Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation
Abstract
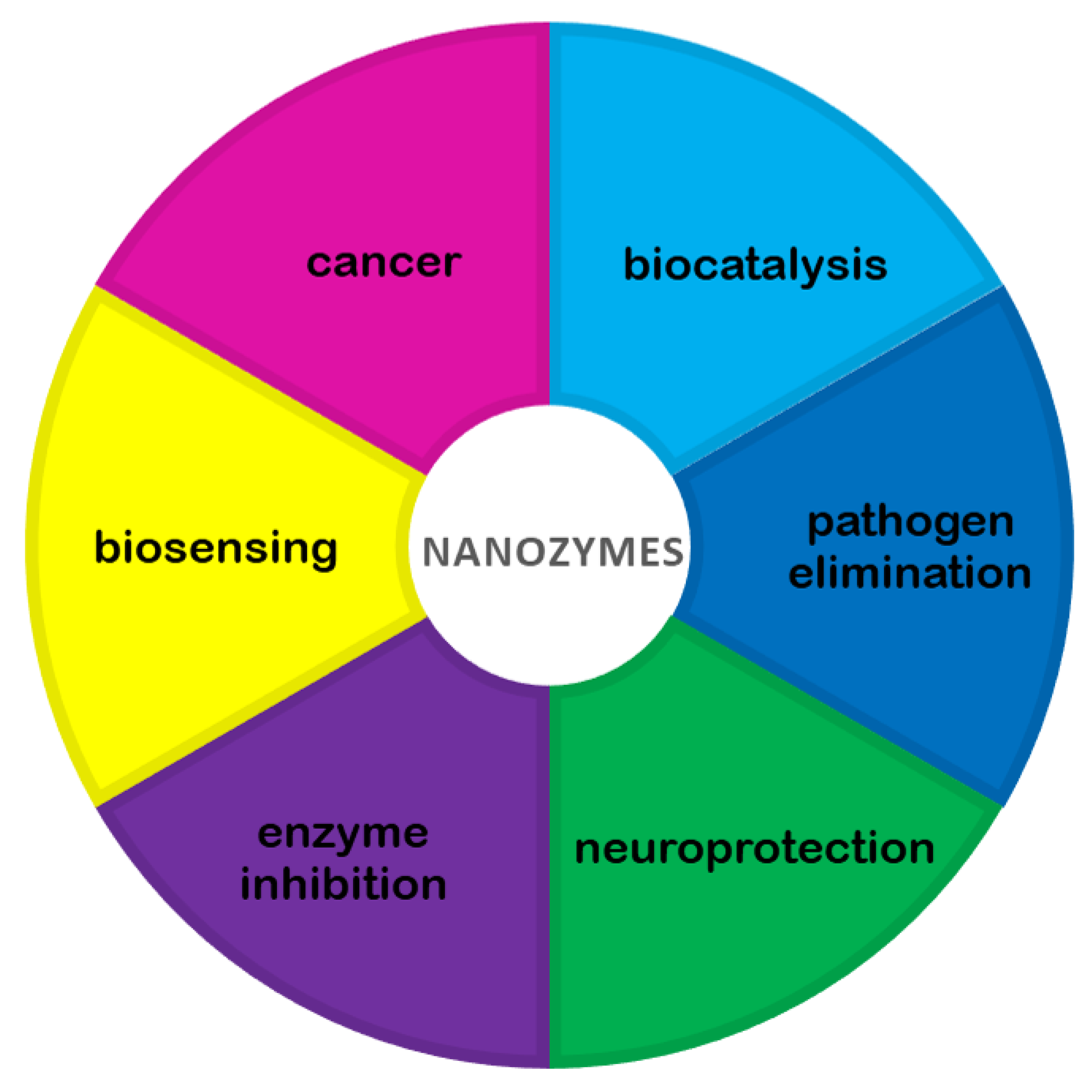
:1. Introduction
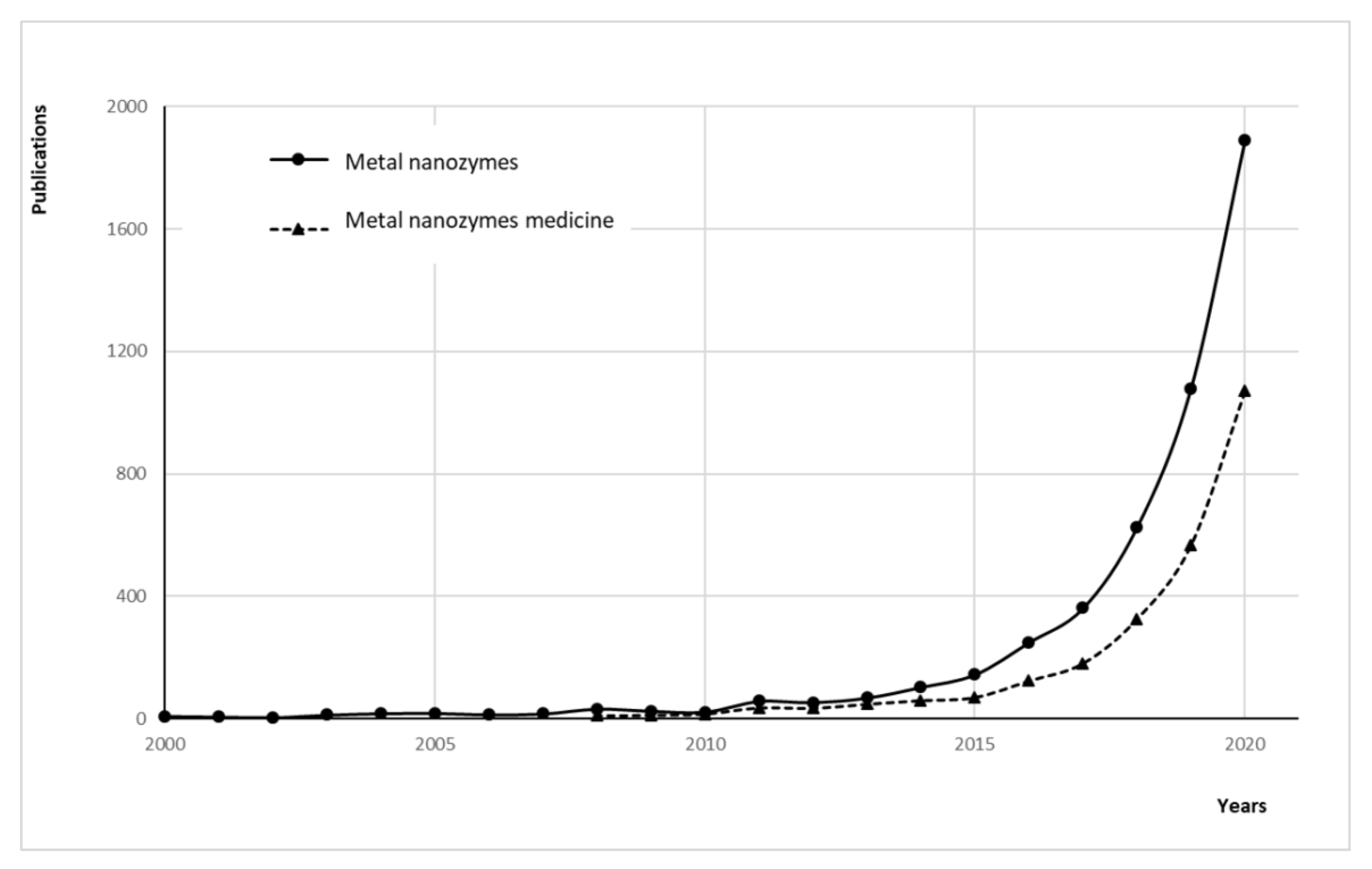
2. A Short History of Nanozymes
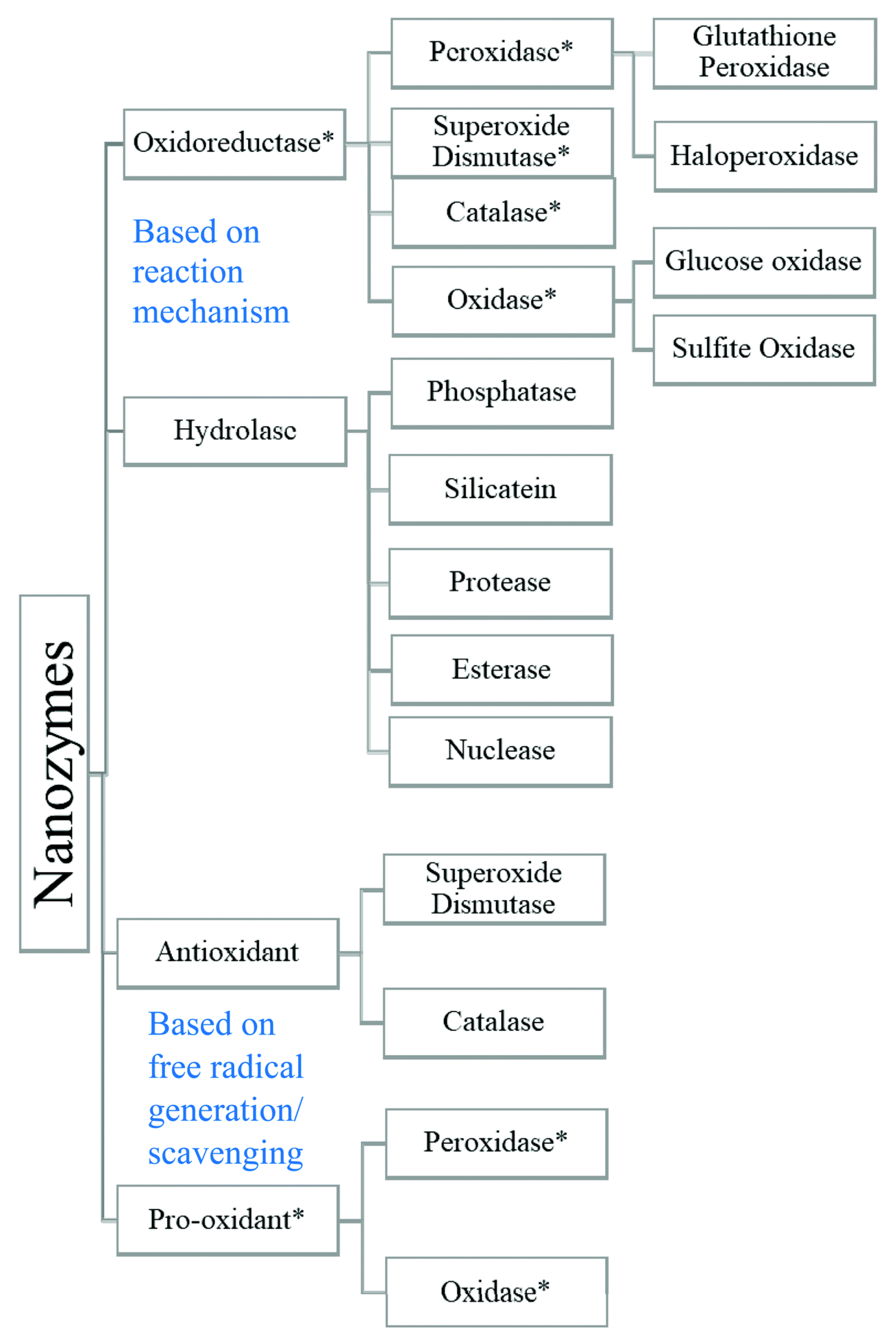
3. Mechanisms of Pro-Oxidative and Antioxidant Action of Nanozymes
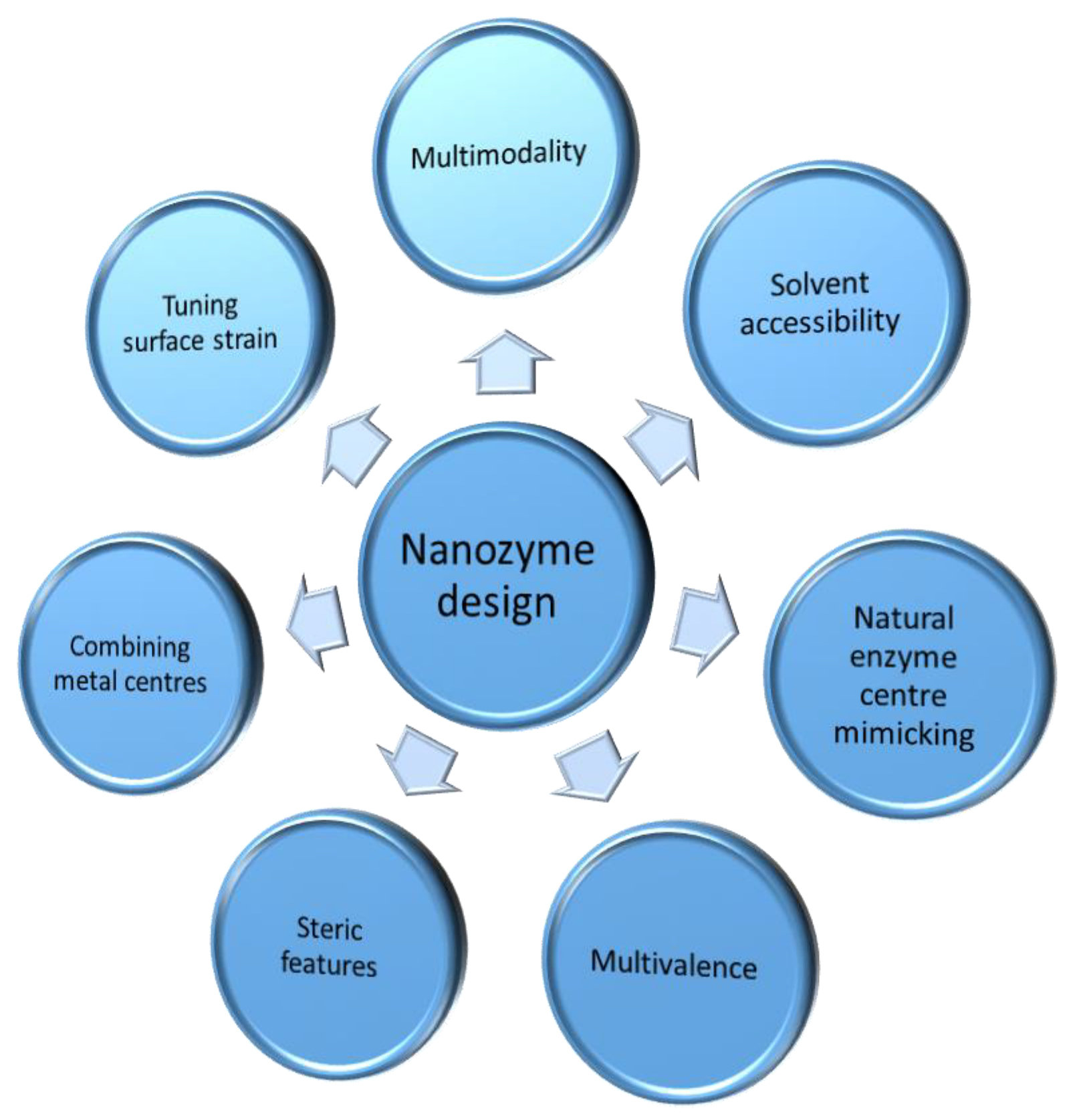
4. Strategies in the Quest for New Nanozymes
5. Metal Nanozymes as Enzyme Inhibitors
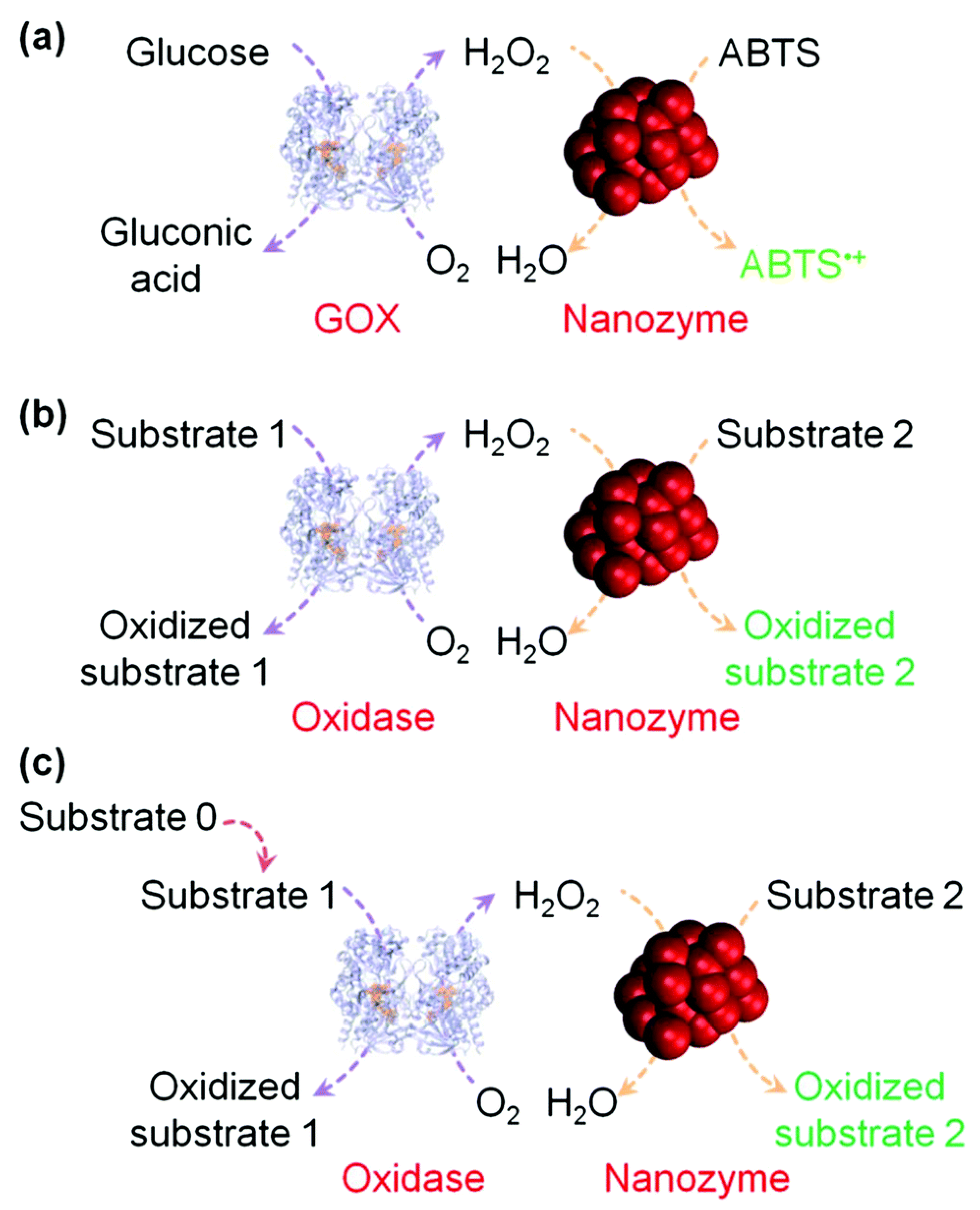
6. Metal Nanozymes as Cellular Probes for Biosensing
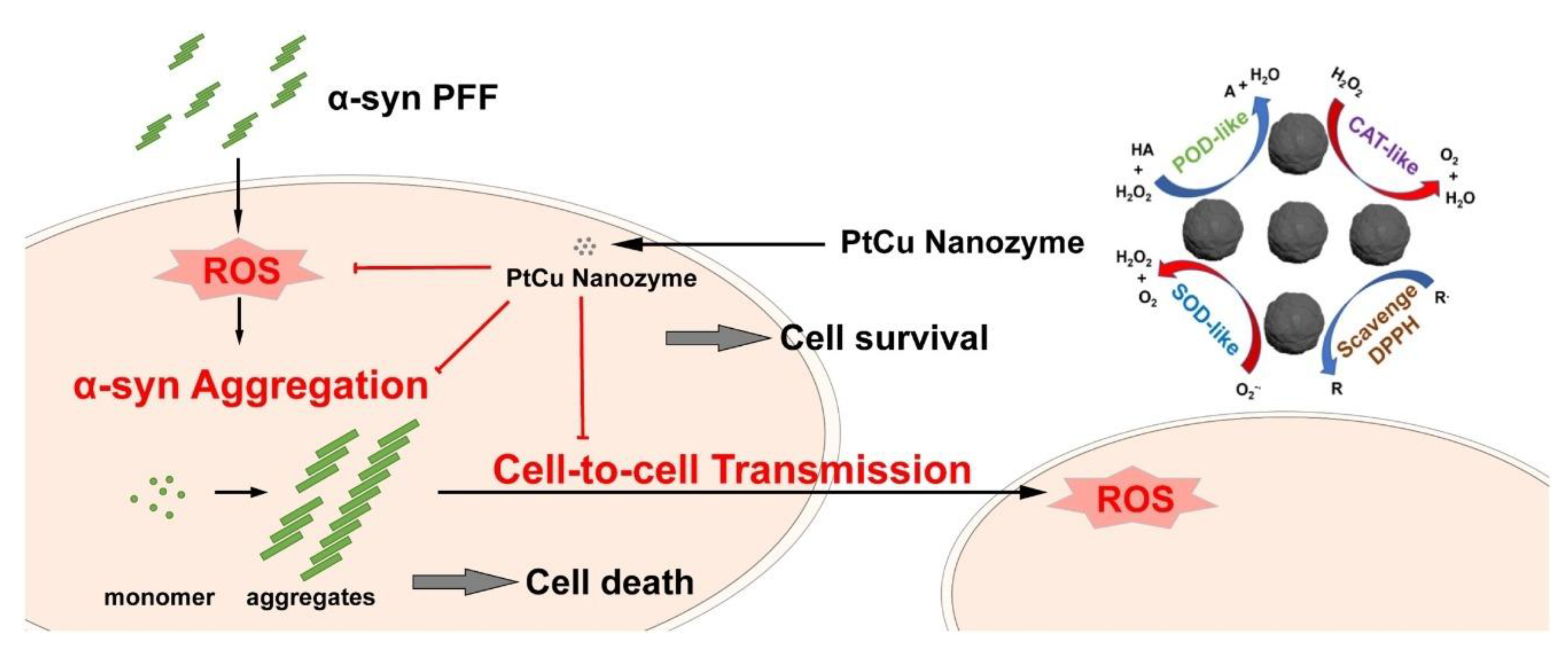
7. Nanozymes in the Fighting of Neurodegenerative Diseases
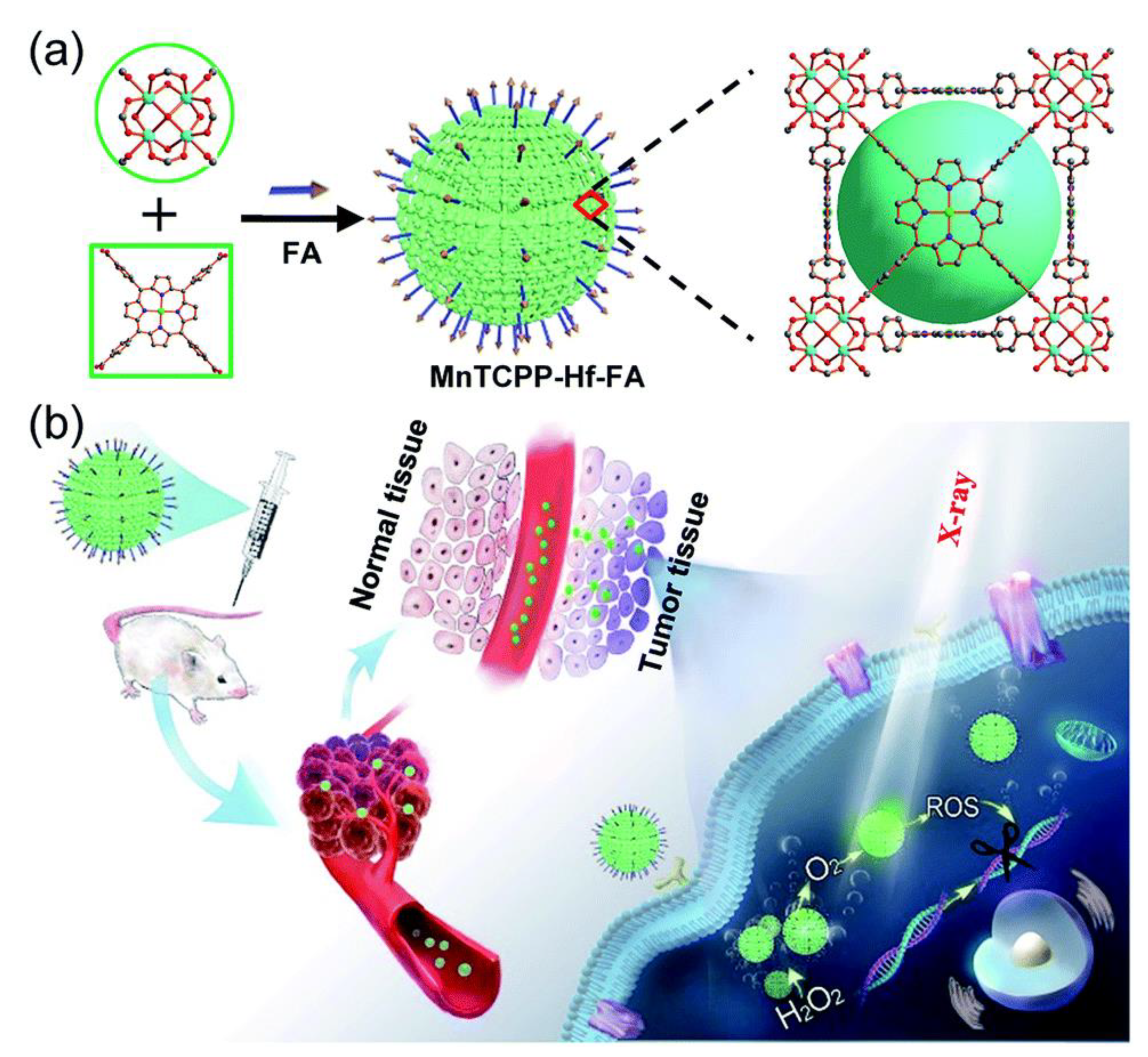
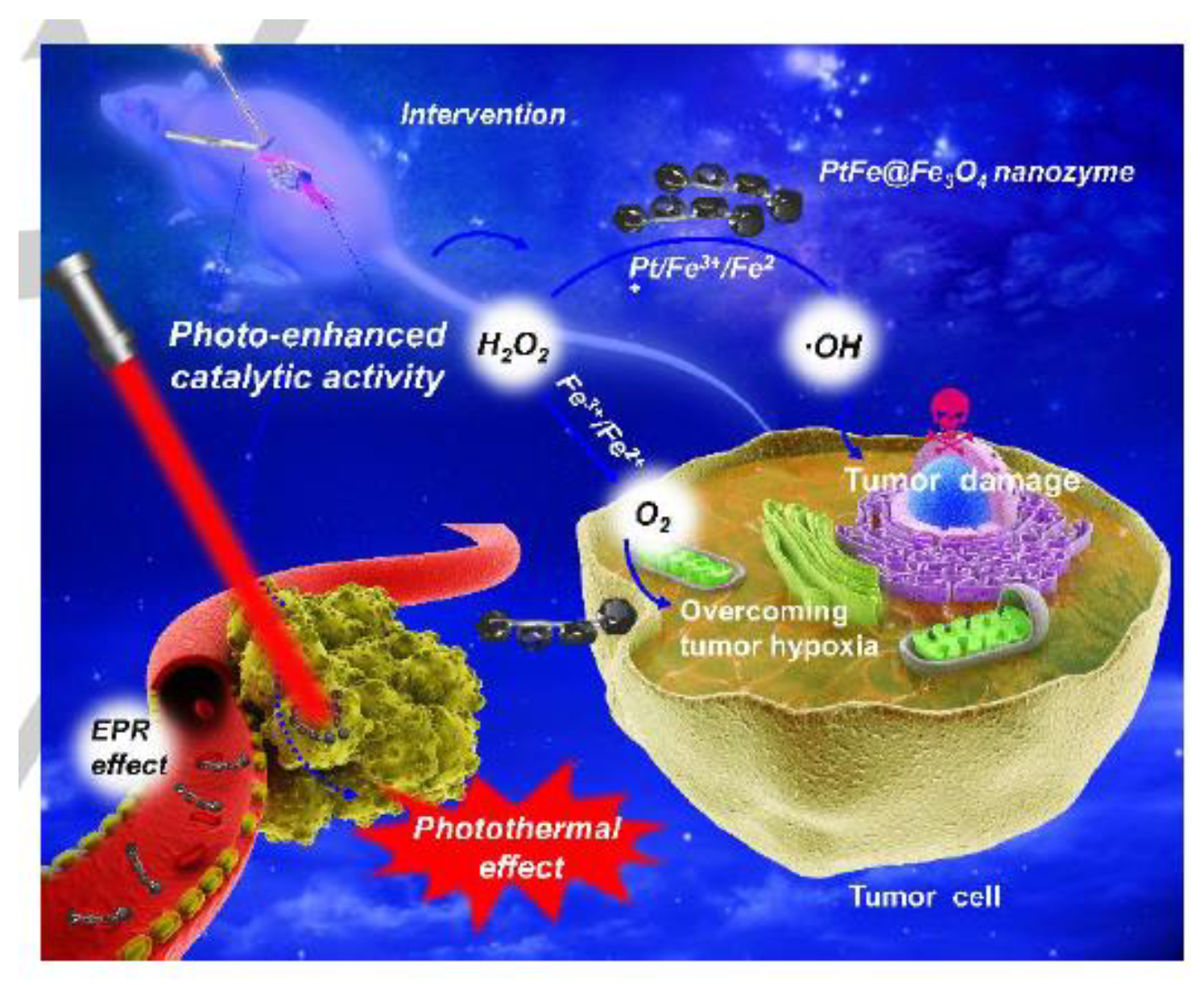
8. Metal Nanozymes in Cancer Treatment
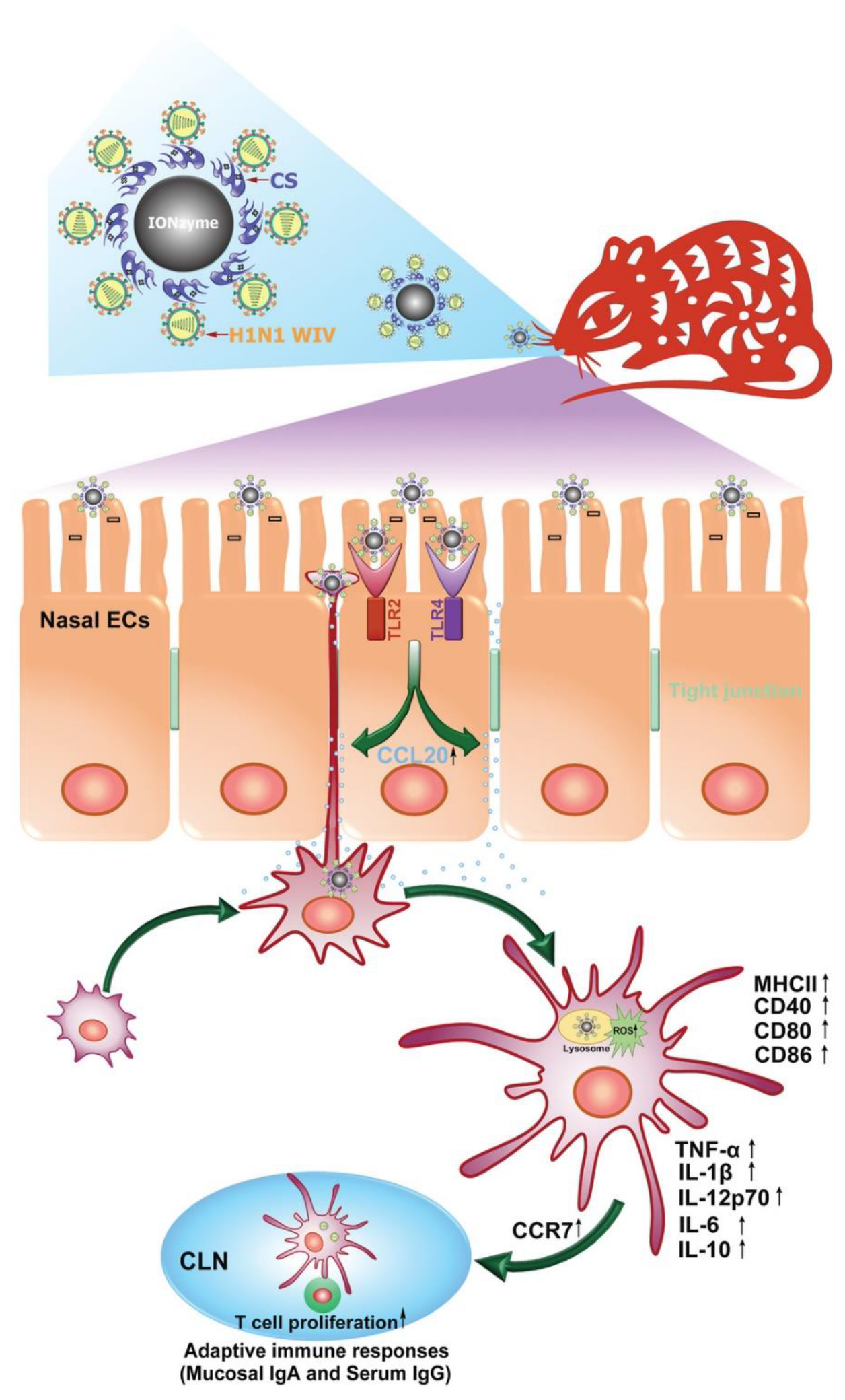
9. Nanozymes Combating Pathogens
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korschelt, K.; Tahir, M.N.; Tremel, W. A Step into the Future: Applications of Nanoparticle Enzyme Mimics. Chemistry 2018, 24, 9703–9713. [Google Scholar] [CrossRef]
- Kuah, E.; Toh, S.; Yee, J.; Ma, Q.; Gao, Z. Enzyme Mimics: Advances and Applications. Chem. Eur. J. 2016, 22, 8404–8430. [Google Scholar] [CrossRef]
- Wiester, M.J.; Ulmann, P.A.; Mirkin, C.A. Enzyme Mimics Based upon Supramolecular Coordination Chemistry. Angew. Chem. Int. Ed. Engl. 2011, 50, 114–137. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes Inspired by Natural Enzymes. Acc. Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Nanozymes: A Promising Horizon for Medical and Environmental Applications. J. Clust. Sci. 2021. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential Applications of Enzymes Immobilized on/in Nano Materials: A Review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Bora, T.; Dutta, J. Applications of Nanotechnology in Wastewater Treatment—A Review. J. Nanosci. Nanotechnol. 2014, 14, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, L.; You, Q.; Chen, Y. PA-Tb-Cu MOF as Luminescent Nanoenzyme for Catalytic Assay of Hydrogen Peroxide. Biosens. Bioelectron. 2017, 96, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Enzyme Mimics for the Catalytic Generation of Nitric Oxide from Endogenous Prodrugs. Small 2020, 16, e1907635. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.; Overman, L.E. “Artificial Enzyme” Combining a Metal Catalytic Group and a Hydrophobic Binding Cavity. J. Am. Chem. Soc. 1970, 92, 1075–1077. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. Int. Ed. 2004, 43, 6165–6169. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal Organic Frameworks Mimicking Natural Enzymes: A Structural and Functional Analogy. Chem. Soc. Rev. 2016, 45, 4127–4170. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme Mimicry for Combating Bacteria and Biofilms. Acc. Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Demkiv, O.; Prokopiv, T.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review. Sensors 2020, 20, 4509. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, R.; Zhang, P.; Qin, Y.; Chen, T.; Ye, F. A Bimetallic (Co/2Fe) Metal-Organic Framework with Oxidase and Peroxidase Mimicking Activity for Colorimetric Detection of Hydrogen Peroxide. Microchim. Acta 2017, 184, 4629–4635. [Google Scholar] [CrossRef]
- Sun, J.; Li, C.; Qi, Y.; Guo, S.; Liang, X. Optimizing Colorimetric Assay Based on V2O5 Nanozymes for Sensitive Detection of H2O2 and Glucose. Sensors 2016, 16, 584. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Lou, D.; Wu, H.; Zhang, X.; Zhu, Y.; Gu, N.; Zhang, Y. A Novel AuNP-Based Glucose Oxidase Mimic with Enhanced Activity and Selectivity Constructed by Molecular Imprinting and O2-Containing Nanoemulsion Embedding. Adv. Mater. Interfaces 2018, 5, 1801070. [Google Scholar] [CrossRef]
- Khade, B.S.; Gawali, P.G.; Waghmare, M.M.; Dongre, P. Adsorption of α-Amylase and Starch on Porous Zinc Oxide Nanosheet: Biophysical Study. Food Biophys. 2021, 16, 280–291. [Google Scholar] [CrossRef]
- Liu, W.; Ruan, M.-L.; Liu, L.; Ji, X.; Ma, Y.; Yuan, P.; Tang, G.; Lin, H.; Dai, J.; Xue, W. Self-Activated in Vivo Therapeutic Cascade of Erythrocyte Membrane-Cloaked Iron-Mineralized Enzymes. Theranostics 2020, 10, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, Y.; Liu, J.; Liu, J. Liposome-Boosted Peroxidase-Mimicking Nanozymes Breaking the PH Limit. Chemistry 2020, 26, 16659–16665. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Dong, Y.; Jia, T.; Liu, S.; Liu, J.; Yang, D.; He, F.; Gai, S.; Yang, P.; Lin, J. GSH-Depleted Nanozymes with Hyperthermia-Enhanced Dual Enzyme-Mimic Activities for Tumor Nanocatalytic Therapy. Adv. Mater. 2020, 32, e2002439. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Garvin, J. Effects of Reactive Oxygen Species on Tubular Transport along the Nephron. Antioxidants 2017, 6, 23. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Z.; Qiu, L.; Hao, L.; Guo, J. Ipatasertib Sensitizes Colon Cancer Cells to TRAIL-Induced Apoptosis through ROS-Mediated Caspase Activation. Biochem. Biophys. Res. Commun. 2019, 519, 812–818. [Google Scholar] [CrossRef]
- Kaminskyy, V.O.; Piskunova, T.; Zborovskaya, I.B.; Tchevkina, E.M.; Zhivotovsky, B. Suppression of Basal Autophagy Reduces Lung Cancer Cell Proliferation and Enhances Caspase-Dependent and -Independent Apoptosis by Stimulating ROS Formation. Autophagy 2012, 8, 1032–1044. [Google Scholar] [CrossRef]
- Mu, W.; Cheng, X.; Zhang, X.; Liu, Y.; Lv, Q.; Liu, G.; Zhang, J.; Li, X. Hinokiflavone Induces Apoptosis via Activating Mitochondrial ROS/JNK/Caspase Pathway and Inhibiting NF-ΚB Activity in Hepatocellular Carcinoma. J. Cell Mol. Med. 2020, 24, 8151–8165. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, F.; Gao, Y.; Tang, G.; Xu, W.; Wang, Y.; Kong, R.; Tuyihong, A.; Wang, Z. ROS Induced by KillerRed Targeting Mitochondria (MtKR) Enhances Apoptosis Caused by Radiation via Cyt c/Caspase-3 Pathway. Oxid. Med. Cell Longev. 2019, 2019, 4528616. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, C.; Gao, J.; Qin, Q.; Zhang, Y.; Zhu, H.; Yang, X.; Yang, D.; Yan, H. Benzoquinone Induces ROS-Dependent Mitochondria-Mediated Apoptosis in HL-60 Cells. Toxicol. Ind. Health 2018, 34, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [Green Version]
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Piñero, E.; Béguin, F.; Kirsch-Volders, M.; Fenoglio, I. Structural Defects Play a Major Role in the Acute Lung Toxicity of Multiwall Carbon Nanotubes: Toxicological Aspects. Chem. Res. Toxicol. 2008, 21, 1698–1705. [Google Scholar] [CrossRef]
- Brzoska, K.; Szczygiel, M.; Drzał, A.; Sniegocka, M.; Michalczyk-Wetula, D.; Biela, E.; Elas, M.; Kapka-Skrzypczak, L.; Lewandowska-Siwkiewicz, H.; Urbańska, K. Transient Vasodilation in Mouse 4T1 Tumors after Intragastric and Intravenous Administration of Gold Nanoparticles. Int. J. Mol. Sci. 2021, 22, 2361. [Google Scholar] [CrossRef]
- Tschopp, J. Mitochondria: Sovereign of Inflammation? Eur. J. Immunol. 2011, 41, 1196–1202. [Google Scholar] [CrossRef]
