagnostic performance of CEUS in TM. Moreover, our analyses addressed two important issues in clinical practice, that are both the differential diagnosis between neoplastic vs. non-neoplastic and malignant vs. benign TM. However, our study is not devoid of limitations. First, CEUS results may be interpreted according to several parameters (wash-out, wash-in, peak intensity, etc.); here, we selected only studies whose authors aimed to report CEUS’s results in the easiest fashion (i.e., just positive vs. negative). Second, some of the eligible studies were retrospective (n = 3) and/or limited in sample size. Third, not all lesions relied on histopathological findings, but follow-up, as well as clinical surveillance were applied as reference standard. However, the present study represents the strongest available evidence, since high-quality, well-designed, and adequately powered longitudinal studies are still lacking.
5. Conclusions
In daily practice, CEUS may represent a promising minimal invasive diagnostic tool for characterization of TM, since it allows clinicians to identify neoplastic lesions and exclude malignant tumors. However, these results need to be validated in multicenter prospective cohorts before definitive conclusions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11198990/s1, Figure S1: Search strategy, Table S1: (a) Quality assessment of the studies according to QUADAS–2: neoplastic vs. non-neoplastic testicular masses; (b) Quality assessment of the studies according to QUADAS–2: malignant vs. benign testicular masses, Table S2: True positive (TP), false positive (FP), true negative (TN), and false negative (FN) values for each study.
Author Contributions
Conceptualization, R.S.F. and A.T.; methodology, L.A.; software, R.M.; validation, L.A. and C.L.; formal analysis, A.T.; investigation, R.M.; resources, A.T.; data curation, G.F.; writing—original draft preparation, A.T.; writing—review and editing, R.S.F., G.F., and V.C.; visualization, C.L.; supervision, G.F.; project administration, V.C.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- EAU Guidelines. EDN Presented at the EAU Annual Congress Milan 2021; EAU: Arnhem, The Netherlands, 2021; ISBN 978-94-92671-13-4. [Google Scholar]
- Cassidy, F.H.; Ishioka, K.M.; McMahon, C.J.; Chu, P.; Sakamoto, K.; Lee, K.S.; Aganovic, L. MR imaging of scrotal tumors and pseudotumors. Radiographics 2010, 30, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, L.; Vinci, V.; Pozza, C.; Saldari, M.; Gianfrilli, D.; Pofi, R.; Bernardo, S.; Cantisani, V.; Lenzi, A.; Scialpi, M.; et al. A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: Distinctive features of Leydig cell tumours. Eur. Radiol. 2015, 25, 3586–3595. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Choi, H. High-efficiency high-voltage class F amplifier for high-frequency wireless ultrasound systems. PLoS ONE 2021, 16, e0249034. [Google Scholar] [CrossRef]
- Hayashi, M.; Matsui, O.; Ueda, K.; Kawamori, Y.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Takashima, T.; Nonomura, A.; Nakanuma, Y. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: Evaluation by CT during intraarterial injection of contrast medium. AJR 1999, 172, 969–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drudi, F.M.; Maghella, F.; Martino, G.; Messineo, D.; Ciccariello, M.; Cantisani, V.; Malpassini, F.; Liberatore, M.; D’Ambrosio, F. Detection of small testicular masses in monorchid patients using US, CPDUS, CEUS and US-guided biopsy. J. Ultrasound 2016, 19, 25–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenuta, M.; Sesti, F.; Bonaventura, I.; Mazzotta, P.; Pofi, R.; Gianfrilli, D.; Pozza, C. Use of contrast enhanced ultrasound in testicular diseases: A comprehensive review. Andrology 2021, 1-14. [Google Scholar] [CrossRef]
- Luzurier, A.; Maxwell, F.; Correas, J.; Benoit, G.; Izard, V.; Ferlicot, S.; Teglas, J.; Bellin, M.; Rocher, L. Qualitative and quantitative contrast-enhanced ultrasonography for the characterisation of non-palpable testicular tumours. Clin. Radiol. 2018, 73, e1–e322. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, M.; Muça, M.; Currò, F.; Bucci, S.; Rocher, L.; Cova, M.A. Multiparametric US for scrotal diseases. Abdom. Radiol. 2018, 43, 899–917. [Google Scholar] [CrossRef] [PubMed]
- Valentino, M.; Bertolotto, M.; Derchi, L.; Bertaccini, A.; Pavlica, P.; Martorana, G.; Barozzi, L. Role of contrast enhanced ultrasound in acute scrotal diseases. Eur. Radiol. 2011, 21, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.; Reitsma, J.B.; Leeflang, M.; Sterne, J.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Mallett, S.; Hopewell, S.; Roderick, P.J.; Deeks, J.J. The Moses-Littenberg meta-analytical method generates systematic differences in test accuracy compared to hierarchical meta-analytical models. J. Clin. Epidemiol. 2016, 80, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Lock, G.; Schmidt, C.; Helmich, F.; Stolle, E.; Dieckmann, K.-P. Early experience with contrast-enhanced ultrasound in the diagnosis of testicular masses: A feasibility study. Urology 2011, 77, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.; Pozza, C.; Gianfrilli, D.; Giannetta, E.; Lemma, A.; Pofi, R.; Barbagallo, F.; Manganaro, L.; Martino, G.; Lombardo, F.; et al. Differential diagnosis of nonpalpable testicular lesions: Qualitative and quantitative contrast-enhanced US of benign and malignant testicular tumors. Radiology 2014, 273, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Lock, G.; Schmidt, C.; Löning, T.; Dieckmann, K.-P. Real-Time Elastography and Contrast-Enhanced Ultrasonography in the Evaluation of Testicular Masses: A Comparative Prospective Study. Ultrasound Med. Biol. 2016, 42, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Lung, P.F.; Fang, C.; Jaffer, O.S.; Deganello, A.; Shah, A.; Hedayati, V.; Obaro, A.; Yusuf, G.T.; Huang, D.Y.; Sellars, M.E.; et al. Vascularity of Intra-testicular Lesions: Inter-observer Variation in the Assessment of Non-neoplastic Versus Neoplastic Abnormalities After Vascular Enhancement With Contrast-Enhanced Ultrasound. Ultrasound Med. Biol. 2020, 46, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.; De Zordo, T.; Dejaco, C.; Gruber, L.; Pichler, R.; Jaschke, W.; Dogra, V.S.; Aigner, F. Value of Multiparametric US in the Assessment of Intratesticular Lesions. Radiology 2017, 285, 640–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerchbaumer, M.H.; Auer, T.A.; Marticorena, G.S.; Stephan, C.; Hamm, B.; Jung, E.-M.; Fischer, T. Diagnostic performance of contrast-enhanced ultrasound (CEUS) in testicular pathologies: Single-center results. Clin. Hemorheol. Microcirc. 2019, 73, 347–357. [Google Scholar] [CrossRef]
- van Kalsbeek, R.J.; van der Pal, H.J.; Hjorth, L.; Winther, J.F.; Michel, G.; Haupt, R.; Uyttebroeck, A.; O’Brien, K.; Kepakova, K.; Follin, C.; et al. The European multistakeholder PanCareFollowUp project: Novel, person-centred survivorship care to improve care quality, effectiveness, cost-effectiveness and accessibility for cancer survivors and caregivers. Eur. J. Cancer 2021, 153, 74–85. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).