A Comparative Study of Cerium- and Ytterbium-Based GO/g-C3N4/Fe2O3 Composites for Electrochemical and Photocatalytic Applications
Abstract
:1. Introduction
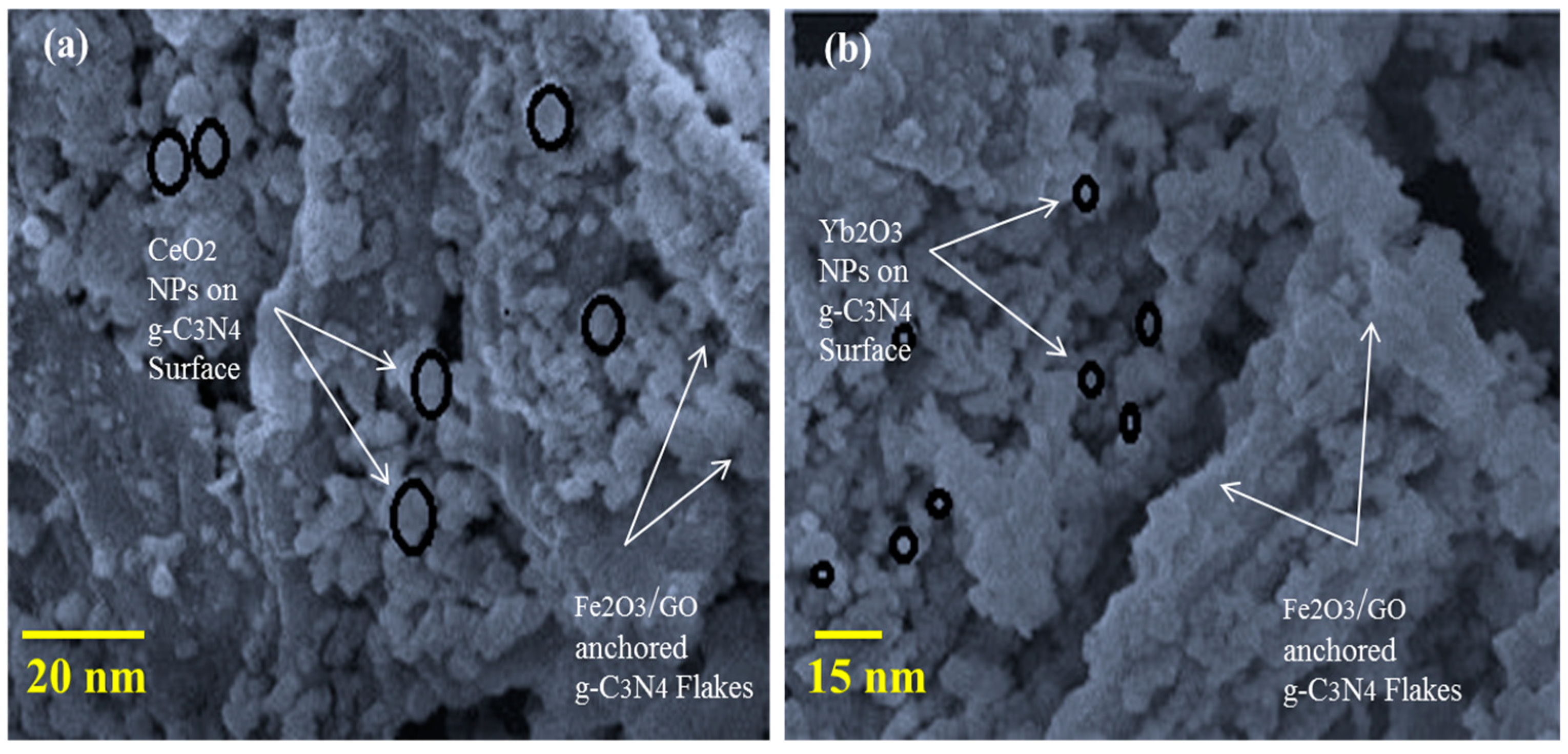
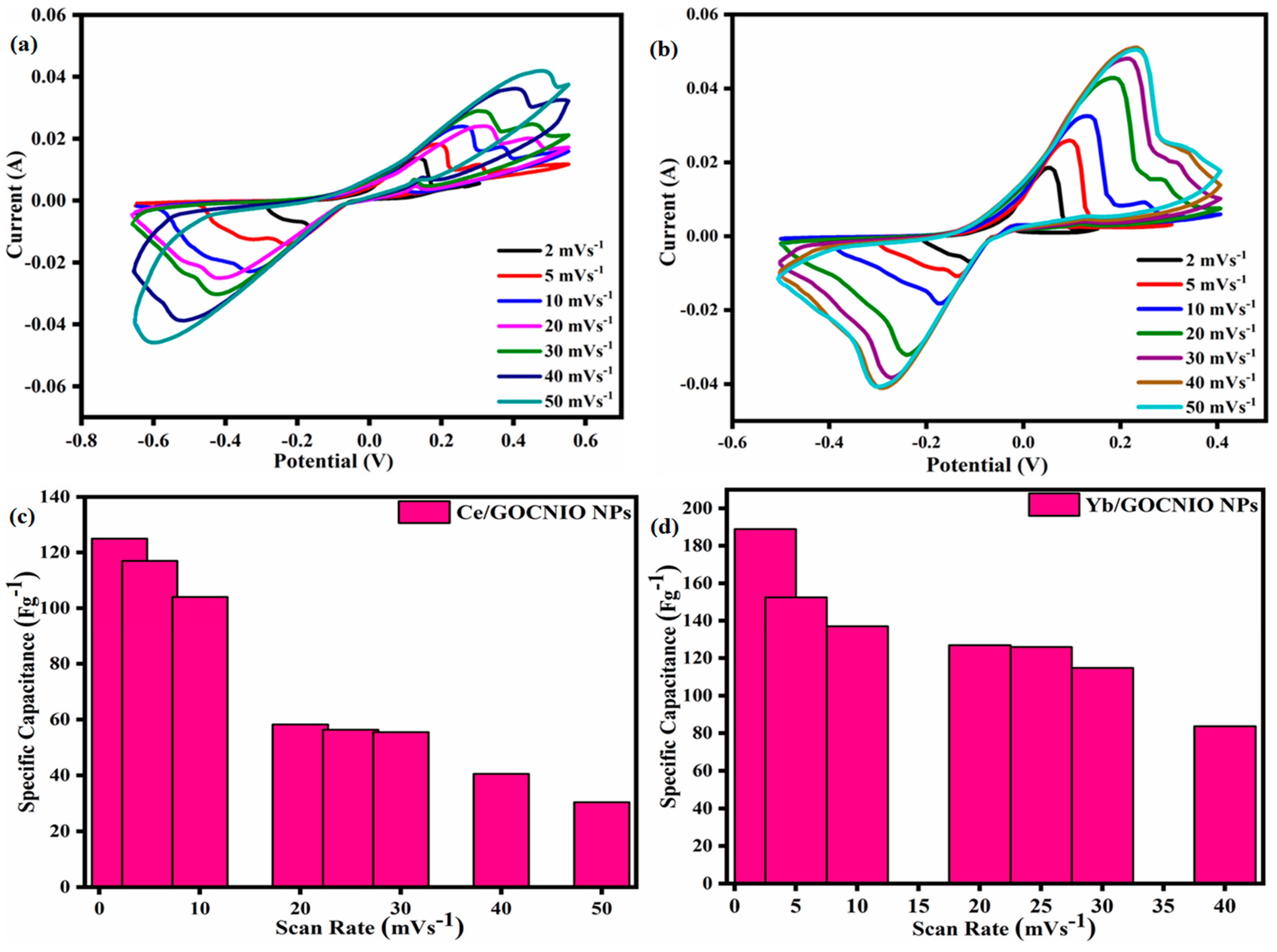
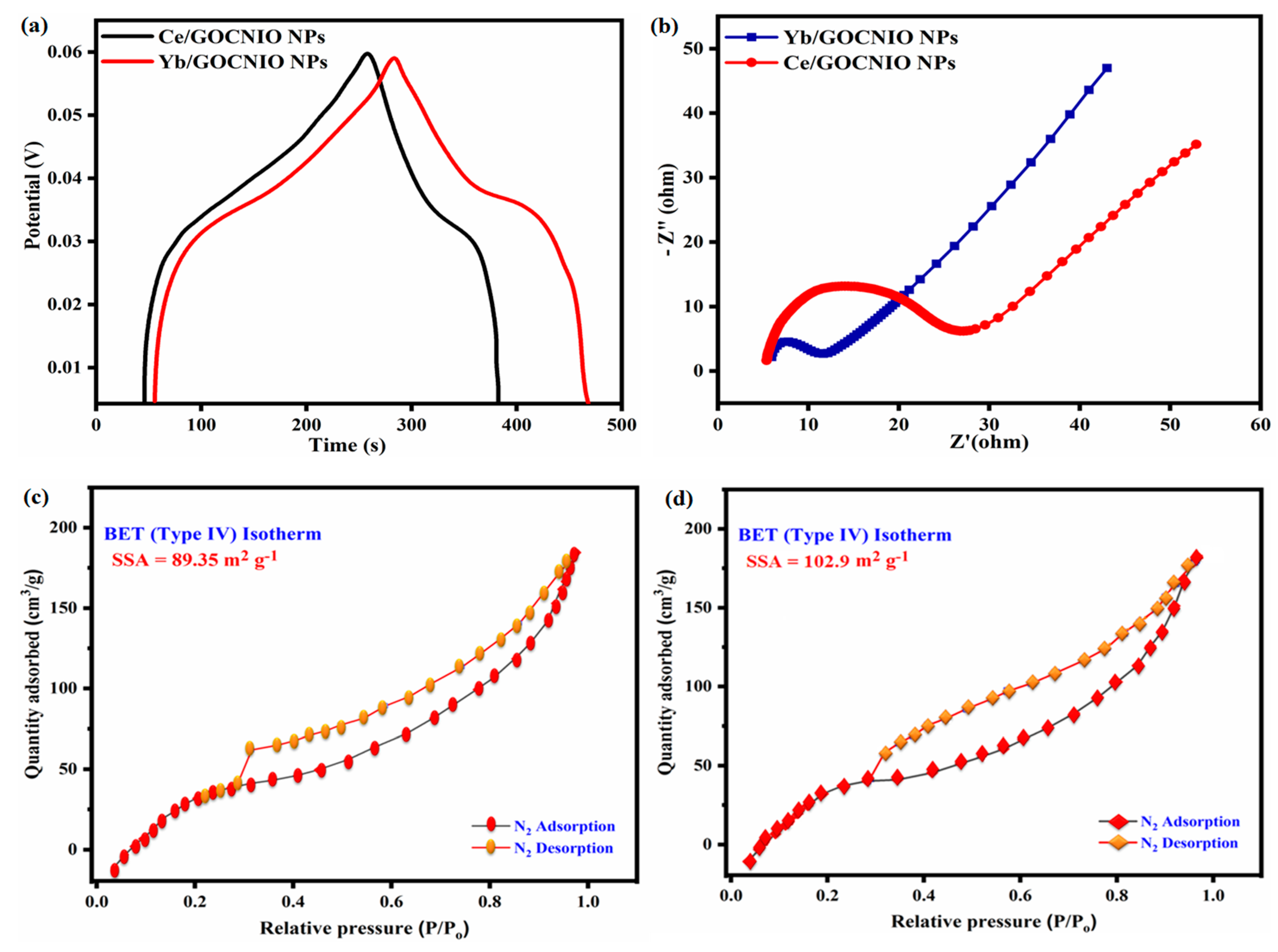
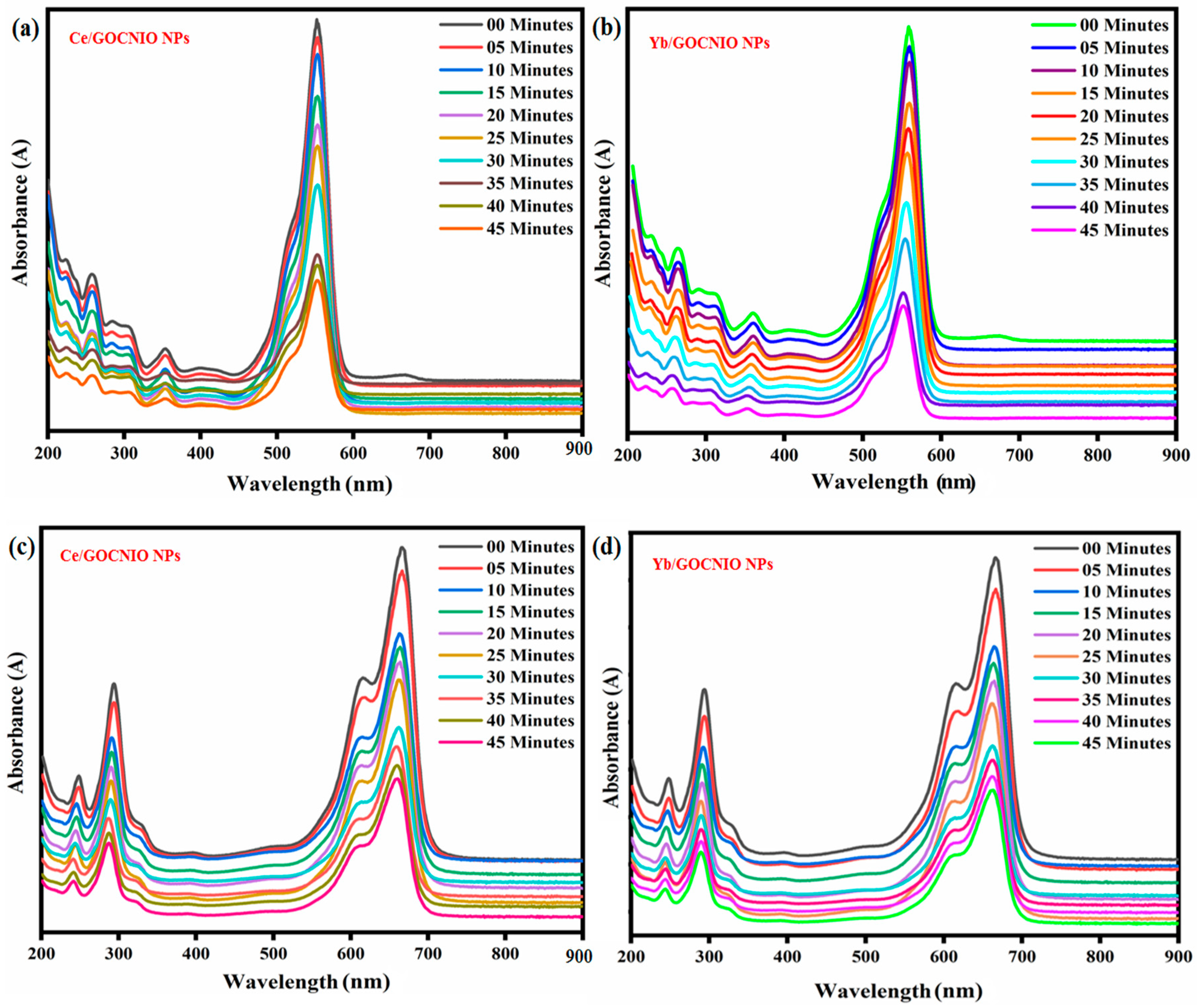
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. Materials Used
4.2. Synthesis of GO, Fe2O3, and Fe2O3/GO Nano-Composite
4.3. Synthesis of Carbon Nitride (g-C3N4) and GOCN IONPs
4.4. Synthesis of CeO2 and Yb2O3 Nanoparticles
4.5. Synthesis of CeO2/GOCN IONPs and Yb2O3/GOCN IONPs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Liu, C.-J. Preparation and application of iron oxide/graphene based composites for electrochemical energy storage and energy conversion devices: Current status and perspective. Nano Energy 2015, 11, 277–293. [Google Scholar] [CrossRef]
- Singh, V.; Patra, M.; Manoth, M.; Gowd, G.; Vadera, S.; Kumar, N. In situ synthesis of graphene oxide and its composites with iron oxide. New Carbon Mater. 2009, 24, 147–152. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Usui, H.; Domi, Y.; Iwama, E.; Kurokawa, H.; Sakaguchi, H. α-Fe2O3 conversion anodes with improved Na-Storage properties by Sb addition. Mater. Chem. Phys. 2021, 272, 125023. [Google Scholar] [CrossRef]
- Choi, D.S.; Lee, H.; Tieves, F.; Lee, Y.W.; Son, E.J.; Zhang, W.; Shin, B.; Hollmann, F.; Park, C.B. Bias-Free In Situ H2O2 Generation in a Photovoltaic-Photoelectrochemical Tandem Cell for Biocatalytic Oxyfunctionalization. ACS Catal. 2019, 9, 10562–10566. [Google Scholar] [CrossRef]
- Sanati, S.; Rezvani, Z. g-C3N4 nanosheet@ CoAl-layered double hydroxide composites for electrochemical energy storage in supercapacitors. Chem. Eng. J. 2019, 362, 743–757. [Google Scholar] [CrossRef]
- Joseph, N.; Bose, A.C. Metallic MoS2 grown on porous g-C3N4 as an efficient electrode material for supercapattery application. Electrochim. Acta 2019, 301, 401–410. [Google Scholar] [CrossRef]
