Bioactive Fraction of Aronia melanocarpa Fruit Inhibits Adipogenic Differentiation of Cultured 3T3-L1 Cells
Abstract
:1. Introduction
2. Materials and Methods
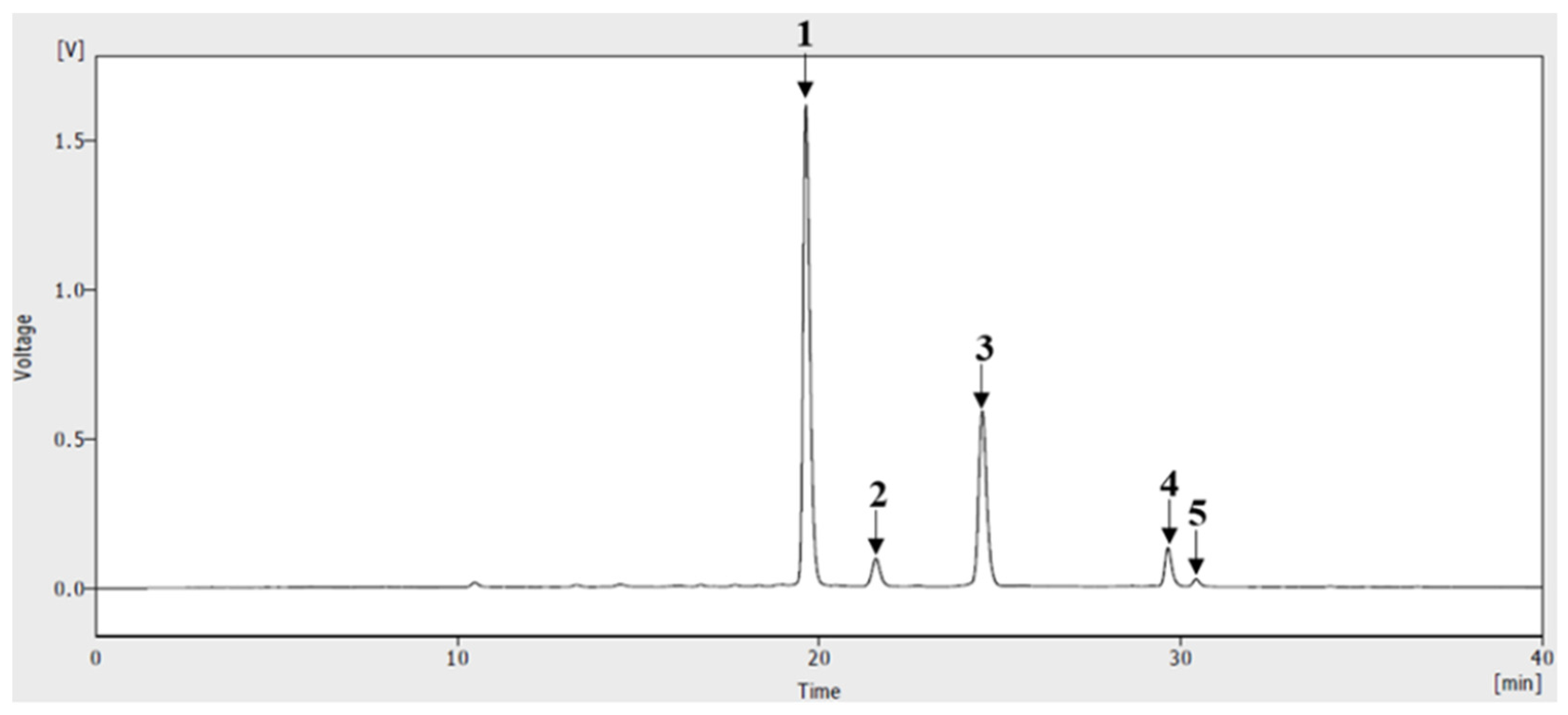
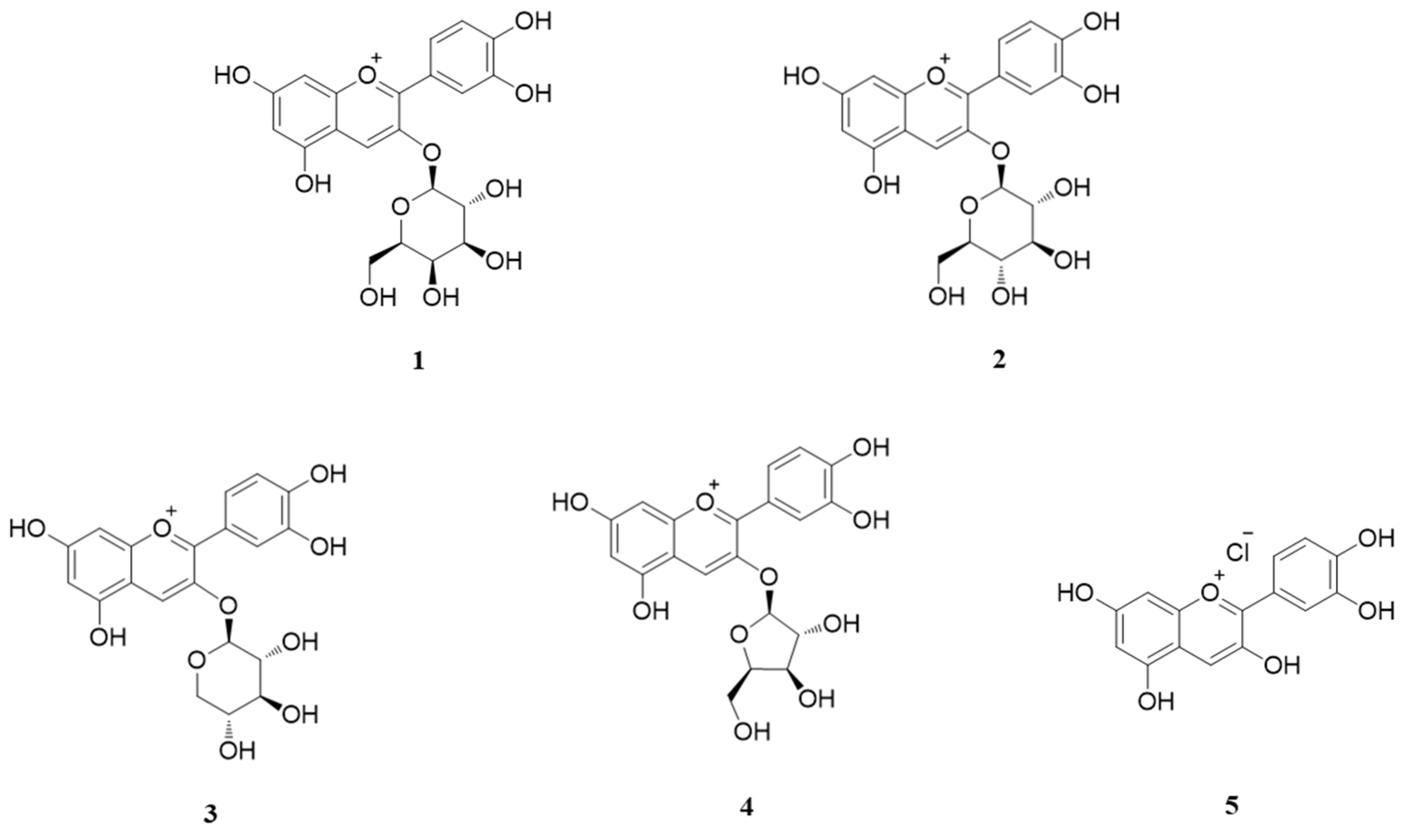
2.1. Plant Preparation and HPLC Analysis of ABF®
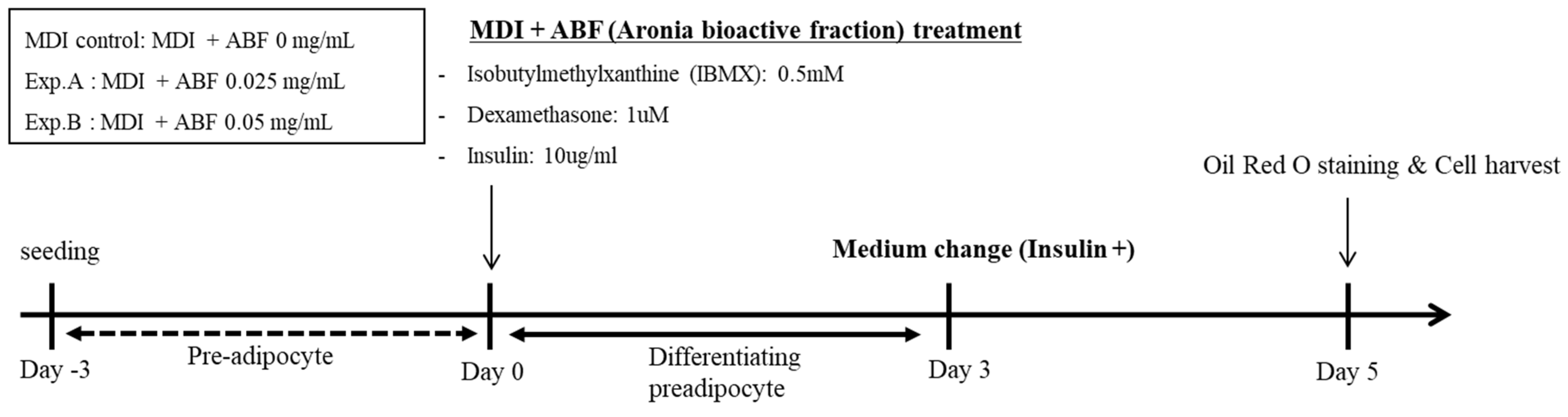
2.2. Cell Culture and Treatment
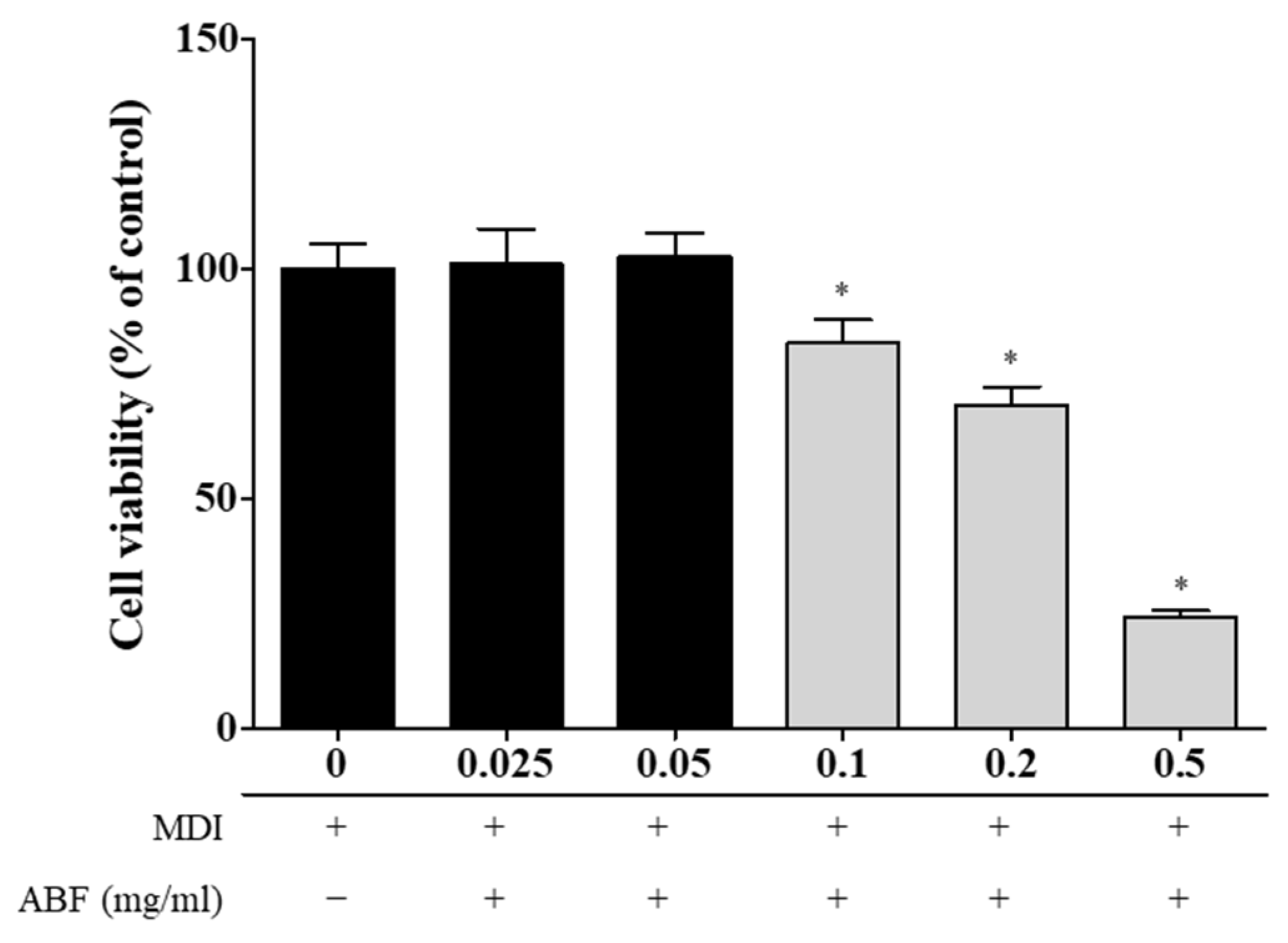
2.3. Determination of Cell Viability
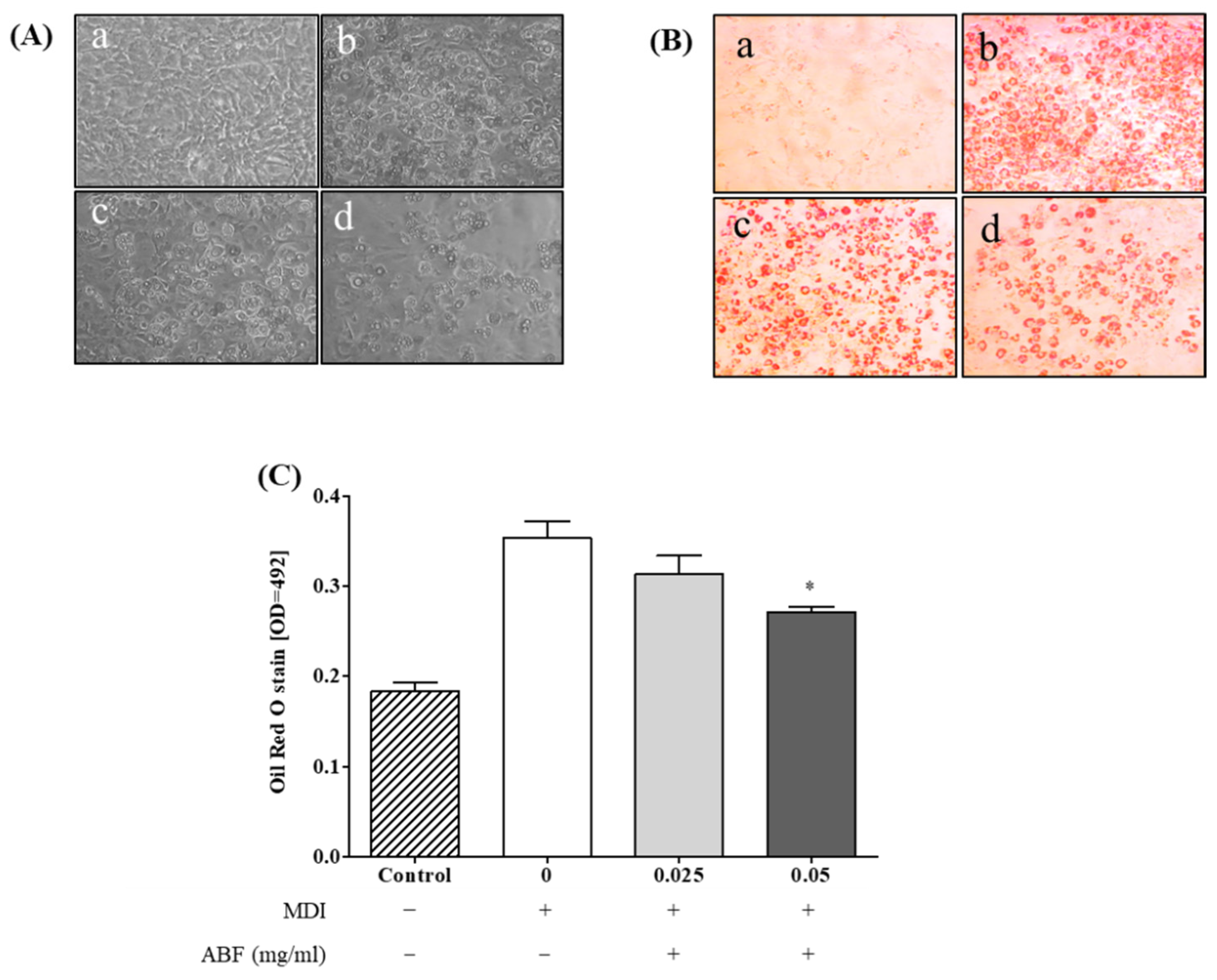
2.4. Oil Red O Staining
2.5. RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.6. Immunoblot Analysis
2.7. Adipokine Secretion Measurement in Cell Supernatant
2.8. Statistical Analysis
3. Results
3.1. HPLC of Anthocyanins from ABF®
3.2. Effects of ABF® on Cell Viability of 3T3-L1 Adipocytes
3.3. Effects of ABF® on Lipid Accumulation in 3T3-L1 Adipocytes
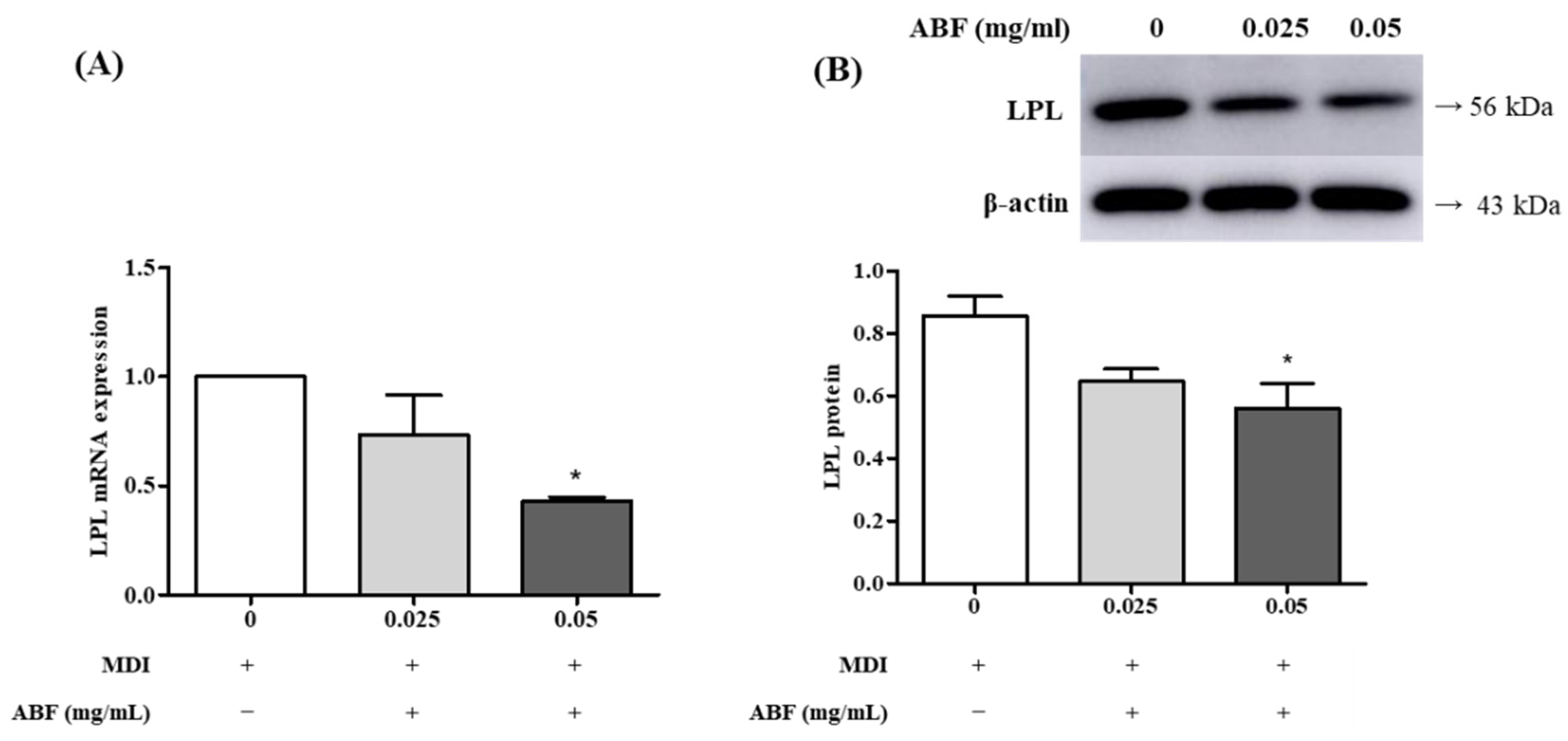
3.4. Effects of ABF® on the mRNA and Protein Expression of Lipoprotein Lipase (LPL) in 3T3-L1 Adipocytes
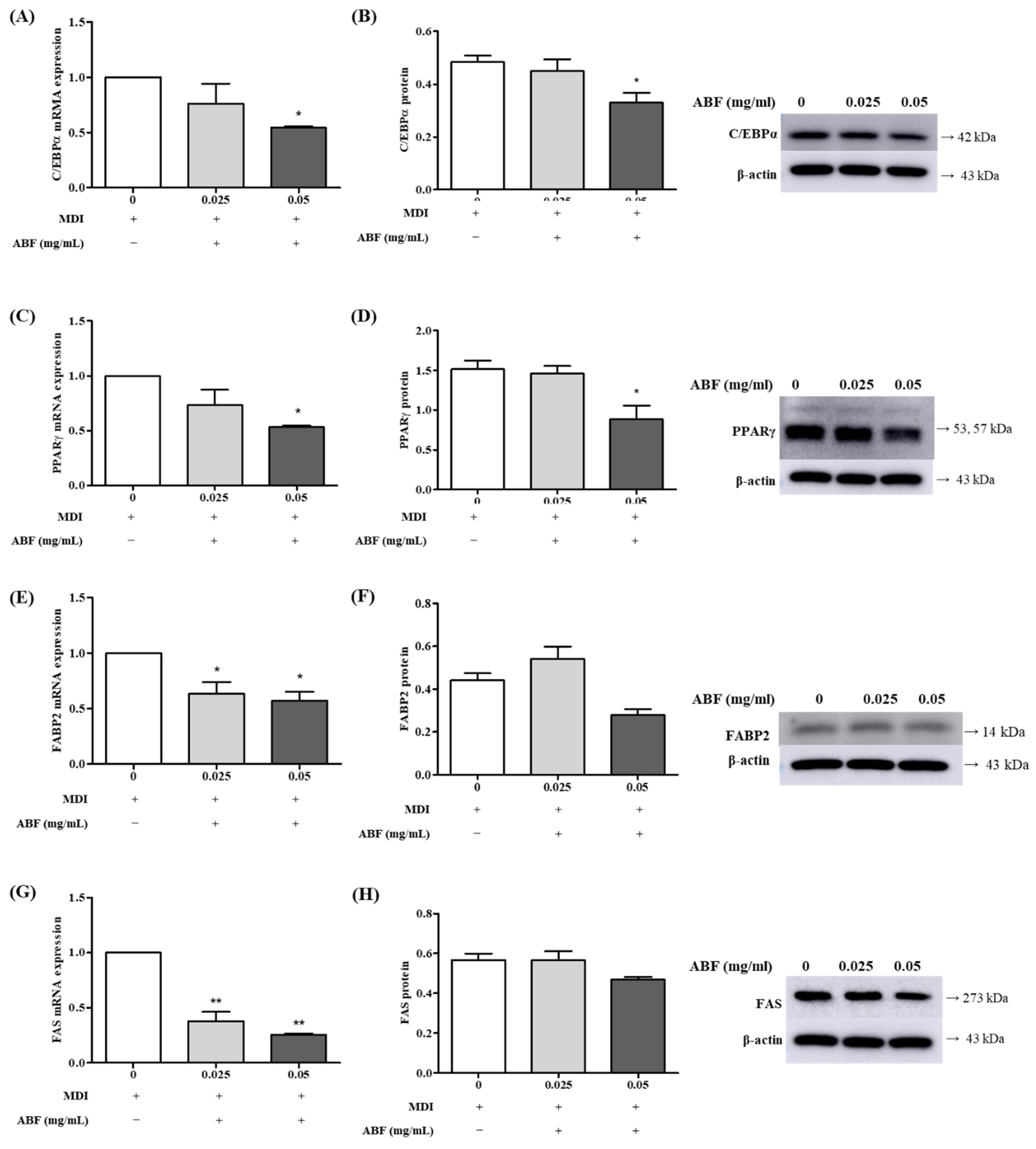
3.5. Effects of ABF® on the mRNA and Protein Expression of Adipogenesis Levels in 3T3-L1 Adipocytes
3.6. Effects of ABF® on Adipokine Secretion in 3T3-L1 Adipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 13 August 2021).