- Karataş, Ö.F.; Sezgin, E.; Aydın, Ö.; Çulha, M. Interaction of Gold Nanoparticles with Mitochondria. Colloids Surf. B Biointerfaces 2009, 71, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-N.; Yang, L.-X.; Shi, X.-Y.; Li, I.-C.; Biazik, J.M.; Ratinac, K.R.; Chen, D.-H.; Thordarson, P.; Shieh, D.-B.; Braet, F. The Selective Growth Inhibition of Oral Cancer by Iron Core-Gold Shell Nanoparticles through Mitochondria-Mediated Autophagy. Biomaterials 2011, 32, 4565–4573. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi, V.; Fischer, U.; Weighardt, H.; Schulze-Osthoff, K.; Nickel, C.; Stahlmecke, B.; Kuhlbusch, T.A.; Scherbart, A.M.; Esser, C.; Schins, R.P. Zinc Oxide Nanoparticles Induce Necrosis and Apoptosis in Macrophages in a P47phox-and Nrf2-Independent Manner. PLoS ONE 2013, 8, e65704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrikossova, N.; Skoglund, C.; Ahrén, M.; Bengtsson, T.; Uvdal, K. Effects of Gadolinium Oxide Nanoparticles on the Oxidative Burst from Human Neutrophil Granulocytes. Nanotechnology 2012, 23, 275101. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, X.; Ji, Z.; Wang, M.; Liao, Y.-P.; Chang, C.H.; Li, R.; Zhang, H.; Nel, A.E.; Xia, T. NADPH Oxidase-Dependent NLRP3 Inflammasome Activation and Its Important Role in Lung Fibrosis by Multiwalled Carbon Nanotubes. Small 2015, 11, 2087–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, F.A.; Schinwald, A.; Poland, C.A.; Donaldson, K. The Mechanism of Pleural Inflammation by Long Carbon Nanotubes: Interaction of Long Fibres with Macrophages Stimulates Them to Amplify pro-Inflammatory Responses in Mesothelial Cells. Part. Fibre Toxicol. 2012, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Culcasi, M.; Benameur, L.; Mercier, A.; Lucchesi, C.; Rahmouni, H.; Asteian, A.; Casano, G.; Botta, A.; Kovacic, H.; Pietri, S. EPR Spin Trapping Evaluation of ROS Production in Human Fibroblasts Exposed to Cerium Oxide Nanoparticles: Evidence for NADPH Oxidase and Mitochondrial Stimulation. Chem.-Biol. Interact. 2012, 199, 161–176. [Google Scholar] [CrossRef]
- Damasco, J.A.; Ravi, S.; Perez, J.D.; Hagaman, D.E.; Melancon, M.P. Understanding Nanoparticle Toxicity to Direct a Safe-by-Design Approach in Cancer Nanomedicine. Nanomaterials 2020, 10, 2186. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. Biomed. Res. Int 2013, 2013, 942916. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-T.; He, W.; Wamer, W.G.; Hu, X.; Wu, X.; Lo, Y.M.; Yin, J.-J. Enzyme-Mimetic Effects of Gold@ Platinum Nanorods on the Antioxidant Activity of Ascorbic Acid. Nanoscale 2013, 5, 1583–1591. [Google Scholar] [CrossRef]
- Palierse, E.; Hélary, C.; Krafft, J.-M.; Génois, I.; Masse, S.; Laurent, G.; Alvarez Echazu, M.I.; Selmane, M.; Casale, S.; Valentin, L.; et al. Baicalein-Modified Hydroxyapatite Nanoparticles and Coatings with Antibacterial and Antioxidant Properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111537. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective Cytotoxicity of Green Synthesized Silver Nanoparticles against the MCF-7 Tumor Cell Line and Their Enhanced Antioxidant and Antimicrobial Properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Mohd Zaffarin, A.S.; Ng, S.-F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef]
- Lewandowska, H.; Kalinowska, M. New Polyphenol-Containing LDL Nano-Preparations in Oxidative Stress and DNA Damage: A Potential Route for Cell-Targeted PP Delivery. Materials 2020, 13, 5106. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Bajić, V.; Spremo-Potparević, B.; Živković, L.; Čabarkapa, A.; Kotur-Stevuljević, J.; Isenović, E.; Sredojević, D.; Vukoje, I.; Lazić, V.; Ahrenkiel, S.P.; et al. Surface-Modified TiO2 Nanoparticles with Ascorbic Acid: Antioxidant Properties and Efficiency against DNA Damage In Vitro. Colloids Surf. B Biointerfaces 2017, 155, 323–331. [Google Scholar] [CrossRef]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver Nanoparticles Mediated by Costus Afer Leaf Extract: Synthesis, Antibacterial, Antioxidant and Electrochemical Properties. Molecules 2017, 22, 701. [Google Scholar] [CrossRef] [Green Version]
- Grace, A.N.; Pandian, K. Organically Dispersible Gold and Platinum Nanoparticles Using Aromatic Amines as Phase Transfer and Reducing Agent and Their Applications in Electro-Oxidation of Glucose. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 113–120. [Google Scholar] [CrossRef]
- Gozdziewska, M.; Cichowicz, G.; Markowska, K.; Zawada, K.; Megiel, E. Nitroxide-Coated Silver Nanoparticles: Synthesis, Surface Physicochemistry and Antibacterial Activity. RSC Adv. 2015, 5, 58403–58415. [Google Scholar] [CrossRef]
- Sadi, G.; Sadi, Ö. Antioxidants and Regulation of Antioxidant Enzymes by Cellular Redox Status. Turk. J. Sci. Rev. 2010, 3, 95–107. [Google Scholar]
- Hikosaka, K.; Kim, J.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Platinum Nanoparticles Have an Activity Similar to Mitochondrial NADH:Ubiquinone Oxidoreductase. Colloids Surf. B Biointerfaces 2008, 66, 195–200. [Google Scholar] [CrossRef]
- Baldim, V.; Yadav, N.; Bia, N.; Graillot, A.; Loubat, C.; Singh, S.; Karakoti, A.S.; Berret, J.-F. Polymer-Coated Cerium Oxide Nanoparticles as Oxidoreductase-like Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 42056–42066. [Google Scholar] [CrossRef]
- Alula, M.T.; Madingwane, M.L. Colorimetric Quantification of Chromium (VI) Ions Based on Oxidoreductase-like Activity of Fe3O4. Sens. Actuators B Chem. 2020, 324, 128726. [Google Scholar] [CrossRef]
- Bonomi, R.; Cazzolaro, A.; Sansone, A.; Scrimin, P.; Prins, L.J. Detection of Enzyme Activity through Catalytic Signal Amplification with Functionalized Gold Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 2307–2312. [Google Scholar] [CrossRef]
- Pasquato, L.; Rancan, F.; Scrimin, P.; Mancin, F.; Frigeri, C. N-Methylimidazole-Functionalized Gold Nanoparticles as Catalysts for Cleavage of a Carboxylic Acid Ester. Chem. Commun. 2000, 2253–2254. [Google Scholar] [CrossRef]
- Pengo, P.; Polizzi, S.; Pasquato, L.; Scrimin, P. Carboxylate−Imidazole Cooperativity in Dipeptide-Functionalized Gold Nanoparticles with Esterase-like Activity. J. Am. Chem. Soc. 2005, 127, 1616–1617. [Google Scholar] [CrossRef]
- Singh, S. Nanomaterials Exhibiting Enzyme-Like Properties (Nanozymes): Current Advances and Future Perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Prousek, J. Fenton Chemistry in Biology and Medicine. Pure Appl. Chem. 2007, 79, 2325–2338. [Google Scholar] [CrossRef]
- Yu, C.-J.; Lin, C.-Y.; Liu, C.-H.; Cheng, T.-L.; Tseng, W.-L. Synthesis of Poly(Diallyldimethylammonium Chloride)-Coated Fe3O4 Nanoparticles for Colorimetric Sensing of Glucose and Selective Extraction of Thiol. Biosens. Bioelectron. 2010, 26, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.I.; Shim, J.; Li, T.; Lee, J.; Park, H.G. Fabrication of Nanoporous Nanocomposites Entrapping Fe3O4 Magnetic Nanoparticles and Oxidases for Colorimetric Biosensing. Chem. Eur. J. 2011, 17, 10700–10707. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, H.; Rahman, Z.U.; Su, L.; Chen, X.; Hu, J.; Chen, X. Graphene Oxide–Fe3O4 Magnetic Nanocomposites with Peroxidase-like Activity for Colorimetric Detection of Glucose. Nanoscale 2012, 4, 3969. [Google Scholar] [CrossRef]
- Huang, F.; Wang, J.; Chen, W.; Wan, Y.; Wang, X.; Cai, N.; Liu, J.; Yu, F. Synergistic Peroxidase-like Activity of CeO2-Coated Hollow Fe3O4 Nanocomposites as an Enzymatic Mimic for Low Detection Limit of Glucose. J. Taiwan Inst. Chem. Eng. 2018, 83, 40–49. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, L.; He, J.; Guo, W.; Zhou, Z.; Zhang, X.; Nie, S.; Wei, H. Integrated Nanozymes with Nanoscale Proximity for in Vivo Neurochemical Monitoring in Living Brains. Anal. Chem. 2016, 88, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, S.; Luo, Y.; Sun, X. Fe(III)-Based Coordination Polymernanoparticles: Peroxidase-like Catalytic Activity and Their Application to Hydrogen Peroxide and Glucose Detection. Catal. Sci. Technol. 2012, 2, 432–436. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, X.J.; Yang, X.X.; Li, Y.F. A Nanosized Metal–Organic Framework of Fe-MIL-88NH2 as a Novel Peroxidase Mimic Used for Colorimetric Detection of Glucose. Analyst 2013, 138, 4526. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.-B.; Shiddiky, M.J. Nanozyme-Based Electrochemical Biosensors for Disease Biomarker Detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef] [PubMed]
- Moons, J.; de Azambuja, F.; Mihailovic, J.; Kozma, K.; Smiljanic, K.; Amiri, M.; Cirkovic Velickovic, T.; Nyman, M.; Parac-Vogt, T.N. Discrete Hf18 Metal-oxo Cluster as a Heterogeneous Nanozyme for Site-Specific Proteolysis. Angew. Chem. 2020, 132, 9179–9186. [Google Scholar] [CrossRef]