- Kaipannan, S.; Marappan, S. Fabrication of 9.6 V high-performance asymmetric supercapacitors stack based on nickel hexacyanoferrate-derived Ni(OH)2 nanosheets and bio-derived activated carbon. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Le, K.; Gao, M.; Liu, J.; Wang, Z.; Wang, F.; Murugadoss, V.; Wu, S.; Ding, T.; Guo, Z. MOF-derived hierarchical core-shell hollow iron-cobalt sulfides nanoarrays on Ni foam with enhanced electrochemical properties for high energy density asymmetric supercapacitors. Electrochim. Acta 2019, 323, 134826. [Google Scholar] [CrossRef]
- Hussain, S.; Javed, M.S.; Asimd, S.; Shaheen, A.; Khan, A.J.; Abbas, Y.; Ullah, N.; Iqbal, A.; Wang, M.; Qiao, G.; et al. Novel gravel-like NiMoO4 nanoparticles on carbon cloth for outstanding supercapacitor applications. Ceram. Int. 2020, 46, 6406–6412. [Google Scholar] [CrossRef]
- Mohammadi, A.; Arsalani, N.; Tabrizi, A.G.; Moosavifard, S.E.; Naqshbandi, Z.; Ghadimi, L.S. Engineering rGO-CNT wrapped Co3S4 nanocomposites for high-performance asymmetric supercapacitors. Chem. Eng. J. 2018, 334, 66–80. [Google Scholar] [CrossRef]
- Zhou, S.-X.; Tao, X.-Y.; Ma, J.; Guo, L.-T.; Zhu, Y.-B.; Fan, H.-L.; Liu, Z.-S.; Wei, X.-Y. Synthesis of flower-like PANI/g-C3N4 nanocomposite as supercapacitor electrode. Vacuum 2018, 149, 175–179. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 reduction: From the electrochemical to photochemical approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef]
- Humayun, M.; Hu, Z.; Khan, A.; Cheng, W.; Yuan, Y.; Zheng, Z.; Fu, Q.; Luo, W. Highly efficient degradation of 2,4-dichlorophenol over CeO2/g-C3N4 composites under visible-light irradiation: Detailed reaction pathway and mechanism. J. Hazard. Mater. 2019, 364, 635–644. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Z.; Huang, S.; Zhang, Y.; Zhang, M.; Zeng, Y.