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A preventable, treatable, but relapsing disease. Nutrition 2020, 71, 110615. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.H. Deaths attributable to obesity. Jama 2005, 293, 1918–1919. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xi, B.; Yang, L.; Sun, J.; Zhao, M.; Bovet, P. Trends in the prevalence of overweight, obesity, and abdominal obesity among Chinese adults between 1993 and 2015. Int. J. Obes. 2021, 45, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Morrison, R.F.; Farmer, S.R. Insights into the transcriptional control of adipocyte differentiation. J. Cell. Biochem. 1999, 75 (Suppl. 32–33), 59–67. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Student, A.K.; Hsu, R.Y.; Lane, M.D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 1980, 255, 4745–4750. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Xu, Q.; Yuan, X.; Dai, W.; Shen, X.; Wang, Z.; Chang, G.; Wang, Z.; Chen, G. The differentiation of preadipocytes and gene expression related to adipogenesis in ducks (Anas platyrhynchos). PLoS ONE 2018, 13, e0196371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakab, J.; Miškić, B.; Mikšić, Š.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Kim, H.J.; Jeong, J.W.; Park, C.; Kim, B.W.; Choi, Y.H. Inhibition of Adipocyte Differentiation by Anthocyanins Isolated from the Fruit of Vitis coignetiae Pulliat is Associated with the Activation of AMPK Signaling Pathway. Toxicol. Res. 2018, 34, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, X.; Liu, P. Lipid droplet proteins and metabolic diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1968–1983. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.V.; Belcheva, A. Current knowledge of Aronia melanocarpa as a medicinal plant. Folia Med. 2006, 48, 11–17. [Google Scholar]
- Platonova, E.Y.; Shaposhnikov, M.V.; Lee, H.-Y.; Lee, J.-H.; Min, K.-J.; Moskalev, A. Black chokeberry (Aronia melanocarpa) extracts in terms of geroprotector criteria. Trends Food Sci. Technol. 2021, 114, 570–584. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [Green Version]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Analysis. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef] [PubMed]
- Oszmianski, J.; Sapis, J.C. Anthocyanins in fruits of Aronia melanocarpa (chokeberry). J. Food Sci. 1988, 53, 1241–1242. [Google Scholar] [CrossRef]