- Hille, R.; Hall, J.; Basu, P. The Mononuclear Molybdenum Enzymes. Chem. Rev. 2014, 114, 3963–4038. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Zhong, L.; Guo, L.; Fu, F.; Chen, G. Seeing Diabetes: Visual Detection of Glucose Based on the Intrinsic Peroxidase-like Activity of MoS2 Nanosheets. Nanoscale 2014, 6, 11856–11862. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Chai, H.; Huang, Y. Recent Advances in the Construction and Analytical Applications of Metal-Organic Frameworks-Based Nanozymes. TrAC Trends Anal. Chem. 2018, 105, 391–403. [Google Scholar] [CrossRef]
- Wu, X.; Chen, T.; Wang, J.; Yang, G. Few-Layered MoSe2 Nanosheets as an Efficient Peroxidase Nanozyme for Highly Sensitive Colorimetric Detection of H2O2 and Xanthine. J. Mater. Chem. B 2018, 6, 105–111. [Google Scholar] [CrossRef]
- Huang, L.; Li, Z.; Guo, L. Colorimetric Assay of Acetylcholinesterase Inhibitor Tacrine Based on MoO2 Nanoparticles as Peroxidase Mimetics. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2020, 224, 117412. [Google Scholar] [CrossRef]
- Yang, Z.-D.; Chang, Z.-W.; Zhang, Q.; Huang, K.; Zhang, X.-B. Decorating Carbon Nanofibers with Mo2C Nanoparticles towards Hierarchically Porous and Highly Catalytic Cathode for High-Performance Li-O2 Batteries. Sci. Bull. 2018, 63, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tang, C.; Liang, T.; Tang, C.; Lv, X.; Tang, K.; Li, C.M. Porous Molybdenum Carbide Nanostructured Catalyst toward Highly Sensitive Biomimetic Sensing of H2O2. Electroanalysis 2020, 32, 1243–1250. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Q.; Zhang, G.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Exfoliated MoS2 Supported Au–Pd Bimetallic Nanoparticles with Core–Shell Structures and Superior Peroxidase-like Activities. RSC Adv. 2015, 5, 10352–10357. [Google Scholar] [CrossRef]
- Zhu, W.; Li, M.; Chen, S.; Wang, C.; Lu, X. Interfacial Engineering Regulating the Peroxidase-like Property of Ternary Composite Nanofibers and Their Sensing Applications. Appl. Surf. Sci. 2019, 491, 138–146. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, M.; Geng, H.; Zhang, P.; Zheng, Z.; Zhou, Y.; He, W. NIR Enhanced Peroxidase-like Activity of Au@CeO2 Hybrid Nanozyme by Plasmon-Induced Hot Electrons and Photothermal Effect for Bacteria Killing. Appl. Catal. B Environ. 2021, 295, 120317. [Google Scholar] [CrossRef]
- Murugan, C.; Murugan, N.; Sundramoorthy, A.K.; Sundaramurthy, A. Nanoceria Decorated Flower-like Molybdenum Sulphide Nanoflakes: An Efficient Nanozyme for Tumour Selective ROS Generation and Photo Thermal Therapy. Chem. Commun. 2019, 55, 8017–8020. [Google Scholar] [CrossRef]
- Ragg, R.; Natalio, F.; Tahir, M.N.; Janssen, H.; Kashyap, A.; Strand, D.; Strand, S.; Tremel, W. Molybdenum Trioxide Nanoparticles with Intrinsic Sulfite Oxidase Activity. ACS Nano 2014, 8, 5182–5189. [Google Scholar] [CrossRef]
- Chen, T.; Zou, H.; Wu, X.; Liu, C.; Situ, B.; Zheng, L.; Yang, G. Nanozymatic Antioxidant System Based on MoS2 Nanosheets. ACS Appl. Mater. Interfaces 2018, 10, 12453–12462. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.; Hu, Z.; Lu, Y.; Liu, Q.; Jin, S.; Zhang, Q.; Zhao, S.; Chou, S. Understanding High-Rate K+-Solvent Co-Intercalation in Natural Graphite for Potassium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 12917–12924. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Zhang, J.; Wang, J.; Yang, J.; Chen, J.; Shen, X.; Deng, J.; Deng, D.; Long, W.; Sun, Y.-M.; et al. Highly Catalytic Nanodots with Renal Clearance for Radiation Protection. ACS Nano 2016, 10, 4511–4519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Cao, W.; Qin, L.; Lin, T.; Chen, W.; Lin, S.; Yao, J.; Zhao, X.; Zhou, M.; Hang, C. Boosting the Peroxidase-like Activity of Nanostructured Nickel by Inducing Its 3+ Oxidation State in LaNiO3 Perovskite and Its Application for Biomedical Assays. Theranostics 2017, 7, 2277. [Google Scholar] [CrossRef] [PubMed]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The Role of Cerium Redox State in the SOD Mimetic Activity of Nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [Green Version]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.; Seal, S.; Self, W.T. Nanoceria Exhibit Redox State-Dependent Catalase Mimetic Activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Q.; Gong, S.-W.; Zhang, Y.; Yang, T.; Wang, C.-Y.; Gu, N. Prussian Blue Modified Iron Oxide Magnetic Nanoparticles and Their High Peroxidase-like Activity. J. Mater. Chem. 2010, 20, 5110–5116. [Google Scholar] [CrossRef]
- Zhao, J.; de Serrano, V.; Dumarieh, R.; Thompson, M.; Ghiladi, R.A.; Franzen, S. The Role of the Distal Histidine in H2O2 Activation and Heme Protection in Both Peroxidase and Globin Functions. J. Phys. Chem. B 2012, 116, 12065–12077. [Google Scholar] [CrossRef]
- Fan, K.; Wang, H.; Xi, J.; Liu, Q.; Meng, X.; Duan, D.; Gao, L.; Yan, X. Optimization of Fe3O4 Nanozyme Activity via Single Amino Acid Modification Mimicking an Enzyme Active Site. Chem. Commun. 2017, 53, 424–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sono, M.; Roach, M.P.; Coulter, E.D.; Dawson, J.H. Heme-Containing Oxygenases. Chem. Rev. 1996, 96, 2841–2888. [Google Scholar] [CrossRef]
- Johnson, D.C.; Dean, D.R.; Smith, A.D.; Johnson, M.K. Structure, Function, and Formation of Biological Iron-Sulfur Clusters. Annu. Rev. Biochem. 2005, 74, 247–281. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A.; Klausner, R.D. Iron-Sulfur Clusters as Biosensors of Oxidants and Iron. Trends Biochem. Sci. 1996, 21, 174–177. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, S.; Yin, J.-J.; He, W.; Lu, W.; Ma, M.; Gu, N.; Zhang, Y. Prussian Blue Nanoparticles as Multienzyme Mimetics and Reactive Oxygen Species Scavengers. J. Am. Chem. Soc. 2016, 138, 5860–5865. [Google Scholar] [CrossRef]
- Hosseini, M.; Mozafari, M. Cerium Oxide Nanoparticles: Recent Advances in Tissue Engineering. Materials 2020, 13, 3072. [Google Scholar] [CrossRef]
- Gorecki, M.; Beck, Y.; Hartman, J.R.; Fischer, M.; Weiss, L.; Tochner, Z.; Slavin, S.; Nimrod, A. Recombinant Human Superoxide Dismutases: Production and Potential Therapeutical Uses. Free Radic. Res. Commun. 1991, 12, 401–410. [Google Scholar] [CrossRef]
- Yao, J.; Cheng, Y.; Zhou, M.; Zhao, S.; Lin, S.; Wang, X.; Wu, J.; Li, S.; Wei, H. ROS Scavenging Mn3O4 Nanozymes for In Vivo Anti-Inflammation. Chem. Sci. 2018, 9, 2927–2933. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-P.; Wu, T.-H.; Lin, Y.-L.; Liu, C.-Y.; Wang, S.; Lin, S.-Y. Tailoring Enzyme-Like Activities of Gold Nanoclusters by Polymeric Tertiary Amines for Protecting Neurons Against Oxidative Stress. Small 2016, 12, 4127–4135. [Google Scholar] [CrossRef]
- He, S.B.; Yang, L.; Lin, M.T.; Balasubramanian, P.; Peng, H.P.; Kuang, Y.; Deng, H.H.; Chen, W. Platinum group element-based nanozymes for biomedical applications: An overview. Biomed. Mater. 2020, 16, 3, 032001. [Google Scholar] [CrossRef]
- Gatto, F.; Moglianetti, M.; Pompa, P.; Bardi, G. Platinum Nanoparticles Decrease Reactive Oxygen Species and Modulate Gene Expression without Alteration of Immune Responses in THP-1 Monocytes. Nanomaterials 2018, 8, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum Nanoparticles in Nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-P.; Kim, S.-H.; Bang, J.-W.; Lee, H.-S.; Kwak, S.-S.; Kwon, S.-Y. Enhanced Tolerance to Oxidative Stress in Transgenic Tobacco Plants Expressing Three Antioxidant Enzymes in Chloroplasts. Plant. Cell Rep. 2007, 26, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, B.J. Catalase and Glutathione Peroxidase Mimics. Biochem. Pharmacol. 2009, 77, 285–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Li, H.; Xia, B.; Ji, W.; Ji, S.; Zhang, W.; Huang, W.; Huo, F.; Xu, H. Selenium-Functionalized Metal-Organic Frameworks as Enzyme Mimics. Nano Res. 2018, 11, 5761–5768. [Google Scholar] [CrossRef]