-J.; Ruan, S. Ce-Doped Graphitic Carbon Nitride Derived from Metal Organic Frameworks as a Visible Light-Responsive Photocatalyst for H2 Production. Nanomaterials 2019, 9, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Qi, W.; Zhao, Y.; Tang, X.; Qiu, Y.; Su, D.; Fan, H.; Wang, G. Hollow CeO2 spheres conformally coated with graphitic carbon for high-performance supercapacitor electrodes. Appl. Surf. Sci. 2019, 463, 244–252. [Google Scholar] [CrossRef]
- Farbod, F.; Mazloum-Ardakani, M.; Naderi, H.R.; Mohammadian-Sarcheshmeh, H. Synthesis of a porous interconnected nitrogen-doped graphene aerogel matrix incorporated with ytterbium oxide nanoparticles and its application in superior symmetric supercapacitors. Electrochim. Acta 2019, 306, 480–488. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Pujar, M.S.; Hunagund, S.M.; Desai, V.R.; Patil, S.; Sidarai, A.H. One-step synthesis and characterizations of cerium oxide nanoparticles in an ambient temperature via Co-precipitation method. Conf. Proc. 2018, 1942, 050026. [Google Scholar]
- Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T. Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method. Rev. Mex. Física 2016, 62, 496–499. [Google Scholar]
- Sun, Y.-H.; Yang, Z.-M.; Xie, C.-N.; Jiang, Z.-H. Comparative Study of Synthesis and Characterization of YAG: Yb3+ Nanoparticles Using Different Precipitator by Co-Precipitation Method. J. Nanosci. Nanotechnol. 2010, 10, 8102–8111. [Google Scholar] [CrossRef]
- Unal, F.; Kaya, F. Modelling of relation between synthesis parameters and average crystallite size of Yb2O3 nanoparticles using Box-Behnken design. Ceram. Int. 2020, 46, 26800–26808. [Google Scholar] [CrossRef]
- Naderi, H.R.; Ganjali, M.R.; Dezfuli, A.S.; Norouzi, P. Sonochemical preparation of a ytterbium oxide/reduced graphene oxide nanocomposite for supercapacitors with enhanced capacitive performance. RSC Adv. 2016, 6, 51211–51220. [Google Scholar] [CrossRef]
- Abdulah, H.I.; Farhan, A.M.; Ali, A.J. Photo-synthesis of nanosized α–Fe2O3. J. Chem. Pharm. Res. 2015, 7, 588–591. [Google Scholar]