- Choi, E.M.; Suh, K.S.; Jung, W.W.; Park, S.Y.; Chin, S.O.; Rhee, S.Y.; Kim Pak, Y.; Chon, S. Glabridin attenuates antiadipogenic activity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in murine 3T3-L1 adipocytes. J. Appl. Toxicol. JAT 2018, 38, 1426–1436. [Google Scholar] [CrossRef]
- Suh, K.S.; Choi, E.M.; Jung, W.W.; Park, S.Y.; Chin, S.O.; Rhee, S.Y.; Pak, Y.K.; Chon, S. 27-Deoxyactein prevents 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cellular damage in MC3T3-E1 osteoblastic cells. J. Environ. Sci. Health Part A 2018, 53, 561–570. [Google Scholar] [CrossRef]
- Kim, Y.I.; Lee, C.Y.; Shin, M.K. Downregulation of activin-signaling gene expression in passaged normal human dermal fibroblasts. Biomed. Rep. 2020, 12, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of Black Chokeberry Aronia melanocarpa in the Prevention of Chronic Diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.K.; Lee, J.W.; Choi, H.; Yim, S.V. Aronia melanocarpa Fruit Bioactive Fraction Attenuates LPS-Induced Inflammatory Response in Human Bronchial Epithelial Cells. Antioxidants 2020, 9, 816. [Google Scholar] [CrossRef] [PubMed]