- Hou, C.; Luo, Q.; Liu, J.; Miao, L.; Zhang, C.; Gao, Y.; Zhang, X.; Xu, J.; Dong, Z.; Liu, J. Construction of GPx Active Centers on Natural Protein Nanodisk/Nanotube: A New Way to Develop Artificial Nanoenzyme. ACS Nano 2012, 6, 8692–8701. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Miao, L.; Li, J.; Fu, S.; An, G.; Si, C.; Dong, Z.; Luo, Q.; Yu, S.; Xu, J.; et al. Self-Assembly of Cricoid Proteins Induced by “Soft Nanoparticles”: An Approach To Design Multienzyme-Cooperative Antioxidative Systems. ACS Nano 2015, 9, 5461–5469. [Google Scholar] [CrossRef]
- Vernekar, A.A.; Sinha, D.; Srivastava, S.; Paramasivam, P.U.; D’Silva, P.; Mugesh, G. An Antioxidant Nanozyme That Uncovers the Cytoprotective Potential of Vanadia Nanowires. Nat. Commun. 2014, 5, 5301. [Google Scholar] [CrossRef]
- Singh, N.; Savanur, M.A.; Srivastava, S.; D’Silva, P.; Mugesh, G. A Redox Modulatory Mn3O4 Nanozyme with Multi-Enzyme Activity Provides Efficient Cytoprotection to Human Cells in a Parkinson’s Disease Model. Angew. Chem. Int. Ed. 2017, 56, 14267–14271. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, J. Structure–Activity Relationship of VOx/CeO2 Nanorod for NO Removal with Ammonia. Appl. Catal. B Environ. 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Singh, N.; NaveenKumar, S.K.; Geethika, M.; Mugesh, G. A Cerium Vanadate Nanozyme with Specific Superoxide Dismutase Activity Regulates Mitochondrial Function and ATP Synthesis in Neuronal Cells. Angew. Chem. Int. Ed. 2021, 60, 3121–3130. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Z.; Liu, C.; Ju, E.; Zhang, Y.; Ren, J.; Qu, X. Self-Assembly of Multi-Nanozymes to Mimic an Intracellular Antioxidant Defense System. Angew. Chem. Int. Ed. 2016, 55, 6646–6650. [Google Scholar] [CrossRef]
- Sui, L.; Wang, J.; Xiao, Z.; Yang, Y.; Yang, Z.; Ai, K. ROS-Scavenging Nanomaterials to Treat Periodontitis. Front. Chem. 2020, 8, 595530. [Google Scholar] [CrossRef]
- Padwal, P.; Bandyopadhyaya, R.; Mehra, S. Polyacrylic Acid-Coated Iron Oxide Nanoparticles for Targeting Drug Resistance in Mycobacteria. Langmuir 2014, 30, 15266–15276. [Google Scholar] [CrossRef]
- Moglianetti, M.; De Luca, E.; Pedone, D.; Marotta, R.; Catelani, T.; Sartori, B.; Amenitsch, H.; Retta, S.F.; Pompa, P.P. Platinum Nanozymes Recover Cellular ROS Homeostasis in an Oxidative Stress-Mediated Disease Model. Nanoscale 2016, 8, 3739–3752. [Google Scholar] [CrossRef] [PubMed]
- Moglianetti, M.; Pedone, D.; Udayan, G.; Retta, S.F.; Debellis, D.; Marotta, R.; Turco, A.; Rella, S.; Malitesta, C.; Bonacucina, G.; et al. Intracellular Antioxidant Activity of Biocompatible Citrate-Capped Palladium Nanozymes. Nanomaterials 2020, 10, 99. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Yin, J.-J.; Ning, B.; Wu, X.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.; Zhao, Y.; Nie, G. Direct Evidence for Catalase and Peroxidase Activities of Ferritin–Platinum Nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, P.; Xu, C.; Ren, J.; Qu, X. Cerium Oxide Caged Metal Chelator: Anti-Aggregation and Anti-Oxidation Integrated H2O2-Responsive Controlled Drug Release for Potential Alzheimer’s Disease Treatment. Chem. Sci. 2013, 4, 2536–2542. [Google Scholar] [CrossRef]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, J.; Li, M.; Wu, L.; Chen, C.; Qu, X. Mesoporous Silica Nanoparticle-based H2O2 Responsive Controlled-release System Used for Alzheimer’s Disease Treatment. Adv. Healthc. Mater. 2012, 1, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Gao, J.; Wang, F.; Yang, J.; Song, N.; Jin, X.; Mi, P.; Tian, J.; Luo, J.; Liang, F. Multistimuli Responsive Core–Shell Nanoplatform Constructed from Fe3O4@ MOF Equipped with Pillar [6] Arene Nanovalves. Small 2018, 14, 1704440. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A. Prussian Blue: A Nanozyme with Versatile Catalytic Properties. Int. J. Mol. Sci. 2021, 22, 5993. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, J.; Wang, Y.; Chen, J.; Xi, J. Nanozymes as Enzyme Inhibitors. Int. J. Nanomed. 2021, 16, 1143–1155. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface Modification of Nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- You, C.-C.; De, M.; Han, G.; Rotello, V.M. Tunable Inhibition and Denaturation of α-Chymotrypsin with Amino Acid-Functionalized Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 12873–12881. [Google Scholar] [CrossRef] [PubMed]
- Sellergren, B. Shaping Enzyme Inhibitors. Nat. Chem. 2010, 2, 7–8. [Google Scholar] [CrossRef] [PubMed]
- You, C.-C.; Agasti, S.S.; De, M.; Knapp, M.J.; Rotello, V.M. Modulation of the Catalytic Behavior of α-Chymotrypsin at Monolayer-Protected Nanoparticle Surfaces. J. Am. Chem. Soc. 2006, 128, 14612–14618. [Google Scholar] [CrossRef]
- Cha, S.-H.; Hong, J.; McGuffie, M.; Yeom, B.; VanEpps, J.S.; Kotov, N.A. Shape-Dependent Biomimetic Inhibition of Enzyme by Nanoparticles and Their Antibacterial Activity. ACS Nano 2015, 9, 9097–9105. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.R.; Pandit, S.; De, M. 2D-MoS2 -Based β-Lactamase Inhibitor for Combination Therapy against Drug-Resistant Bacteria. ACS Appl. Biol. Mater. 2018, 1, 967–974. [Google Scholar] [CrossRef]
- Taubes, G. The Bacteria Fight Back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef]
- Karunakaran, S.; Pandit, S.; De, M. Functionalized Two-Dimensional MoS2 with Tunable Charges for Selective Enzyme Inhibition. ACS Omega 2018, 3, 17532–17539. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wei, H. Multifunctional Nanozymes: Enzyme-like Catalytic Activity Combined with Magnetism and Surface Plasmon Resonance. Nanoscale Horiz. 2018, 3, 367–382. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A Review on Metal-and Metal Oxide-Based Nanozymes: Properties, Mechanisms, and Applications. Nanomicro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Feng, Y.-B.; Hong, L.; Chen, Q.-Y.; Wu, L.-F.; Lin, X.-H.; Xia, X.-H. Peroxidase-like Activity of Water-Soluble Cupric Oxide Nanoparticles and Its Analytical Application for Detection of Hydrogen Peroxide and Glucose. Analyst 2012, 137, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-M.; Zhong, X.-L.; Wen, S.-H.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. Colorimetric Detection of Methyltransferase Activity Based on the Enhancement of CoOOH Nanozyme Activity by SsDNA. Sens. Actuators B Chem. 2019, 281, 1073–1079. [Google Scholar] [CrossRef]
- Sharma, T.K.; Ramanathan, R.; Weerathunge, P.; Mohammadtaheri, M.; Daima, H.K.; Shukla, R.; Bansal, V. Aptamer-Mediated ‘Turn-off/Turn-on’Nanozyme Activity of Gold Nanoparticles for Kanamycin Detection. Chem. Commun. 2014, 50, 15856–15859. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Lin, S.; Muhammad, F.; Lin, Y.-W.; Wei, H. Rationally Modulate the Oxidase-like Activity of Nanoceria for Self-Regulated Bioassays. ACS Sens. 2016, 1, 1336–1343. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Z.; Jia, S.; Lei, Z.; Lin, S.; Zhang, H.; Ma, Y.; Tian, Z.; Yang, C.J. Au@ Pt Nanoparticle Encapsulated Target-responsive Hydrogel with Volumetric Bar-chart Chip Readout for Quantitative Point-of-care Testing. Angew. Chem. Int. Ed. 2014, 53, 12503–12507. [Google Scholar]
- Duan, D.; Fan, K.; Zhang, D.; Tan, S.; Liang, M.; Liu, Y.; Zhang, J.; Zhang, P.; Liu, W.; Qiu, X. Nanozyme-Strip for Rapid Local Diagnosis of Ebola. Biosens. Bioelectron. 2015, 74, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, L.; Hu, L.; Xing, K.; Lu, X.; Huang, Y.; Zhang, J.; Lai, W.; Chen, T. Nanozyme-Based Lateral Flow Assay for the Sensitive Detection of Escherichia coli O157: H7 in Milk. J. Dairy Sci. 2018, 101, 5770–5779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Qin, L.; Zhou, M.; Lou, Z.; Wei, H. Nanozyme Sensor Arrays for Detecting Versatile Analytes from Small Molecules to Proteins and Cells. Anal. Chem. 2018, 90, 11696–11702. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Wu, D.; Li, T.; Yin, Y.; Li, G. Improvement of Enzyme-Linked Immunosorbent Assay for the Multicolor Detection of Biomarkers. Chem. Sci. 2016, 7, 3011–3016. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Xu, Z.; Long, L.; Chen, Y.; Chen, M.-L.; Cheng, Y.-H. A Nanozyme-Linked Immunosorbent Assay Based on Metal–Organic Frameworks (MOFs) for Sensitive Detection of Aflatoxin B1. Food Chem. 2021, 338, 128039. [Google Scholar] [CrossRef]