- Chen, Y.; Liu, X.; Hou, L.; Guo, X.; Fu, R.; Sun, J. Construction of covalent bonding oxygen-doped carbon nitride/graphitic carbon nitride Z-scheme heterojunction for enhanced visible-light-driven H2 evolution. Chem. Eng. J. 2020, 383, 123132. [Google Scholar] [CrossRef]
- Aleksandrzak, M.; Kukulka, W.; Mijowska, E. Graphitic carbon nitride/graphene oxide/reduced graphene oxide nanocomposites for photoluminescence and photocatalysis. Appl. Surf. Sci. 2017, 398, 56–62. [Google Scholar] [CrossRef]
- Baig, U.; Khan, A.; Gondal, M.A.; Dastageer, M.A.; Falath, W.S. Laser induced anchoring of nickel oxide nanoparticles on polymeric graphitic carbon nitride sheets using pulsed laser ablation for efficient water splitting under visible light. Nanomaterials 2020, 10, 1098. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Bose, A.C.; Rameshbabu, N. X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson–Hall analysis. Phys. B Condens. Matter 2010, 405, 4256–4261. [Google Scholar] [CrossRef]
- Hosokawa, S.; Iwamoto, S.; Inoue, M. Synthesis of Mesoporous Needle-Shaped Ytterbium Oxide Crystals by Solvothermal Treatment of Ytterbium Chloride. J. Am. Ceram. Soc. 2007, 90, 1215–1221. [Google Scholar] [CrossRef]
- Bosund, M.; Mizohata, K.; Hakkarainen, T.; Putkonen, M.; Söderlund, M.; Honkanen, S.; Lipsanen, H. Atomic layer deposition of ytterbium oxide using β-diketonate and ozone precursors. Appl. Surf. Sci. 2009, 256, 847–851. [Google Scholar] [CrossRef]
- Dakhel, A. Annealing effect on the structural, optical and electrical properties of Yb–Mn oxide thin films. J. Alloys Compd. 2009, 476, 28–32. [Google Scholar] [CrossRef]
- Dezfuli, A.S.; Ganjali, M.R.; Norouzi, P.; Faridbod, F. Facile sonochemical synthesis and electrochemical investigation of ceria/graphene nanocomposites. J. Mater. Chem. B 2015, 3, 2362–2370. [Google Scholar] [CrossRef]