- Karschner, V.A. Post-Transcriptional Regulation of mRNA Metabolism during Differentiation of 3T3-L1 Cells: Role of HuR; East Carolina University: Greenville, NC, USA, 2010. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.niddk.nih.gov/health-information/weight-management/prescription-medications-treat-overweight-obesity (accessed on 13 August 2021).
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Health Information Service. Available online: https://www.nhsinform.scot/illnesses-and-conditions/nutritional/obesity (accessed on 12 August 2021).
- University of California San Francisco Health. Available online: https://www.ucsfhealth.org/conditions/obesity/treatment (accessed on 12 August 2021).
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef]
- Cheung, B.M.Y.; Cheung, T.T.; Samaranayake, N.R. Safety of antiobesity drugs. Ther. Adv. Drug Saf. 2013, 4, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Rhyu, D.Y.; Sharma, B.R.; Yokozawa, T. Inhibition of preadipocyte differentiation and lipid accumulation by 7-O-galloyl-d-sedoheptulose treatment in 3T3-L1 adipocytes. Biomed. Prev. Nutr. 2013, 3, 319–324. [Google Scholar] [CrossRef]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.H.; Jegal, J.; Kim, Y.N.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. Chokeberry Extract and Its Active Polyphenols Suppress Adipogenesis in 3T3-L1 Adipocytes and Modulates Fat Accumulation and Insulin Resistance in Diet-Induced Obese Mice. Nutrients 2018, 10, 1734. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Sharma, A.; Lee, H.J. Anti-Adipogenic Effects of Delphinidin-3-O-β-Glucoside in 3T3-L1 Preadipocytes and Primary White Adipocytes. Molecules 2019, 24, 1848. [Google Scholar] [CrossRef] [Green Version]
- Amiri, M.; Yousefnia, S.; Seyed Forootan, F.; Peymani, M.; Ghaedi, K.; Nasr Esfahani, M.H. Diverse roles of fatty acid binding proteins (FABPs) in development and pathogenesis of cancers. Gene 2018, 676, 171–183. [Google Scholar] [CrossRef]
- Ordovas, J.M. Genetic influences on blood lipids and cardiovascular disease risk: Tools for primary prevention. Am. J. Clin. Nutr. 2009, 89, 1509S–1517S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, C.; Ordovas, J.M. Recent Advances in Nutrigenetics and Nutrigenomics; Academic Press: London, UK, 2012. [Google Scholar]
- Montoudis, A.; Seidman, E.; Boudreau, F.; Beaulieu, J.F.; Menard, D.; Elchebly, M.; Mailhot, G.; Sane, A.T.; Lambert, M.; Delvin, E.; et al. Intestinal fatty acid binding protein regulates mitochondrion beta-oxidation and cholesterol uptake. J. Lipid Res. 2008, 49, 961–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajda, A.M.; Storch, J. Enterocyte fatty acid-binding proteins (FABPs): Different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot. Essent. Fat. Acids 2015, 93, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halldén, G.; Aponte, G.W. Evidence for a role of the gut hormone PYY in the regulation of intestinal fatty acid-binding protein transcripts in differentiated subpopulations of intestinal epithelial cell hybrids. J. Biol. Chem. 1997, 272, 12591–12600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudkowska, I.; Pérusse, L. Individualized weight management: What can be learned from nutrigenomics and nutrigenetics? Progress Mol. Biol. Transl. Sci. 2012, 108, 347–382. [Google Scholar] [CrossRef]
- Dave, S.; Kaur, N.J.; Nanduri, R.; Dkhar, H.K.; Kumar, A.; Gupta, P. Inhibition of adipogenesis and induction of apoptosis and lipolysis by stem bromelain in 3T3-L1 adipocytes. PLoS ONE 2012, 7, e30831. [Google Scholar] [CrossRef] [Green Version]
- Pirahanchi, Y.; Anoruo, M.; Sharma, S. Biochemistry, Lipoprotein Lipase. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef]
- MedlinePlus_Lipoprotein Lipase Gene. Available online: https://medlineplus.gov/genetics/gene/lpl/#conditions (accessed on 13 August 2021).