- Jin, T.; Li, Y.; Jing, W.; Li, Y.; Fan, L.; Li, X. Cobalt-Based Metal Organic Frameworks: A Highly Active Oxidase-Mimicking Nanozyme for Fluorescence “Turn-on” Assays of Biothiol. Chem. Commun. 2020, 56, 659–662. [Google Scholar] [CrossRef]
- Miao, L.; Jiao, L.; Tang, Q.; Li, H.; Zhang, L.; Wei, Q. A Nanozyme-Linked Immunosorbent Assay for Dual-Modal Colorimetric and Ratiometric Fluorescent Detection of Cardiac Troponin I. Sens. Actuators B Chem. 2019, 288, 60–64. [Google Scholar] [CrossRef]
- Lin, T.; Qin, Y.; Huang, Y.; Yang, R.; Hou, L.; Ye, F.; Zhao, S. A Label-Free Fluorescence Assay for Hydrogen Peroxide and Glucose Based on the Bifunctional MIL-53 (Fe) Nanozyme. Chem. Commun. 2018, 54, 1762–1765. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhang, L.; Du, W.; Li, H.; Yang, D.; Zhu, C. Hierarchical Manganese Dioxide Nanoflowers Enable Accurate Ratiometric Fluorescence Enzyme-Linked Immunosorbent Assay. Nanoscale 2018, 10, 21893–21897. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Kaittanis, C.; Santra, S.; Perez, J.M. PH-Tunable Oxidase-like Activity of Cerium Oxide Nanoparticles Achieving Sensitive Fluorigenic Detection of Cancer Biomarkers at Neutral PH. Anal. Chem. 2011, 83, 2547–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, W.; Peng, Y.; Yang, H.; Tan, Y.; Ma, M.; Xie, Q.; Chen, S. Ruthenium Ion-Complexed Carbon Nitride Nanosheets with Peroxidase-like Activity as a Ratiometric Fluorescence Probe for the Detection of Hydrogen Peroxide and Glucose. ACS Appl. Mater. Interfaces 2019, 11, 29072–29077. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small Molecule-Based Ratiometric Fluorescence Probes for Cations, Anions, and Biomolecules. Chem. Soc. Rev. 2015, 44, 4185. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Wang, K.; Wang, M.; Yu, P.; Mao, L. Mitochondria Targeted Nanoscale Zeolitic Imidazole Framework-90 for ATP Imaging in Live Cells. J. Am. Chem. Soc. 2017, 139, 5877–5882. [Google Scholar] [CrossRef]
- Winarta, J.; Shan, B.; Mcintyre, S.M.; Ye, L.; Wang, C.; Liu, J.; Mu, B. A Decade of UiO-66 Research: A Historic Review of Dynamic Structure, Synthesis Mechanisms, and Characterization Techniques of an Archetypal Metal–Organic Framework. Cryst. Growth Des. 2020, 20, 1347–1362. [Google Scholar] [CrossRef]
- Koo, K.M.; Carrascosa, L.G.; Shiddiky, M.J.A.; Trau, M. Poly(A) Extensions of MiRNAs for Amplification-Free Electrochemical Detection on Screen-Printed Gold Electrodes. Anal. Chem. 2016, 88, 2000–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masud, M.K.; Umer, M.; Hossain, M.S.A.; Yamauchi, Y.; Nguyen, N.-T.; Shiddiky, M.J.A. Nanoarchitecture Frameworks for Electrochemical MiRNA Detection. Trends Biochem. Sci. 2019, 44, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Kanjanawarut, R.; Su, X. Colorimetric Detection of DNA Using Unmodified Metallic Nanoparticles and Peptide Nucleic Acid Probes. Anal. Chem. 2009, 81, 6122–6129. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lim, E.; Fields, T.; Wu, H.; Xu, Y.; Wang, Y.A.; Mao, H. Improving Sensitivity and Specificity of Amyloid-β Peptides and Tau Protein Detection with Antibiofouling Magnetic Nanoparticles for Liquid Biopsy of Alzheimer’s Disease. ACS Biomater. Sci. Eng. 2019, 5, 3595–3605. [Google Scholar] [CrossRef]
- Blair, E.O.; Corrigan, D.K. A Review of Microfabricated Electrochemical Biosensors for DNA Detection. Biosens. Bioelectron. 2019, 134, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.-S.; Zhang, Z.-H.; Chen, M.; Zhao, H.; Duan, F.-H.; Chen, D.-M.; Wang, M.-H.; Zhang, S.; Du, M. Pore Modulation of Zirconium–Organic Frameworks for High-Efficiency Detection of Trace Proteins. Chem. Commun. 2017, 53, 3941–3944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Hu, M.; Huang, X.; Wang, M.; He, L.; Song, Y.; Jia, Q.; Zhou, N.; Zhang, Z.; Du, M. Multicomponent Zirconium-Based Metal-Organic Frameworks for Impedimetric Aptasensing of Living Cancer Cells. Sens. Actuators B Chem. 2020, 306, 127608. [Google Scholar] [CrossRef]
- Qiao, X.; Su, B.; Liu, C.; Song, Q.; Luo, D.; Mo, G.; Wang, T. Selective Surface Enhanced Raman Scattering for Quantitative Detection of Lung Cancer Biomarkers in Superparticle@ MOF Structure. Adv. Mater. 2018, 30, 1702275. [Google Scholar] [CrossRef]
- Ansari, I.; Raddatz, G.; Gutekunst, J.; Ridnik, M.; Cohen, D.; Abu-Remaileh, M.; Tuganbaev, T.; Shapiro, H.; Pikarsky, E.; Elinav, E.; et al. The Microbiota Programs DNA Methylation to Control Intestinal Homeostasis and Inflammation. Nat. Microbiol. 2020, 5, 610–619. [Google Scholar] [CrossRef]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in α-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef]
- Musgrove, R.E.; Helwig, M.; Bae, E.-J.; Aboutalebi, H.; Lee, S.-J.; Ulusoy, A.; Di Monte, D.A. Oxidative Stress in Vagal Neurons Promotes Parkinsonian Pathology and Intercellular α-Synuclein Transfer. J. Clin. Investig. 2019, 129, 3738–3753. [Google Scholar] [CrossRef]
- Scudamore, O.; Ciossek, T. Increased Oxidative Stress Exacerbates α-Synuclein Aggregation In Vivo. J. Neuropathol. Exp. Neurol. 2018, 77, 443–453. [Google Scholar] [CrossRef]
- Luk, K.C. Oxidative Stress and α-Synuclein Conspire in Vulnerable Neurons to Promote Parkinson’s Disease Progression. J. Clin. Investig. 2019, 129, 3530–3531. [Google Scholar] [CrossRef]
- McCormack, A.L.; Thiruchelvam, M.; Manning-Bog, A.B.; Thiffault, C.; Langston, J.W.; Cory-Slechta, D.A.; Di Monte, D.A. Environmental Risk Factors and Parkinson’s Disease: Selective Degeneration of Nigral Dopaminergic Neurons Caused by the Herbicide Paraquat. Neurobiol. Dis. 2002, 10, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kam, T.-I.; Mao, X.; Park, H.; Chou, S.-C.; Karuppagounder, S.S.; Umanah, G.E.; Yun, S.P.; Brahmachari, S.; Panicker, N.; Chen, R.; et al. Poly(ADP-Ribose) Drives Pathologic α-Synuclein Neurodegeneration in Parkinson’s Disease. Science 2018, 362, eaat8407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-Q.; Mao, Y.; Xu, E.; Jia, H.; Zhang, S.; Dawson, V.L.; Dawson, T.M.; Li, Y.-M.; Zheng, Z.; He, W.; et al. Nanozyme Scavenging ROS for Prevention of Pathologic α-Synuclein Transmission in Parkinson’s Disease. Nano Today 2021, 36, 101027. [Google Scholar] [CrossRef]
- Hao, C.; Qu, A.; Xu, L.; Sun, M.; Zhang, H.; Xu, C.; Kuang, H. Chiral Molecule-Mediated Porous CuxO Nanoparticle Clusters with Antioxidation Activity for Ameliorating Parkinson’s Disease. J. Am. Chem. Soc. 2019, 141, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, Z.; Gao, N.; Pi, Z.; Du, X.; Ren, J.; Qu, X. Self-Protecting Biomimetic Nanozyme for Selective and Synergistic Clearance of Peripheral Amyloid-β in an Alzheimer’s Disease Model. J. Am. Chem. Soc. 2020, 142, 21702–21711. [Google Scholar] [CrossRef]
- Perry, G.; Cash, A.D.; Smith, M.A. Alzheimer Disease and Oxidative Stress. J. Biomed. Biotechnol. 2002, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Patnode, C.D.; Perdue, L.A.; Rossom, R.C.; Rushkin, M.C.; Redmond, N.; Thomas, R.G.; Lin, J.S. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force; U.S. Preventive Services Task Force Evidence Syntheses, Formerly Systematic Evidence Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2020.