- Prabaharan, D.M.D.M.; Sadaiyandi, K.; Mahendran, M.; Sagadevan, S. Structural, optical, morphological and dielectric properties of cerium oxide nanoparticles. Mater. Res. 2016, 19, 478–482. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Kim, Y.-H.; Park, C.-J. One step hydrothermal synthesis of a carbon nanotube/cerium oxide nanocomposite and its electrochemical properties. Nanotechnology 2013, 24, 365401. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.-P.; Dai, R.-R.; Liu, X.; Weerasooriya, R.; Hong, Z.-Y.; Chen, X.; Wu, Y.-C. New strategy for fabricating Cd (II) sensing electrochemical interface based on enhanced adsorption followed by redox processes: Ferro-cerium oxide nanocomposite as an example. J. Alloys Compd. 2020, 829, 154551. [Google Scholar] [CrossRef]
- Sabeeh, H.; Zulfiqar, S.; Aadil, M.; Shahid, M.; Shakir, I.; Khan, M.A.; Warsi, M.F. Flake-like MoS2 nano-architecture and its nanocomposite with reduced Graphene Oxide for hybrid supercapacitors applications. Ceram. Int. 2020, 46, 21064–21072. [Google Scholar] [CrossRef]
- Aghazadeh, M. Cathodic electrochemical deposition of nanostructured metal oxides/hydroxides and their composites for supercapacitor applications: A review. Anal. Bioanal. Electrochem 2019, 11, 211–266. [Google Scholar]
- Yadav, N.; Singh, M.K.; Yadav, N.; Hashmi, S.A. High performance quasi-solid-state supercapacitors with peanut-shell-derived porous carbon. J. Power Sources 2018, 402, 133–146. [Google Scholar] [CrossRef]
- Nazir, M.A.; Bashir, M.S.; Jamshaid, M.; Anum, A.; Najam, T.; Shahzad, K.; Imran, M.; Shah, S.S.A.; Rehman, A.U. Synthesis of porous secondary metal-doped MOFs for removal of Rhodamine B from water: Role of secondary metal on efficiency and kinetics. Surf. Interfaces 2021, 25, 101261. [Google Scholar] [CrossRef]