- He, Y.; Niu, W.; Xia, C.; Cao, B. Daidzein reduces the proliferation and adiposeness of 3T3-L1 preadipocytes via regulating adipogenic gene expression. J. Funct. Foods 2016, 22, 446–453. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.; Gao, Y.; Shi, Z.; Hu, Y.; Ren, G. Suppressive effects of saponin-enriched extracts from quinoa on 3T3-L1 adipocyte differentiation. Food Funct. 2015, 6, 3282–3290. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Y.; Gong, Z.; Sheng, X.; Li, Z.; Zhang, W.; Qin, Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem. Biophys. Res. Commun. 2006, 348, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [Green Version]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Freitag Luglio, H. Genetic Variation of Fatty Acid Oxidation and Obesity, A Literature Review. Int. J. Biomed. Sci. IJBS 2016, 12, 1–8. [Google Scholar]
- Khan, M.; Joseph, F. Adipose tissue and adipokines: The association with and application of adipokines in obesity. Scientifica 2014, 2014, 328592. [Google Scholar] [CrossRef]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, S.; Kim, H.Y.; Cho, E.J. Acer okamotoanum inhibits adipocyte differentiation by the regulation of adipogenesis and lipolysis in 3T3-L1 cells. Int. J. Mol. Med. 2020, 45, 589–596. [Google Scholar] [CrossRef] [PubMed]

| Genes | Accession No. | Forward Primer | Reverse Primer | Product Size (bp) |
|---|---|---|---|---|
| C/EBPα 1 | NM_007678.3 | 5′-AAACAACGCAACGTGGAGA-3′ | 5′-GCGGTCATTGTCACTGGTC-3′ | 60 |
| PPARγ2 2 | EF062476.1 | 5’-TTATAGCTGTCATTATTC TCAGTGGAG-3’ | 5’-ACTCTGGGTGGTTCAGCTTG-3’ | 123 |
| FABP2 3 | NM_007980.2 | 5′-ACGGAACGGAGCTCACTG-3′ | 5′-TTACCAGAAACCTCTCGGACA-3′ | 112 |
| FAS 4 | NM_007988.3 | 5′- GCTCCTCGCTTGTCGTCT-3′ | 5′- GCAACTTCCCCGACATACC-3′ | 86 |
| LPL 5 | NM_008509.2 | 5′-TTTGTGAAATGCCATGACAAG-3′ | 5′-CAGATGCTTTCTTCTCTTGTTTGT-3′ | 76 |
| GAPDH | AY618199.1 | 5’-AGGCAAAAGACACCGTCAAG-3’ | 5’-CACAAGAAGATGCGGCTGT-3’ | 64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-Y.; Suh, K.S.; Kim, Y.I.; Jang, B.-K.; Kim, B.-H.; Yim, S.-V. Bioactive Fraction of Aronia melanocarpa Fruit Inhibits Adipogenic Differentiation of Cultured 3T3-L1 Cells. Appl. Sci. 2021, 11, 9224. https://doi.org/10.3390/app11199224
Lee H-Y, Suh KS, Kim YI, Jang B-K, Kim B-H, Yim S-V. Bioactive Fraction of Aronia melanocarpa Fruit Inhibits Adipogenic Differentiation of Cultured 3T3-L1 Cells. Applied Sciences. 2021; 11(19):9224. https://doi.org/10.3390/app11199224
Chicago/Turabian StyleLee, Hwa-Young, Kwang Sik Suh, Young Il Kim, Bong-Keun Jang, Bo-Hyung Kim, and Sung-Vin Yim. 2021. "Bioactive Fraction of Aronia melanocarpa Fruit Inhibits Adipogenic Differentiation of Cultured 3T3-L1 Cells" Applied Sciences 11, no. 19: 9224. https://doi.org/10.3390/app11199224