- Swedish Council on Health Technology Assessment Dementia—Caring, Ethics, Ethnical and Economical Aspects: A Systematic Review; SBU Systematic Reviews; Swedish Council on Health Technology Assessment (SBU): Stockholm, Sweden, 2008; ISBN 978-91-85413-23-2.
- Tomita, T. Aberrant Proteolytic Processing and Therapeutic Strategies in Alzheimer Disease. Adv. Biol. Regul. 2017, 64, 33–38. [Google Scholar] [CrossRef]
- Shin, S.J.; Jeon, S.G.; Kim, J.; Jeong, Y.; Kim, S.; Park, Y.H.; Lee, S.-K.; Park, H.H.; Hong, S.B.; Oh, S.; et al. Red Ginseng Attenuates Aβ-Induced Mitochondrial Dysfunction and Aβ-Mediated Pathology in an Animal Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.J.; Cha, M.-Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Li, M.; Dong, K.; Gao, N.; Ren, J.; Zheng, Y.; Qu, X. Ceria/POMs Hybrid Nanoparticles as a Mimicking Metallopeptidase for Treatment of Neurotoxicity of Amyloid-β Peptide. Biomaterials 2016, 98, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Duan, T.; Xu, C.; Qu, X. Transition-Metal-Substituted Polyoxometalate Derivatives as Functional Anti-Amyloid Agents for Alzheimer’s Disease. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Z.; Li, X.; Wang, L.; Yin, M.; Wang, L.; Chen, N.; Fan, C.; Song, H. Dietary Iron Oxide Nanoparticles Delay Aging and Ameliorate Neurodegeneration in Drosophila. Adv. Mater. 2016, 28, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Moloney, A.; Sattelle, D.B.; Lomas, D.A.; Crowther, D.C. Alzheimer’s Disease: Insights from Drosophila Melanogaster Models. Trends Biochem. Sci. 2010, 35, 228–235. [Google Scholar] [CrossRef]
- Ha, C.; Ryu, J.; Park, C.B. Metal Ions Differentially Influence the Aggregation and Deposition of Alzheimer’s β-Amyloid on a Solid Template. Biochemistry 2007, 46, 6118–6125. [Google Scholar] [CrossRef]
- Ejaz, H.W.; Wang, W.; Lang, M. Copper Toxicity Links to Pathogenesis of Alzheimer’s Disease and Therapeutics Approaches. Int. J. Mol. Sci. 2020, 21, 7660. [Google Scholar] [CrossRef]
- Kenche, V.B.; Barnham, K.J. Alzheimer’s Disease & Metals: Therapeutic Opportunities. Br. J. Pharmacol. 2011, 163, 211–219. [Google Scholar]
- Dickinson, B.C.; Chang, C.J. Chemistry and Biology of Reactive Oxygen Species in Signaling or Stress Responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Klotz, L.-O.; Steinbrenner, H. Cellular Adaptation to Xenobiotics: Interplay between Xenosensors, Reactive Oxygen Species and FOXO Transcription Factors. Redox Biol. 2017, 13, 646–654. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. In Ultraviolet Light in Human Health, Diseases and Environment; Advances in Experimental Medicine and Biology; Ahmad, S.I., Ed.; Springer International Publishing: Cham, Switzerland, 2017; Volume 996, pp. 15–23. ISBN 978-3-319-56016-8. [Google Scholar]
- Opie, L.H. Cardiac Metabolism in Health and Disease. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 23–36. ISBN 978-0-12-405206-2. [Google Scholar]
- Alili, L.; Sack, M.; von Montfort, C.; Giri, S.; Das, S.; Carroll, K.S.; Zanger, K.; Seal, S.; Brenneisen, P. Downregulation of Tumor Growth and Invasion by Redox-Active Nanoparticles. Antioxid. Redox Signal. 2013, 19, 765–778. [Google Scholar] [CrossRef] [Green Version]
- Arnott, J.A.; Planey, S.L. The Influence of Lipophilicity in Drug Discovery and Design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Forte, E.; Fiorenza, D.; Torino, E.; Costagliola di Polidoro, A.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2020, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Kochebina, O.; Halty, A.; Taleb, J.; Kryza, D.; Janier, M.; Sadr, A.B.; Baudier, T.; Rit, S.; Sarrut, D. In Vivo Gadolinium Nanoparticle Quantification with SPECT/CT. EJNMMI Phys. 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Xu, J.; Cui, H. Functional Nanoparticles for Magnetic Resonance Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 814–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piergies, N.; Oćwieja, M.; Paluszkiewicz, C.; Kwiatek, W.M. Spectroscopic Insights into the Effect of PH, Temperature, and Stabilizer on Erlotinib Adsorption Behavior onto Ag Nanosurface. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 117737. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The Enhanced Permeability and Retention (EPR) Effect in Tumor Vasculature: The Key Role of Tumor-Selective Macromolecular Drug Targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Yang, C.; Liu, H.Z.; Fu, Z.X.; Lu, W.D. Oxaliplatin Long-Circulating Liposomes Improved Therapeutic Index of Colorectal Carcinoma. BMC Biotechnol. 2011, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Matsumura, Y. Tumoritropic and Lymphotropic Principles of Macromolecular Drugs. Crit. Rev. Ther. Drug Carr. Syst. 1988, 6, 193–210. [Google Scholar]
- Maeda, H. SMANCS and Polymer-Conjugated Macromolecular Drugs: Advantages in Cancer Chemotherapy. Adv. Drug Deliv. Rev. 2001, 46, 169–185. [Google Scholar] [CrossRef]
- Maeda, H.; Seymour, L.W.; Miyamoto, Y. Conjugates of Anticancer Agents and Polymers: Advantages of Macromolecular Therapeutics In Vivo. Bioconj. Chem. 1992, 3, 351–362. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Hu, Y.; Wu, J.; Wei, H. Nanozymes: Next Wave of Artificial Enzymes. In SpringerBriefs in Molecular Science; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-53066-5. [Google Scholar]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.-J.; Zhang, J. Ultrasmall Gold Nanoparticles in Cancer Diagnosis and Therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- He, J.; Liu, S.; Zhang, Y.; Chu, X.; Lin, Z.; Zhao, Z.; Qiu, S.; Guo, Y.; Ding, H.; Pan, Y.; et al. The Application of and Strategy for Gold Nanoparticles in Cancer Immunotherapy. Front. Pharmacol. 2021, 12, 687399. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G. Applications of Gold Nanoparticles in Cancer Imaging and Treatment. In Noble and Precious Metals—Properties, Nanoscale Effects and Applications; Seehra, M.S., Bristow, A.D., Eds.; InTech: London, UK, 2018; ISBN 978-1-78923-292-9. [Google Scholar]
- García Calavia, P.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-Gold Nanoparticle Conjugates for Photodynamic Therapy of Cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef] [Green Version]
- Dan, Q.; Hu, D.; Ge, Y.; Zhang, S.; Li, S.; Gao, D.; Luo, W.; Ma, T.; Liu, X.; Zheng, H.; et al. Ultrasmall Theranostic Nanozymes to Modulate Tumor Hypoxia for Augmenting Photodynamic Therapy and Radiotherapy. Biomater. Sci. 2020, 8, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, H.; Wang, C.; Gu, L.; Chen, H.; Jana, D.; Feng, L.; Liu, J.; Wang, X.; Xu, P.; et al. Self-Assembled Single-Site Nanozyme for Tumor-Specific Amplified Cascade Enzymatic Therapy. Angew. Chem. Int. Ed. 2021, 60, 3001–3007. [Google Scholar] [CrossRef]
- Guo, Z.; Li, C.; Gao, M.; Han, X.; Zhang, Y.; Zhang, W.; Li, W. Inside Cover: Mn−O Covalency Governs the Intrinsic Activity of Co-Mn Spinel Oxides for Boosted Peroxymonosulfate Activation (Angew. Chem. Int. Ed. 1/2021). Angew. Chem. Int. Ed. 2021, 60, 2. [Google Scholar] [CrossRef]
- Cramer, F.; Kampe, W. Inclusion Compounds. XVII.1 Catalysis of Decarboxylation by Cyclodextrins. A Model Reaction for the Mechanism of Enzymes. J. Am. Chem. Soc. 1965, 87, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Sutrisno, L.; Hu, Y.; Hou, Y.; Cai, K.; Li, M.; Luo, Z. Progress of Iron-Based Nanozymes for Antitumor Therapy. Front. Chem. 2020, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin Nanoparticles for Targeting and Visualizing Tumour Tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fan, K.; Yan, X. Ferritin Drug Carrier (FDC) for Tumor Targeting Therapy. J. Control. Release 2019, 311–312, 288–300. [Google Scholar] [CrossRef]
- Chen, D.; Li, B.; Jiang, L.; Duan, D.; Li, Y.; Wang, J.; He, J.; Zeng, Y. Highly Efficient Colorimetric Detection of Cancer Cells Utilizing Fe-MIL-101 with Intrinsic Peroxidase-like Catalytic Activity over a Broad PH Range. RSC Adv. 2015, 5, 97910–97917. [Google Scholar] [CrossRef]