- Farooq, N.; ur Rehman, A.; Qureshi, A.M.; ur Rehman, Z.; Ahmad, A.; Aslam, M.K.; Javed, H.M.A.; Hussain, S.; Habila, M.A.; AlMasoud, N. Au@ GO@ g-C3N4 and Fe2O3 nanocomposite for efficient photocatalytic and electrochemical applications. Surf. Interfaces 2021, 26, 101399. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Xue, N.; Wang, P.; Pei, M.; Guo, W. Ultra-Highly Efficient Removal of Methylene Blue Based on Graphene Oxide/TiO2/Bentonite Sponge. Materials 2020, 13, 824. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Uresti, D.; Vázquez, A.; Sanchez-Martinez, D.; Obregón, S. Performance of the polymeric g-C3N4 photocatalyst through the degradation of pharmaceutical pollutants under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2016, 324, 47–52. [Google Scholar] [CrossRef]
- Monga, D.; Basu, S. Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: Role of shape of TiO2. Adv. Powder Technol. 2019, 30, 1089–1098. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.; Allabadi, O.; Aljarrah, M.T. Photocatalytic activity of graphene oxide/zinc oxide nanocomposites with embedded metal nanoparticles for the degradation of organic dyes. ACS Omega 2020, 5, 28046–28055. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, A.; Mark, J.A.M.; Deivatamil, D.; Revathi, J.; Prince Jesuraj, J. Sunlight active photocatalytic studies of Fe2O3 based nanocomposites developed via two-pot synthesis technique. Inorg. Chem. Commun. 2021, 124, 108417. [Google Scholar] [CrossRef]
- Keerthana, S.; Yuvakkumar, R.; Ravi, G.; Kumar, P.; Elshikh, M.S.; Alkhamis, H.H.; Alrefaei, A.F.; Velauthapillai, D. A strategy to enhance the photocatalytic efficiency of α-Fe2O3. Chemosphere 2021, 270, 129498. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Cao, J.; Tang, Q.; Li, M.; Kang, P.; Shi, C.; Ma, M. Ultrasonic-assisted synthesis of 2D α-Fe2O3@ g-C3N4 composite with excellent visible light photocatalytic activity. Catalysts 2018, 8, 457. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, K.; Najam, T.; Bashir, M.S.; Nazir, M.A.; Rehman, A.U.; Bashir, M.A.; Shah, S.S.A. Fabrication of Periodic Mesoporous Organo Silicate (PMOS) composites of Ag and ZnO: Photo-catalytic degradation of methylene blue and methyl orange. Inorg. Chem. Commun. 2021, 123, 108357. [Google Scholar] [CrossRef]
- Jamshaid, M.; Rehman, A.U.; Kumar, O.P.; Iqbal, S.; Nazir, M.A.; Anum, A.; Khan, H.M. Design of dielectric and photocatalytic properties of Dy–Ni substituted Ca0.5Pb0.5−x Fe12−yO19 M-type hexaferrites. J. Mater. Sci. Mater. Electron. 2021, 32, 16255–16268. [Google Scholar] [CrossRef]
- Wang, J.; Guo, P.; Guo, Q.; Jönsson, P.G.; Zhao, Z. Fabrication of novel gC3N4/nanocage ZnS composites with enhanced photocatalytic activities under visible light irradiation. CrystEngComm 2014, 16, 4485–4492. [Google Scholar] [CrossRef]
- Kolesnyk, I.; Kujawa, J.; Bubela, H.; Konovalova, V.; Burban, A.; Cyganiuk, A.; Kujawski, W. Photocatalytic properties of PVDF membranes modified with g-C3N4 in the process of Rhodamines decomposition. Sep. Purif. Technol. 2020, 250, 117231. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Q.; Cui, D. Preparation and photocatalytic properties of g-C3N4/TiO2 hybrid composite. J. Mater. Sci. Technol. 2010, 26, 925–930. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Qiblawey, H.; Khraisheh, M.; Atieh, M.; Al-Ghouti, M. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int. 2020, 46, 23997–24007. [Google Scholar] [CrossRef]
- Hadadian, S.; Masoudpanah, S.; Alamolhoda, S. Solution combustion synthesis of Fe3O4 powders using mixture of CTAB and citric acid fuels. J. Supercond. Nov. Magn. 2019, 32, 353–360. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule self-assembly synthesis of porous few-layer carbon nitride for highly efficient photoredox catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, L.; Škuta, R.; Matějka, V.; Dvorský, R.; Matýsek, D.; Henych, J.; Mančík, P.; Praus, P. Graphene oxide and graphitic carbon nitride nanocomposites assembled by electrostatic attraction forces: Synthesis and characterization. Mater. Chem. Phys. 2019, 228, 228–236. [Google Scholar] [CrossRef]






| Material | Electro- Negativity (eV) | Band Gap (eV) | Valence Band Potential (eV) | Conduction Band Potential (eV) |