- Chen, Y.; Zhong, H.; Wang, J.; Wan, X.; Li, Y.; Pan, W.; Li, N.; Tang, B. Catalase-like Metal–Organic Framework Nanoparticles to Enhance Radiotherapy in Hypoxic Cancer and Prevent Cancer Recurrence. Chem. Sci. 2019, 10, 5773–5778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munjal, T.; Dutta, S. Biocompatible Nanoreactors of Catalase and Nanozymes for Anticancer Therapeutics. Nano Select 2021. nano.202100040. [Google Scholar] [CrossRef]
- Li, S.; Shang, L.; Xu, B.; Wang, S.; Gu, K.; Wu, Q.; Sun, Y.; Zhang, Q.; Yang, H.; Zhang, F.; et al. A Nanozyme with Photo-Enhanced Dual Enzyme-Like Activities for Deep Pancreatic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 12624–12631. [Google Scholar] [CrossRef]
- Petty, H.R. Could Nanoparticles That Mimic the NADPH Oxidase Be Used to Kill Tumor Cells? Nanomedicine 2016, 11, 1631–1634. [Google Scholar] [CrossRef] [Green Version]
- Clark, A.J.; Petty, H.R. WO 3 /Pt Nanoparticles Promote Light-Induced Lipid Peroxidation and Lysosomal Instability within Tumor Cells. Nanotechnology 2016, 27, 075103. [Google Scholar] [CrossRef]
- Clark, A.J.; Coury, E.L.; Meilhac, A.M.; Petty, H.R. WO3/Pt Nanoparticles Are NADPH Oxidase Biomimetics That Mimic Effector Cells In Vitro and In Vivo. Nanotechnology 2016, 27, 065101. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Cheng, L.; Yang, N.; Betzer, O.; Feng, L.; Zhou, Q.; Li, Y.; Chen, R.; Popovtzer, R.; Liu, Z. Ultrasmall Oxygen-Deficient Bimetallic Oxide MnWOX Nanoparticles for Depletion of Endogenous GSH and Enhanced Sonodynamic Cancer Therapy. Adv. Mater. 2019, 31, 1900730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Deng, Y.; Xiang, G.; Liu, Y. Nanoparticle-Assisted Sonosensitizers and Their Biomedical Applications. IJN 2021, 16, 4615–4630. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Song, J.; Chen, X.; Yang, H. Ultrasound-Activated Sensitizers and Applications. Angew. Chem. Int. Ed. 2020, 59, 14212–14233. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; Jing, X.; Yang, D.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. Oxygen-Deficient Black Titania for Synergistic/Enhanced Sonodynamic and Photoinduced Cancer Therapy at Near Infrared-II Biowindow. ACS Nano 2018, 12, 4545–4555. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.; Bai, L.; Xu, J.; Gong, F.; Dong, Z.; Yang, Z.; Zeng, Z.; Liu, Z.; Cheng, L. Ultrafine Titanium Monoxide (TiO1+x) Nanorods for Enhanced Sonodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper Oxide Nanoparticles Induced Mitochondria Mediated Apoptosis in Human Hepatocarcinoma Cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Li, Z.; Xu, P.; Xiao, L.; Yang, Z. Nanosized Copper Oxide Induces Apoptosis through Oxidative Stress in Podocytes. Arch. Toxicol. 2013, 87, 1067–1073. [Google Scholar] [CrossRef]
- Perreault, F.; Melegari, S.P.; da Costa, C.H.; Rossetto, A.L.D.O.F.; Popovic, R.; Matias, W.G. Genotoxic Effects of Copper Oxide Nanoparticles in Neuro 2A Cell Cultures. Sci. Total Environ. 2012, 441, 117–124. [Google Scholar] [CrossRef]
- Mkandawire, M.M.; Lakatos, M.; Springer, A.; Clemens, A.; Appelhans, D.; Krause-Buchholz, U.; Pompe, W.; Rödel, G.; Mkandawire, M. Induction of Apoptosis in Human Cancer Cells by Targeting Mitochondria with Gold Nanoparticles. Nanoscale 2015, 7, 10634–10640. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Liu, S.; Hu, M.; Zhang, H.; Duan, D.; He, J.; Hong, J.; Lv, R.; Choi, H.S.; Yan, X. Peroxidase-Like Nanozymes Induce a Novel Form of Cell Death and Inhibit Tumor Growth In Vivo. Adv. Funct. Mater. 2020, 30, 2000647. [Google Scholar] [CrossRef]
- Chen, S.; Quan, Y.; Yu, Y.-L.; Wang, J.-H. Graphene Quantum Dot/Silver Nanoparticle Hybrids with Oxidase Activities for Antibacterial Application. ACS Biomater. Sci. Eng. 2017, 3, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, K.; Liu, Z.; Zhang, Y.; Chen, Z.; Sun, H.; Ren, J.; Qu, X. Corrigendum to “Activation of Biologically Relevant Levels of Reactive Oxygen Species by Au/g-C3N4 Hybrid Nanozyme for Bacteria Killing and Wound Disinfection” [Biomaterials, 113(2017)145–157]. Biomaterials 2020, 233, 119754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, Y.; Cui, H.; Yan, X.; Fan, K. Nanozyme-Based Catalytic Theranostics. RSC Adv. 2020, 10, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Dong, K.; Yan, Z.; Ding, C.; Chen, Z.; Ren, J.; Qu, X. Bacterial Hyaluronidase Self-Triggered Prodrug Release for Chemo-Photothermal Synergistic Treatment of Bacterial Infection. Small 2016, 12, 6200–6206. [Google Scholar] [CrossRef]
- Herget, K.; Hubach, P.; Pusch, S.; Deglmann, P.; Götz, H.; Gorelik, T.E.; Gural’skiy, I.A.; Pfitzner, F.; Link, T.; Schenk, S.; et al. Haloperoxidase Mimicry by CeO2−x Nanorods Combats Biofouling. Adv. Mater. 2017, 29, 1603823. [Google Scholar] [CrossRef]
- Gao, L.; Giglio, K.M.; Nelson, J.L.; Sondermann, H.; Travis, A.J. Ferromagnetic Nanoparticles with Peroxidase-like Activity Enhance the Cleavage of Biological Macromolecules for Biofilm Elimination. Nanoscale 2014, 6, 2588–2593. [Google Scholar] [CrossRef] [Green Version]
- Xi, J.; Wei, G.; An, L.; Xu, Z.; Xu, Z.; Fan, L.; Gao, L. Copper/Carbon Hybrid Nanozyme: Tuning Catalytic Activity by the Copper State for Antibacterial Therapy. Nano Lett. 2019, 19, 7645–7654. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Li, J.; Chu, H. Research Progress in Nanozyme-Based Composite Materials for Fighting against Bacteria and Biofilms. Colloids Surf. B Biointerfaces 2020, 198, 111465. [Google Scholar] [CrossRef]
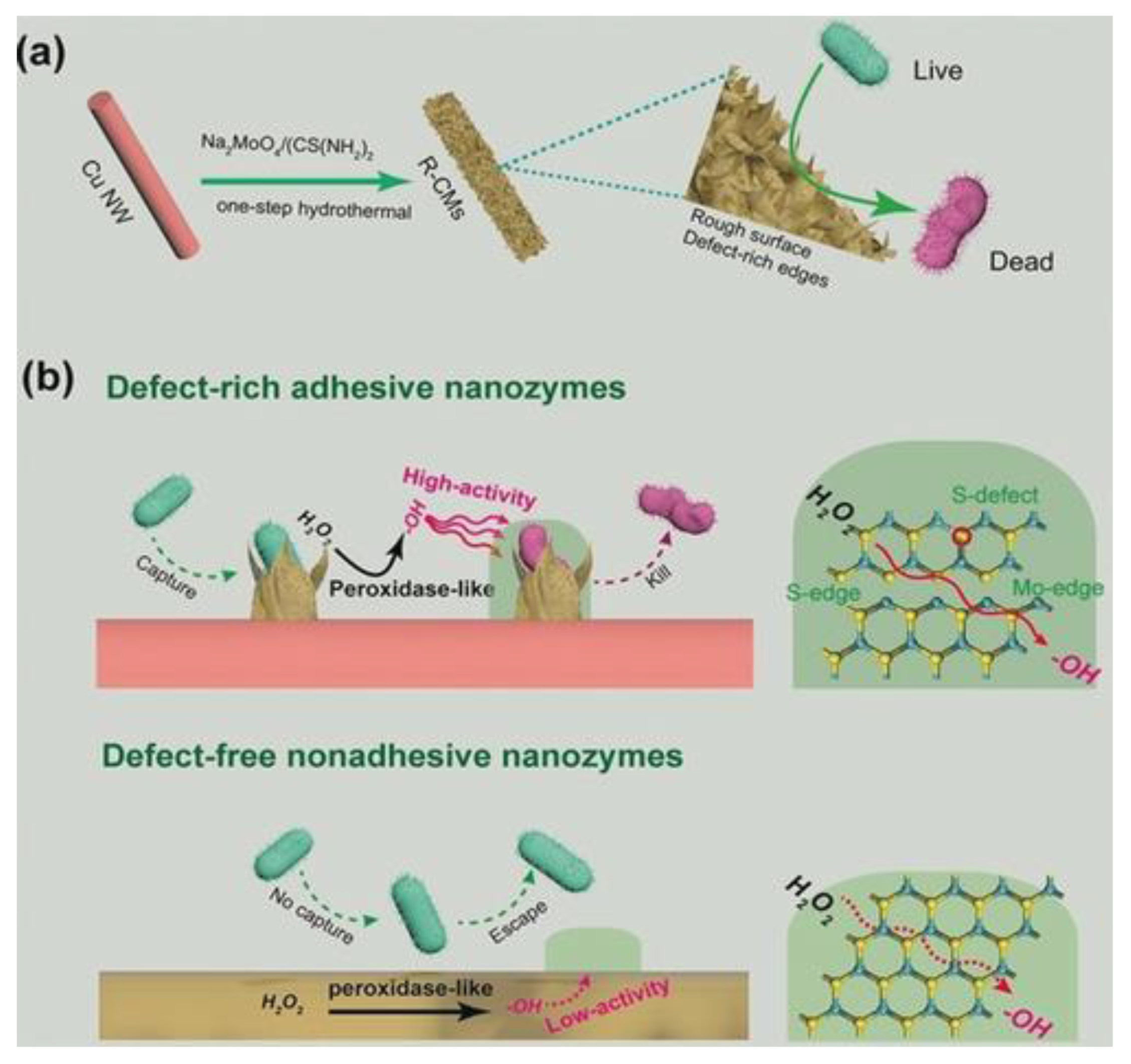
- Cao, F.; Zhang, L.; Wang, H.; You, Y.; Wang, Y.; Gao, N.; Ren, J.; Qu, X. Defect-Rich Adhesive Nanozymes as Efficient Antibiotics for Enhanced Bacterial Inhibition. Angew. Chem. Int. Ed. 2019, 58, 16236–16242. [Google Scholar] [CrossRef]
- Wang, L.; Gao, F.; Wang, A.; Chen, X.; Li, H.; Zhang, X.; Zheng, H.; Ji, R.; Li, B.; Yu, X. Defect-Rich Adhesive Molybdenum Disulfide/RGO Vertical Heterostructures with Enhanced Nanozyme Activity for Smart Bacterial Killing Application. Adv. Mater. 2020, 32, 2005423. [Google Scholar] [CrossRef]
- Chen, Z.; Ji, H.; Liu, C.; Bing, W.; Wang, Z.; Qu, X. A Multinuclear Metal Complex Based DNase-Mimetic Artificial Enzyme: Matrix Cleavage for Combating Bacterial Biofilms. Angew. Chem. Int. Ed. 2016, 55, 10732–10736. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts Promote Streptococcus Mutans Biofilm Matrix Degradation and Enhance Bacterial Killing to Suppress Dental Caries In Vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Qiu, Z.; Liu, Q.; Huang, Y.; Li, D.; Shen, X.; Fan, K.; Xi, J.; Gu, Y.; Tang, Y.; et al. Converting Organosulfur Compounds to Inorganic Polysulfides against Resistant Bacterial Infections. Nat. Commun. 2018, 9, 3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Jia, Y.; Li, J.; Dong, R.; Zhang, J.; Ma, C.; Wang, H.; Rui, Y.; Jiang, X. Indole Derivative-Capped Gold Nanoparticles as an Effective Bactericide in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 29398–29406. [Google Scholar] [CrossRef]
- Cai, S.; Jia, X.; Han, Q.; Yan, X.; Yang, R.; Wang, C. Porous Pt/Ag Nanoparticles with Excellent Multifunctional Enzyme Mimic Activities and Antibacterial Effects. Nano Res. 2017, 10, 2056–2069. [Google Scholar] [CrossRef]
- Mo, S.; Chen, X.; Chen, M.; He, C.; Lu, Y.; Zheng, N. Two-Dimensional Antibacterial Pd@ Ag Nanosheets with a Synergetic Effect of Plasmonic Heating and Ag+ Release. J. Mater. Chem. B 2015, 3, 6255–6260. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Lin, J.-Y.; Chen, W.-J.; Luo, L.; Wei-Guang Diau, E.; Chen, Y.-C. Functional Gold Nanoclusters as Antimicrobial Agents for Antibiotic-Resistant Bacteria. Nanomedicine 2010, 5, 755–764. [Google Scholar] [CrossRef]
- Boomi, P.; Prabu, H.G.; Mathiyarasu, J. Synthesis and Characterization of Polyaniline/Ag–Pt Nanocomposite for Improved Antibacterial Activity. Colloids Surf. B Biointerfaces 2013, 103, 9–14. [Google Scholar] [CrossRef]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-Dependent Antimicrobial Effects of Novel Palladium Nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dong, K.; Liu, Z.; Zhang, Y.; Chen, Z.; Sun, H.; Ren, J.; Qu, X. Activation of Biologically Relevant Levels of Reactive Oxygen Species by Au/g-C3N4 Hybrid Nanozyme for Bacteria Killing and Wound Disinfection. Biomaterials 2017, 113, 145–157. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Ren, J.; Qu, X. A Series of MOF/Ce-Based Nanozymes with Dual Enzyme-like Activity Disrupting Biofilms and Hindering Recolonization of Bacteria. Biomaterials 2019, 208, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, H.; Wang, Y.; Wang, Y.; Huang, Z.; Su, H.; Liu, J. CeO2 Nanoparticle Transformation to Nanorods and Nanoflowers in Acids with Boosted Oxidative Catalytic Activity. ACS Appl. Nano Mater. 2021, 4, 2098–2107. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, L.; Li, Q.; Jiao, L.; Yu, X.; Gao, X.; Qiu, H.; Zhang, Z.; Bing, W. Will the Bacteria Survive in the CeO2 Nanozyme-H2O2 System? Molecules 2021, 26, 3747. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Liu, F.; Wilson, J.; Lelieveld, S.; Korschelt, K.; Wang, T.; Wang, Y.; Reich, T.; Pöschl, U.; Tremel, W.; et al. Antioxidant Activity of Cerium Dioxide Nanoparticles and Nanorods in Scavenging Hydroxyl Radicals. RSC Adv. 2019, 9, 11077–11081. [Google Scholar] [CrossRef] [Green Version]
- Tammina, S.K.; Wan, Y.; Li, Y.; Yang, Y. Synthesis of N, Zn-Doped Carbon Dots for the Detection of Fe3+ Ions and Bactericidal Activity against Escherichia Coli and Staphylococcus Aureus. J. Photochem. Photobiol. B Biol. 2020, 202, 111734. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Pang, X.; Cheng, Y.; Ming, J.; Xiang, S.; Zhang, C.; Lv, P.; Chu, C.; Chen, X.; Liu, G. Ultrasound-Switchable Nanozyme Augments Sonodynamic Therapy against Multidrug-Resistant Bacterial Infection. ACS Nano 2020, 14, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of Nanozyme-hydrogel for Enhanced Capture and Elimination of Bacteria. Adv. Funct. Mater. 2019, 29, 1900518. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Deng, Q.; Sang, Y.; Dong, K.; Ren, J.; Qu, X. Nature-Inspired Construction of MOF@ COF Nanozyme with Active Sites in Tailored Microenvironment and Pseudopodia-Like Surface for Enhanced Bacterial Inhibition. Angew. Chem. Int. Ed. 2021, 60, 3469–3474. [Google Scholar] [CrossRef]
- Roberts, R.E.; Hallett, M.B. Neutrophil Cell Shape Change: Mechanism and Signalling during Cell Spreading and Phagocytosis. Int. J. Mol. Sci. 2019, 20, 1383. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Fan, K.; Yan, X. Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetics for Biomedical Application. Nanozymology 2020, 105–140. [Google Scholar] [CrossRef]
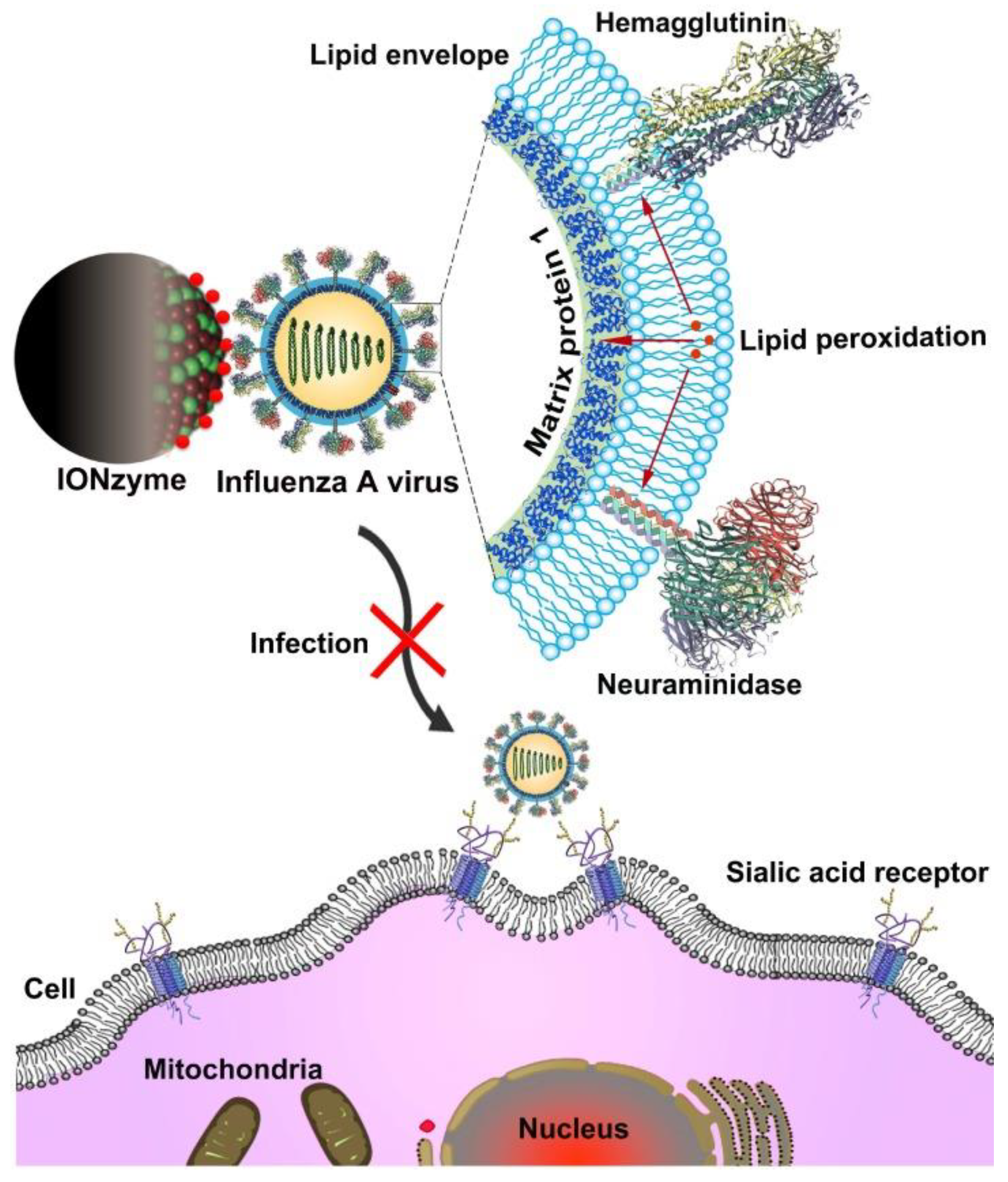
- Qin, T.; Ma, R.; Yin, Y.; Miao, X.; Chen, S.; Fan, K.; Xi, J.; Liu, Q.; Gu, Y.; Yin, Y. Catalytic Inactivation of Influenza Virus by Iron Oxide Nanozyme. Theranostics 2019, 9, 6920. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Ma, S.; Miao, X.; Tang, Y.; Huangfu, D.; Wang, J.; Jiang, J.; Xu, N.; Yin, Y.; Chen, S.; et al. Mucosal Vaccination for Influenza Protection Enhanced by Catalytic Immune-Adjuvant. Adv. Sci. 2020, 7, 2000771. [Google Scholar] [CrossRef]
- Ravishankar Rai, V. Nanoparticles and Their Potential Application as Antimicrobials. 2011.
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Fouad, G.I. A Proposed Insight into the Anti-Viral Potential of Metallic Nanoparticles against Novel Coronavirus Disease-19 (COVID-19). Bull. Natl. Res. Cent. 2021, 45, 1–22. [Google Scholar]
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral Nanoparticles for Sanitizing Surfaces: A Roadmap to Self-Sterilizing against COVID-19. Nano Today 2021, 101267. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Munoz, R.; Lopez-Ribot, J.L. Nanotechnology as an Alternative to Reduce the Spread of COVID-19. Challenges 2020, 11, 15. [Google Scholar] [CrossRef]
- Vahedifard, F.; Chakravarthy, K. Nanomedicine for COVID-19: The Role of Nanotechnology in the Treatment and Diagnosis of COVID-19. Emergent Mater. 2021, 4, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, M.; Xu, Y.; Zhang, J.; Meng, X.; An, X.; Sun, L.; Guo, L.; Shan, X.; Ge, J.; et al. Novel Gold Nanorod-Based HR1 Peptide Inhibitor for Middle East Respiratory Syndrome Coronavirus. ACS Appl. Mater. Interfaces 2019, 11, 19799–19807. [Google Scholar] [CrossRef]
- Antoine, T.E.; Mishra, Y.K.; Trigilio, J.; Tiwari, V.; Adelung, R.; Shukla, D. Prophylactic, Therapeutic and Neutralizing Effects of Zinc Oxide Tetrapod Structures against Herpes Simplex Virus Type-2 Infection. Antivir. Res. 2012, 96, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Tabish, T.A.; Hamblin, M.R. Multivalent Nanomedicines to Treat COVID-19: A Slow Train Coming. Nano Today 2020, 35, 100962. [Google Scholar] [CrossRef]
- Zhao, S.; Duan, H.; Yang, Y.; Yan, X.; Fan, K. Fenozyme Protects the Integrity of the Blood–Brain Barrier against Experimental Cerebral Malaria. Nano Lett. 2019, 19, 8887–8895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fan, K.; Yan, X. Nanozymes: Created by Learning from Nature. Sci. China Life Sci. 2020, 63, 1183–1200. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.F.; Yang, W.; Jiang, W.; Geng, S.; Peng, T.; Li, J.L. Magnetosomes Eliminate Intracellular Reactive Oxygen Species in Magnetospirillum Gryphiswaldense MSR-1. Environ. Microbiol. 2012, 14, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Fang, L.; Wu, K.; Yan, X.; Fan, K. Ferritins as Natural and Artificial Nanozymes for Theranostics. Theranostics 2020, 10, 687–706. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, H.; Zhang, Y.; Li, Z.; Lin, Y. Enzyme-Mimic Activity of Ferric Nano-Core Residing in Ferritin and Its Biosensing Applications. Anal. Chem. 2011, 83, 8611–8616. [Google Scholar] [CrossRef] [PubMed]


| Nanozymes | Substrate | Km (mM) | Vmax (M × s−1) | Ref. |
|---|---|---|---|---|
| V2O5 | TMB H2O2 | 0.738 0.232 | 1.85 × 10−5 1.29 × 10−5 | [21] |
| Fe3O4 MNPs | TMB H2O2 | 0.434 154 | 10.00 × 10−8 9.78 × 10−8 | [21] |
| MOF(Co/2Fe0) | TMB H2O2 | 0.25 4.22 | 0.38 × 10−7 0.49 × 10−7 | [20] |
| AuNPs | glucose | 0.41 | 0.10 × 10−6 | [22] |
| ZnO | starch | 0.022 | 1.81 × 10−6 | [23] |
| GOx-Fe0@EM-A | H2O2 | 29.59 | 3.81 × 10−8 | [24] |
| Nanozyme | Ref. | Target Enzyme | Enzyme-Nanozyme Interaction Type | Application |
|---|---|---|---|---|
| AuNPs | [132] | ChT | electrostatic interactions | Protein interaction |
| ZnONPs | [135] | Beta-Gal | Hydrogen bonding, Van der Vaals, molecular shape | Ovarian cancer, cell aging |
| 2DMoS2 | [138] | β-Gal, ChT | electrostatic interactions | diagnosis, treatment |
| 2DMoS2 | [136] | β-lactamase | electrostatic interactions, steric hindrance | overcoming penicillin resistance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, H.; Wójciuk, K.; Karczmarczyk, U. Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation. Appl. Sci. 2021, 11, 9019. https://doi.org/10.3390/app11199019
Lewandowska H, Wójciuk K, Karczmarczyk U. Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation. Applied Sciences. 2021; 11(19):9019. https://doi.org/10.3390/app11199019
Chicago/Turabian StyleLewandowska, Hanna, Karolina Wójciuk, and Urszula Karczmarczyk. 2021. "Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation" Applied Sciences 11, no. 19: 9019. https://doi.org/10.3390/app11199019
APA StyleLewandowska, H., Wójciuk, K., & Karczmarczyk, U. (2021). Metal Nanozymes: New Horizons in Cellular Homeostasis Regulation. Applied Sciences, 11(19), 9019. https://doi.org/10.3390/app11199019