|---|---|---|---|---|
| Fe2O3 | 5.27 | 2.3 | 2.1 | −0.13 |
| g-C3N4 | 4.67 | 2.7 | 1.77 | −0.93 |
| Photocatalyst | Quantity (mg) | Dyes Used | Removal (%) | Time (Min) | Reference |
|---|---|---|---|---|---|
| Fe2O3@MgO | 0.1 | RhB/MB | 73/91 | 120 | [45] |
| Co-Fe3O4 | 25 | MB | 92 | 120 | [46] |
| Fe2O3/g-C3N4 | 1 | RhB | 90 | 120 | [47] |
| Ag-PMOS | 10 | MB | 81 | 60 | [48] |
| CaPbFe12O19 Composite | 50 | MB | 85 | 120 | [49] |
| ZnS/g-C3N4 | 5 | RhB | 90 | 90 | [50] |
| PVDF/g-C3N4 | 5 | RhB | 80 | 120 | [51] |
| TiO2/g-C3N4 | 10 | RhB | 80 | 300 | [52] |
| Yb2O3/GOCNIONPs | 5 | RhB/MB | 67.11/83.5 | 45/45 | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooq, N.; Luque, R.; Hessien, M.M.; Qureshi, A.M.; Sahiba, F.; Nazir, M.A.; ur Rehman, A. A Comparative Study of Cerium- and Ytterbium-Based GO/g-C3N4/Fe2O3 Composites for Electrochemical and Photocatalytic Applications. Appl. Sci. 2021, 11, 9000. https://doi.org/10.3390/app11199000
Farooq N, Luque R, Hessien MM, Qureshi AM, Sahiba F, Nazir MA, ur Rehman A. A Comparative Study of Cerium- and Ytterbium-Based GO/g-C3N4/Fe2O3 Composites for Electrochemical and Photocatalytic Applications. Applied Sciences. 2021; 11(19):9000. https://doi.org/10.3390/app11199000
Chicago/Turabian StyleFarooq, Nosheen, Rafael Luque, Mahmoud M. Hessien, Ashfaq Mahmood Qureshi, Farzana Sahiba, Muhammad Altaf Nazir, and Aziz ur Rehman. 2021. "A Comparative Study of Cerium- and Ytterbium-Based GO/g-C3N4/Fe2O3 Composites for Electrochemical and Photocatalytic Applications" Applied Sciences 11, no. 19: 9000. https://doi.org/10.3390/app11199000
APA StyleFarooq, N., Luque, R., Hessien, M. M., Qureshi, A. M., Sahiba, F., Nazir, M. A., & ur Rehman, A. (2021). A Comparative Study of Cerium- and Ytterbium-Based GO/g-C3N4/Fe2O3 Composites for Electrochemical and Photocatalytic Applications. Applied Sciences, 11(19), 9000. https://doi.org/10.3390/app11199000








